Co-option of stress mechanisms in the origin of evolutionary novelties
Abstract
It is widely accepted that stressful conditions can facilitate evolutionary change. The mechanisms elucidated thus far accomplish this with a generic increase in heritable variation that facilitates more rapid adaptive evolution, often via plastic modifications of existing characters. Through scrutiny of different meanings of stress in biological research, and an explicit recognition that stressors must be characterized relative to their effect on capacities for maintaining functional integrity, we distinguish between: (1) previously identified stress-responsive mechanisms that facilitate evolution by maintaining an adaptive fit with the environment, and (2) the co-option of stress-responsive mechanisms that are specific to stressors leading to the origin of novelties via compensation. Unlike standard accounts of gene co-option that identify component sources of evolutionary change, our model documents the cost-benefit trade-offs and thereby explains how one mechanism—an immediate response to acute stress—is transformed evolutionarily into another—routine protection from recurring stressors. We illustrate our argument with examples from cell type origination as well as processes and structures at higher levels of organization. These examples suggest a general principle of evolutionary origination based on the capacity to switch between regulatory states related to reproduction and proliferation versus survival and differentiation.
“The rationale for any multilayered biological process can only be derived from the order in which its component parts evolved.”
Pauline Schaap, 2011
What “Everyone” Knows About Stress and Evolution
A large and growing literature has documented that stressful conditions can facilitate evolutionary change in populations of organisms (see, e.g., Hoffmann and Hercus 2000; Nevo 2001; Badyaev 2005a,b; Chevin and Hoffmann 2017; Eyck et al. 2019). The mechanisms that underlie this evolutionary facilitation are diverse and include: global hypermutation in bacterial taxa under starvation (Foster 2007; MacLean et al. 2013; Swings et al. 2017), increased translational readthrough due to prion presence (True and Lindquist 2000; Tyedmers et al. 2008), the revealing of cryptic genetic variation (Rutherford and Lindquist 1998; Zabinsky et al. 2019), and transposable element activation leading to higher mutation rates (Saier and Zhang 2014; Vandecraen et al. 2016). In these situations, stressful conditions either promote a higher mutation rate or are a special case of genotype-by-environment interactions that yield new or hidden genetic variation. The latter (i.e., G×E interactions) are often manifested in plastic modifications of existing traits that release hidden genetic variation regardless of whether the overall level of buffering is affected (Bergman and Siegal 2003; Hermisson and Wagner 2004; Levy and Siegal 2008).
A common effect of all these mechanisms is a generic increase in heritable variation available for selection to act upon, even in cases where the effect is more localized, such as in hypermutation of V(D)J genes for antibody formation in the vertebrate adaptive immune system (Jacobs and Bross 2001) or mutator alleles that hitchhike with beneficial alleles through effects due to a particular mutational spectrum (Couce et al. 2013). Despite sometimes being more localized to certain parts of the genome (Martincorena and Luscombe 2012; Martincorena et al. 2012), mutational effects are not matched with the particular form of stress conditions being experienced (e.g., starvation vs. prolonged temperature elevation). Metaphorically speaking, a generalized increase of phenotypic variation is an empirical bet that populations make to identify beneficial variants more quickly or efficiently under stress than would be available normally due to standard mutation, recombination, and other mechanisms that generate heritable genetic variation during the evolutionary process.
The urgency of understanding stress-induced evolvability derives from the severe environmental challenges facing a variety of taxa because of climate change (Chevin and Hoffmann 2017; Grant et al. 2017; Kingsolver and Buckley 2017; van de Pol et al. 2017). Whether these mechanisms harbor sufficient resources to prevent large-scale extinction remains to be seen. However, this facilitation of evolution in situations of stress primarily concentrates on if and how rates of adaptive change track increasing rates of environmental alteration (e.g., temperature increases). The role of stress in these conditions relates to the increase in heritable phenotypic variation visible to selection (Paaby and Rockman 2014; Vihervaara et al. 2018). This variation is typically quantitative in nature, modifying many traits through augmentation or diminishment among which at least one variant (ideally) may be beneficial. For example, a mutation that increases the expression of chaperone proteins can compensate for deleterious misfolding effects in a target protein in the case of high-temperature conditions (Maisnier-Patin et al. 2005). Models for this stress-facilitated evolution fall under the larger family of models focused on environmentally induced changes to existing characters (i.e., phenotypic plasticity). In these “plasticity first” models, the environment first causes a set of genotype-specific changes in a phenotypic character and then selection favors some range of this plastic change for a long enough time that the induced phenotype is genetically assimilated to become a recurring feature independent of the changes in environmental variables (Levis and Pfennig 2016, 2019, 2020; Pfennig 2021). Stressful conditions are environmental changes that induce plastic responses in traits. However, whether stress-facilitated evolution should always be conceptualized as a subset of responses to environmental change in plasticity first models is unclear because there has not been sustained attention to the diverse meanings associated with the concept of stress (see STRESS: CHARACTERIZED AND CONTEXTUALIZED and MODELS OF EVOLUTIONARY CHANGE FACILITATED BY STRESS).
What these models neglect is the possibility of a creative role for stress in evolution related to the origin of new organismal features, especially in multicellular taxa (Nedelcu and Michod 2006, 2020; Wagner et al. 2019; Davison and Michod 2021). Instead of a general increase in variation due to phenotypic plasticity under conditions of stress, a specific stabilization of preexisting regulatory variation prompted by stressful conditions could yield a new trait that specifically compensates for the conditions of stress. Accounting for how and why specific mechanisms might be recruited or co-opted via stabilization in this way is the key challenge. The model of Stress-Induced Evolutionary Innovation (SIEI), which elaborates an earlier statement (Wagner et al. 2019), suggests that stress-response mechanisms are co-opted (i.e., permanently stabilized) to control the development of novel features. This yields a model of how one mechanism—an immediate response to acute stress—is transformed evolutionarily into another via permanent control of the development of a novel character that provides protection from recurring stressors (What is Stress? and Co-option: Descriptive and Explanatory).
This distinctive mode of evolutionary change has been more significant in the history of life than previously appreciated, an insight anticipated in a number of research fields (e.g., Nedelcu and Michod 2006, 2020; Ritchie et al. 2008; Schaap 2011, 2021; König and Nedelcu 2016, 2020; Erkenbrack et al. 2018). Below we summarize a number of diverse examples that illustrate the wide applicability of this model: from cell type origination, such as germ-soma differentiation in algae or fruiting body constituents in slime molds, to the emergence of processes and structures at higher levels of organization, such as dorsal closure in insect morphogenesis, metazoan eyes, and the cetacean epidermis (Examples of Evolution via the Co-option of Stress Response Mechanisms). Although these examples are heterogeneous, a number of empirical tests can be pursued to validate the SIEI model (Testing SIEI). Additionally, they share an abstract pattern that suggests a principle for evolutionary origination: stress-induced state switching. This principle involves a homology of process between the binary phenotypic and gene regulatory states related to either (a) reproduction and proliferation or (b) survival and differentiation. We label these processes “generative dyads” because shifting between the two states, especially a permanent stabilization of stress-induced regulation, is evolutionarily productive (i.e., can yield new characters). These homologous processes facilitate new iterations of the same type of interactions in diverse contexts and across levels of organization in biological systems (A Principle of Evolutionary Origination?). In summary, we elaborate and situate the SIEI model in a conceptual framework of stress research, discuss its status with respect to standard treatments of co-option, and review a diverse set of examples across different taxa. This makes it possible to go beyond what everyone knows about the role of stress in evolution and formulate a principle of character origination with broad applicability that suggests new empirical tests for a research program on the creative role of stress.
What is Stress?
HISTORICAL AMBIGUITY
Stress has a complicated conceptual history (Csermely 1998; Abbott 2001). The concept originated in the context of qualitative medical diagnoses of mental or nervous diseases and later was adopted in the quantitative physiology of organisms and human sociology. Contained within these diverse contexts is an ambiguity reflected in the use of “stress” as both noun and verb (Kültz 2020). The original context exhibited a split between a concern with stressors of modern life and the experience of or response to stress (Abbott 2001, ch. 2). In biological research, this ambiguity grew in two ways. First, stress as a phenomenon can exist at different levels of organization in living systems (genome, organelle, cells, tissues, organs, organisms, populations), including social groups and societal institutions (e.g., “stress tests” for banks). The same applies to the stress response (e.g., the genomic, cellular, or organismal stress responses). Therefore, stressor and response may not be confined to the same level of organization, which leaves an increased number of possibilities to track both empirically and theoretically (i.e., many-many relationships). Second, these multi-level stressors and responses were studied by diverse disciplinary approaches that gave specialized meaning to the concept, especially for the purposes of facilitating measurement (i.e., operationalization) and mathematical modeling. This yielded contradictory terminologies in different branches of science studying stress. These distinct conceptions, sometimes captured by subtle changes in language (e.g., distress), are in various ways interdependent and resist a simple core or essential definition of the term “stress.”
A less-observed facet of this complicated history is a common commitment to the generic nature of stress, both as phenomenon and response. Whether an increased pace of “modern life” or elevated temperatures during ontogeny, stressors were typically general environmental phenomena (e.g., “noise”) and evoked a generic stress response. This was codified in the work of Hans Selye who founded the modern physiological investigation of stress (Selye 1932, 1955). Unlike a bacterial pathogen that colonized and affected a particular organ or functional system, stress applied ubiquitously to the organism. This nonspecificity of stress combined with another ambiguity for stress: the direction of its valence (i.e., positive or negative). Although most research into stress conceives of it as negative, such as high levels of toxins that induce protein unfolding or the production of reactive oxygen species (ROS), it also has been recognized as beneficial to a degree, especially in phenomena like dose-response or hormesis (Calabrese 2018). Just as some but not too much friction is important for locomotion, a moderate amount of challenge is necessary for normal functioning and even protective against the negative impact derived from intense stressors later in life.
These historical considerations might suggest that attempts to univocally answer the question “what is stress?” are misguided and even that using the term “stress” in evolutionary explanations might lead to more confusion than insight. However, the emphasis on the generic nature of stress and the ambiguities manifested by the concept can serve as a guide to investigating the evolutionary relevance and significance of stress. To pursue this task, we need an explicit characterization of stress and several allied concepts. Importantly, we do not claim that our characterization is uniquely accurate; in fact, many of our distinctions are broadly accepted but not always represented with the same terminology (see, e.g., Selye 1955; Romero et al. 2009; Koolhaas et al. 2011; Del Giudice et al. 2018; Kültz 2020). Instead, we seek to resolve ambiguity with respect to how stress as a phenomenon may have played distinctive roles in evolution and how stress responses at different levels of organization are conducive to evolutionary innovation (e.g., Erkenbrack et al. 2018; König and Nedelcu 2020; Schaap 2021).
STRESS: CHARACTERIZED AND CONTEXTUALIZED
In a recent attempt to clarify the meaning of stress for physiological investigation, Kültz recognized the difficulties associated with the concept: “Establishing a succinct definition of ‘stress’ that manages to capture its scientific basis in a universal and unambiguous manner is challenging” (Kültz 2020, p. 1). Kültz sought a complete derivation of stress and allied concepts from analogies with physics. This has some distinct advantages in offering a precise vocabulary, but it also exposes a lacuna: a framework built from the theoretical resources of materials science lacks any account of autopoiesis—the capacity of and need for a biological system to engage in self-maintenance (Maturana and Varela 1972).
Autopoiesis is a form of autonomy and often taken to be distinctive of living organisms (Moreno and Mossio 2015). Although absent from many biological discussions of stress, in part because they concentrate on neuroendocrine systems and behavior in mammalian model organisms (e.g., Romero et al. 2009; Koolhaas et al. 2011), it is crucial for modeling the main features of both stress phenomena and responses, and therefore prompts a specific characterization of stress-related concepts. The damage that stressors cause and the organism's attempt to counteract it by stress responses only make sense when recognizing that organisms maintain their integrity through continual re-constitution of their molecular component parts.
A stressor is any deviation in the value of an external environmental or internal milieu variable from the range of values that is favorable for the autopoietic stability of an entity. This use of “autopoiesis” diverges somewhat from standard uses because we also apply it to organismal subunits, such as organs or tissues—autopoietic stability obtains at different levels of organization. However, it retains core elements associated with the designation: (1) a thermodynamically unlikely material system that needs energy and material turnover (metabolism) to maintain itself; (2) a homeostatic system that is organized so as to compensate for environmental and internally generated perturbations, thereby attaining and sustaining a dynamic equilibrium (e.g., homeothermy); and (3) a self-producing and self-organizing system that continuously replaces components and replenishes activities in the process of maintenance (e.g., protein turnover in a cell or cellular replacement in an epithelium).1 The implication is that the category “stressor” is inherently relational because its members (i.e., internal or external environmental deviations) are defined in relation to a normal autopoietic range of states. In other words, the notion of a stressor is not reducible to the magnitude or intensity of a physical or chemical variable; it must be characterized with respect to the effect of such a variable on an entity with a capacity for maintaining its functioning and integrity. A stressor is always defined in terms of the context of the organic system that is affected by it; 60°C is not stressful for simple thermophiles, but it is for a mammal.
Strain is the metabolic and physiological cost imposed on the organism or other autopoietic entity by attempts to counter the effects of a stressor at some level of organization. The response of the self-maintaining entity to a stressor can be divided into three regimes: homeostatic, stress (sensu stricto), and failure. In the homeostatic regime, regulatory mechanisms compensate for the effect of a stressor such that the strain is below a threshold at which autopoietic processes of the entity incur damage (i.e., the rate of replacement compensates for the damage happening to the system). Homeostatic mechanisms, unlike stress response mechanisms, attempt to directly control the stressor variable (e.g., body temperature or fluid osmolarity).
In the stress regime, the entity or system experiences a higher degree or rate of damage due to the extent of deviation from equilibrium (e.g., body temperature is high despite homeostatic effort, leading to damage) or collateral damage due to the strain induced by homeostatic effort (e.g., the associated metabolic strain leads to an accumulation of ROS that damages DNA, proteins, and lipids). Here, the amount or rate of damage starts to exceed compensation by normal homeostatic mechanisms. In response to this damage, the system deploys mechanisms that attempt to compensate once the threshold between homeostatic and stress regimes is crossed (e.g., response and repair pathways for both DNA and protein damage): this is the stress response in the strict sense. The negative metabolic and physiological effects of the stress regime are generic (i.e., not specific to the nature of the stressor) and include DNA damage, protein misfolding, and chemical degradation of lipids (e.g., peroxidation), as well as programmed cell death in multicellular organisms.
In the failure regime, the accumulated damage reaches a breaking point where the investment in homeostatic effort is causing more damage than can be repaired by the stress response. The failure regime refers to a declining state of the self-maintaining entity that is a consequence of the effects of a stressor, which leads to a progressively increasing accumulation of damage from which the entity cannot recover.2
The term “stress response” picks out the processes by which the self-maintaining unit seeks to limit and repair the damage caused by excessive strain (i.e., in the stress regime). These processes are often generic precisely because different stressors can have similar kinds of negative metabolic and physiological consequences. Thus, both the strain and the stress response are typically generic in character because there are numerous relationships between different normal autopoietic ranges of states at distinct levels of organization and the impact of a stressor on those ranges at one or more levels. Consequently, the stress response is not usually matched to the stressor with any specificity; it is a generalized attempt on the part of the autopoietic entity to avoid entering the failure regime.
In behavioral and physiological stress research, the term allostasis has been introduced to describe “maintaining viability through change,” such as changes in the activation of the vertebrate hypothalamic-pituitary-adrenal axis in response to environmental cues that typically signal the potential presence of threats even when no genuine threat (yet) exists (Sterling and Eyer 1988; Wingfield and Sapolsky 2003; Del Giudice et al. 2013, 2018). This has parallels with the stress response because it also is a change in the mode of reaction to environmental challenges. However, allostasis goes beyond a stress response to actual damage because it is anticipatory—the stress response is activated prior to stressor exposure and therefore prior to metabolic and physiological effects that accompany the stress regime. For instance, the availability of free fatty acids (FFAs) to a cell can lead to the overproduction of ROS and thus genes expressed in the oxidative stress response can be activated by molecules that sense the presence of FFAs, so-called xenosensors, even before ROS levels in the cell actually increase (Schönfeld and Wojtczak 2008; Klotz and Steinbrenner 2017; Sies and Jones 2020). These anticipatory mechanisms are critical for the co-option of stress mechanisms into normal development and physiology.
This characterization of stress and allied concepts might not be satisfactory to many practitioners in various fields where stress plays a central conceptual role, such as mammalian physiology or behavioral biology and psychology. In these fields, stress responses manifest in highly specialized and complex ways that only have a distant echo to the stress phenomenon that must have occurred in our distant unicellular ancestors. We think the emphasis on anticipatory stress responses in areas of physiology and behavioral sciences is justified in those contexts (Levine et al. 1991; McEwen and Wingfield 2003; Romero et al. 2009; Koolhaas et al. 2011). However, these are evolutionarily derived states. The multiplicity of stress concepts in various fields reflects the fact that different disciplines work with systems harboring different levels of evolved complexity and thus focus on different manifestations of the way living systems deal with actual or perceived stressors.
EVOLUTIONARY IMPLICATIONS
Two features emerge from our characterization of the stress concept and allied notions (Box 1). First, the stress response (activated in the stress regime), in contrast to regulation in the homeostatic regime (part of normal physiology), involves a radical reprogramming of metabolism and gene expression in the autopoietic entity, such as a cell or in physiological endocrine signaling. Thus, stress responses are under the control of regulatory mechanisms that switch a gene expression or metabolic profile to an alternative state. Importantly, this ability to switch between different regulatory states is also necessary during the development of multicellular organisms, especially for cell fate decisions. Regulatory state switching is a key reason why cellular stress response mechanisms are likely to be co-opted into the evolution of novel cell types, thereby facilitating cell fate decisions that underlie new cell type identities (see below, Cell Types and Cell Type Differentiation).
Box 1 “Stress” Terminology
Stressor: any deviation in the value of an external environmental or internal milieu variable from the range of values that is favorable for the autopoietic stability of an entity.
Strain: the metabolic and physiological cost that is imposed on the organism or other autopoietic entity by its attempt to counter the effects of a stressor at a level of organization.
Homeostatic regime: when the regulatory mechanisms of an entity can compensate for the effects of a stressor such that the strain is below a threshold at which regulatory efforts become damaging to the autopoietic equilibrium of the entity.
Stress regime: the experience of damage by the autopoietic entity due to the extent of deviation from equilibrium (e.g., body temperature is high despite homeostatic effort) or collateral damage due to the strain induced by homeostatic effort (e.g., the associated metabolic strain leads to an accumulation of ROS that damages DNA, proteins, and lipids). In the stress regime, the entity seeks to limit and reverse the damage by activating a stress response that targets the damage rather than the stressor itself.
Failure regime: when the amount of damage reaches a breaking point, the autopoietic entity enters the failure regime where the investment in homeostatic effort itself is causing more damage than can be repaired by the stress response. The failure regime refers to this declining ability for self-maintenance that is a consequence of the effects of a stressor.
Stress response: processes activated when the self-maintaining entity enters the stress regime and by which it seeks to limit and repair the damage caused by excessive strain (i.e., in the stress regime).
Allostasis: a change of the physiological state of an autopoietic entity in anticipation of the arrival of an expected stressor.
Second, the possibility of allostatic anticipation can facilitate more specific matching relationships between stressors (or any other signals) and a stress response. This can normalize stress response mechanisms under predictable life history events—the stressor can be replaced by an endocrine or paracrine signal and thereby become integrated into the normal physiology or development of a species. Consider again the anticipation of oxidative stress, which depends on the detection of molecules in the environment that, once incorporated into cellular metabolism, will lead to the production of ROS, such as FFAs (Sies and Jones 2020). These are broken down preferentially in the peroxisomes of cells where each FFA molecule leads to the production of many molecules of hydrogen peroxide (one H2O2 for each two carbon atoms). The detection of FFAs is performed by proteins that belong to a class of transcription factors called nuclear receptors (NRs), which function both as signal receptors (e.g., for steroids like testosterone, estrogen, or progesterone) and as xenosensors in anticipatory allostasis. NRs comprise a gene family, having evolved from a single ancestral NR. A phylogenetic reconstruction of the gene family showed that the oldest known NR lineage belongs to a FFA receptor in a sponge species (Bridgham et al. 2010). This finding is consistent with the hypothesis that the role of NR as signal receptors in development and physiology is derived from an ancestral function as a xenosensor. The developmental functions of steroid receptors are evolutionarily derived from preexisting xenosensor functioning because of anticipatory allostasis mechanisms.
MODELS OF EVOLUTIONARY CHANGE FACILITATED BY STRESS
We are now able to distinguish two different models of evolutionary change that are facilitated by stress (Fig. 1). There is a large body of evidence showing a generalized increase of phenotypic variation when organisms or distinct subsystems at different levels of organization experience strain from a stressor, pushing an autopoietic entity into the stress regime. The corresponding stress response increases the availability of potentially beneficial variants, which makes it possible for adaptation to occur more rapidly than via standing genetic variation (Fig. 1A) (Rutherford and Lindquist 1998). As noted (What “Everyone” Knows about Stress and Evolution), this model—stress-induced evolutionary adaptation—is a member of a broader family of models of evolutionary change based on plasticity where the stress response is one type of plastic response to environmental variation (Fig. 1B; Weber and Depew 2003; West-Eberhard 2003; Badyaev 2005b; Badyaev Alexander 2009; Ehrenreich and Pfennig 2016; Badyaev et al. 2017). An environmentally induced trait becomes undergirded by heritable genetic variation, such as when individuals with different genotypes show different phenotypic responses or different degrees of plasticity (e.g., Rothman et al. 2021), and then natural selection can preserve, augment, and refine these traits occurring in a beneficial direction or remove detrimental plasticity. Eventually, these phenotypes become anchored by genetic alterations and no longer require the distinctive environmental factor to trigger the relevant trait (Fig. 1A, B).
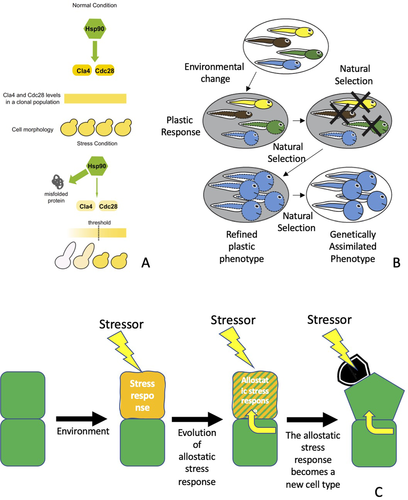
Evolutionary adaptation due to induced plasticity is a more encompassing category because the environmental change need not arise from a stressor that pushes an entity beyond homeostasis into the stress regime (Scheiner 1993; Pigliucci 2001; Murren et al. 2015). The only requirement is some degree of genotype by environment interaction. However, the same basic principle applies: a generalized increase of phenotypic variation that facilitates more rapid adaptive evolutionary change. These models illuminate how quantitative traits, such as size or shape, change through time to maintain an adaptive fit with the environment, indicating how adaptation takes advantage of the consequences of stress. Although it is possible that this quantitative transformation might yield a qualitatively distinct phenotype (West-Eberhard 2003; Levis and Pfennig 2019; Levis et al. 2020; Pfennig 2021), the qualitative change is typically not a new character but rather a novel character state (i.e., a polyphenism), such as a distinct color morph or a different feeding strategy within a life history (Wagner 2014).
In contrast, a distinct type of model for evolutionary change facilitated by stress is SIEI (sensu Wagner et al. 2019). This model incorporates the fact that the stress response involves a switch between regulatory states during the transition from homeostatic to stress regime (Evolutionary Implications). During the evolutionary process, this regulatory shift can lead to a change from one form of variability to another because a distinct set of regulatory genes is active under stress conditions and therefore becomes subject to natural selection. An evolutionary scenario of SIEI in three steps can be illustrated by reference to examples from the evolution of NRs and mammalian embryo attachment: (1) evolution of anticipatory allostatic mechanisms, (2) integration of these allostatic mechanisms into normal physiology, and (3) evolutionary transformation of the stress response into a novel character (e.g., a new cell type). This scenario can apply to any autopoietic unit found in living systems (Fig. 1C).
When stressors are a regular feature of an organism's environment, mechanisms that allow organisms to anticipate future stress evolve. This is possible because features of the environment can be used to predict the arrival of a stressor to activate the stress response network; these features predict strain before the stressor can cause real damage. At the cellular level, this is often mediated by xenosensors, which detect the presence of toxins in the environment that eventually would lead to cell stress of one sort or another. Many xenosensors are NRs, a class of proteins that also play a key role in endocrine and paracrine signaling in multicellular organisms (see above). It is precisely this overlap that is pertinent for the next step of the SIEI scenario: the integration of allostatic mechanisms into normal physiology.
A further refinement of the allostatic response occurs when xenosensors come under the control of internal physiological signals. Well known examples are steroid receptors that mediate hormonal signals from sex hormones and metabolic hormones, such as glucocorticoids. A phylogenetic study of these proteins demonstrated that xenosensors and hormone receptors are related as a monophyletic group of genes (Bridgham et al. 2010). Furthermore, a key cell type identity molecule (Hepatic Nuclear Factor 4) is closely related to FFA receptors and can still bind these molecules (Bridgham et al. 2010). This implies that the specialized hormone and other NRs that are physiological signals evolved from xenosensors that ancestrally regulated the anticipatory, allostatic stress response.
Another scenario that points in this direction is the role of inflammatory mediators in normal cell differentiation. The attachment of an embryo to the uterine lining leads to a transient inflammatory process (the “attachment reaction”) in which typical inflammatory mediators are expressed, such as IL1, IL6, and PGE2 (Mor et al. 2011). Additionally, stromal cells of the endometrium differentiate into decidual cells that are necessary for successful implantation and placenta formation. This differentiation requires PGE2 signals along with the ovarian hormone progesterone (Stadtmauer and Wagner 2021). One of the primary molecules of the inflammatory signal cascade, triggered by embryo attachment, has been co-opted into the differentiation of a novel cell type: decidual cells. The likely change that facilitated the co-option of PGE2 into cell differentiation was the progesterone receptor evolving to switch the PGE2 receptor type from EP4 to EP2. EP2 activates the PKA pathway and turns off the PI4K/Akt pathway; PKA signaling is necessary for decidual cell differentiation (Fig. 2; Stadtmauer and Wagner 2021).
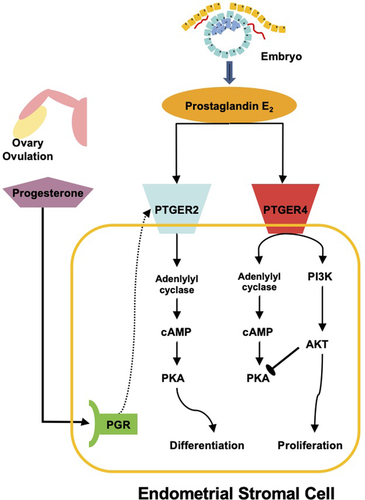
Once the activation of the regulatory network that controls the stress response is integrated into appropriate developmental or physiological conditions, the stabilized output of the stress response network can become subject to natural selection. In the case of eutherians, “appropriate physiological conditions” have been identified for the endometrium. First, progesterone indicates to the uterus that an ovulation has occurred and thus a preimplantation embryo could be in route. The second signal comes from the inflammatory attachment reaction, which yields the production of PGE2. A combination of progesterone and PGE2 indicates that an embryo is arriving, and the uterus prepares for embryo implantation by differentiation of decidual cells. However, a sustained implantation of the embryo requires that these signals not activate the wound healing process; they should only lead to a tissue state that tolerates the presence of the embryo. If the functional output of the decidual cells was wound-activated fibroblasts, then the uterus would seek to repair the wound caused by the embryo. Instead, decidual cells must permit and regulate the implantation process, which is accomplished by the suppression of gene expression in decidual cells that supports wound healing (Nancy et al. 2018) and production of effector and signaling genes such as Laminin, prolactin, and IGFBP1 (Gellersen and Brosens 2014). These features suggest that transcription factor networks controlling the stress response can be co-opted into controlling new phenotypes for new functions.
Thus, the SIEI model concentrates on the mechanistic nature of the stress response and addresses its evolutionary transformation into a derived trait. The stress response of an autopoietic unit is initially protective, mitigating or shielding that unit from damage. In the regular stress response, this halts movement of the autopoietic unit from the stress regime toward the failure regime and returns it to a homeostatic regime. However, over evolutionary time, the regulatory response can become a source for and eventually directly control a new phenotype. Xenosensors (i.e., NRs that sense the presence of toxins) can evolve to become receptors for intra-organismal signals, such as hormones and prostaglandins, thereby integrating stress response mechanisms into the normal physiology of the organism.
Crucially, SIEI models involve both the evolutionary emergence of a new character and the replacement of an environmental trigger (i.e., the stressor) by a physiological signal. What initially was a generic stress response becomes co-opted or stabilized into a new gene regulatory state that represents a novel trait. This transforms the process of protection into a state of compensation. The new trait is specifically poised for activation in the context of a signal or a combination of signals, rather than generally ready to respond to stress. Although variation is always present in this evolutionary process, its generic increase within the stress regime is not in view on the SIEI model. Rather, the existing ability to switch between regulatory states associated with homeostatic and stress regimes, such as inflammation, is incorporated into a specific organismal context through permanent allostatic anticipation. This yields a new, individualized trait that can be fine-tuned by selection for this compensatory role. A cellular response to toxic by-products of metabolism, such as ROS, can become a signal to initiate routine cell differentiation or physiological changes during an organism's life history—the stress response mechanisms themselves are co-opted (Arbiser et al. 2002; Cliff and Dalton 2017).
Co-option: Descriptive and Explanatory
As the comparative study of gene expression became possible through the discovery of conserved genes and introduction of methods for detecting RNA expression in nonmodel organisms (e.g., in situ hybridization, RT-PCR, RNAseq), the notion of genetic co-option has been a mainstay of evolutionary narratives (True and Carroll 2002; Piatigorsky 2007; McLennan 2008; Erwin 2020). However, there are two different senses of genetic co-option that should be distinguished: descriptive and explanatory. The descriptive sense of genetic co-option pertains to a situation in which a gene that is expressed in a certain developmental or anatomical context in an outgroup species, taken to represent the ancestral state, acquires a novel expression domain in a derived lineage. A paradigmatic example is the recruitment of enzymes as crystallins in the eyes of vertebrates (Wistow et al. 1987). Biologists have long suspected that the cellular stress response to UV radiation was co-opted as a genetic resource in the origin of vertebrate lens crystallins from heat-shock proteins and metabolic enzymes (Piatigorsky and Wistow 1991; Piatigorsky 1992, 1993, 2007). The descriptive sense of genetic co-option denotes an evolutionary change in the pattern of gene expression (that co-option has occurred) but does not play a broader explanatory role beyond the fact of redeployment of a particular gene.
The explanatory sense of genetic co-option requires showing how the gene redeployment contributed to the origin of another character. This may involve a documentation of the recruitment of whole gene regulatory networks (GRNs) rather than only single genes (Erwin 2020; McQueen and Rebeiz 2020). Paradigmatic examples of the explanatory sense are the genetic co-option of the limb signaling network to explain the origin of beetle horns (Wasik and Moczek 2011) or butterfly eye spots (Keys et al. 1999). Another example is the genetic co-option of the spiracle GRN to explain the origin of the posterior lobe in the male genitalia of some drosophilids (Glassford et al. 2015). In these examples, one not only claims that certain genes have a distinct, derived expression domain, but also that a genetic network co-option accounts for a novel structure. It is not just that decapentaplegic is expressed in beetle horns, but the network of gene expression has a capacity for coordinating appendage growth relevant to producing beetle horns.
In order for the explanatory sense of genetic co-option to go beyond description, more is needed than just documenting gene network recruitment into a novel context (McLennan 2008). In addition, there must be the right kind of mechanistic detail for why a certain feature arises in the new location, such as a new pigment pattern or cell type. We need to understand the conditions under which the genetic co-option of network components and their organization explains the origin of a new character. These conditions can be fulfilled when three distinct questions are answered (see Box 2): What are the consequences of co-option? What is the cost of co-option? What opportunities does co-option afford?
Box 2 Co-option Distinctions
Descriptive co-option: an account of the fact that a gene expressed in a certain developmental or anatomical context in an outgroup species, taken to represent the ancestral state, acquires a novel expression domain or context in a derived lineage.
Explanatory co-option: the recruitment of a gene or a GRN into a new anatomical or developmental context that explains how a novel feature above the level of the gene or GRN itself evolved. A classic example is beetle horns, which are explained by the recruitment of a leg outgrowth module into the pronotum of a beetle.
Co-option consequences: the direct developmental consequence of co-opting a gene or GRN that contributes to the novel character. For example, that the leg GRN can initiate outgrowth means it can be used to cause other extensions from the body surface that are not legs (for instance beetle horns).
Co-option cost: the side effects of co-opting a gene or GRN that do not contribute to the novel character. These side effects need to be suppressed and/or eliminated after the initial co-option event.
Co-option opportunities: novel evolutionary possibilities that result from a co-option event but are not its direct consequences. These lead to the evolutionary elaboration of the developmental outcome of the initial co-option event.
WHAT ARE THE CONSEQUENCES OF CO-OPTION?
The consequences of co-option are the set of causal powers that a gene or GRN components and their organization contribute to the mechanistic basis of a new character, such as beetle horns, a pigment pattern, the posterior lobe, or a novel cell type (DiFrisco et al. 2020). In the case of a beetle horn, the claim is that the expression of the leg outgrowth GRN has the consequence of directly endowing a region on the back of the beetle with the capacity to have localized outgrowth and proximo-distal differentiation. Explanatory co-option claims typically do not imply that the new structure is a duplicate of the ancestral structure controlled by the GRN; if so, we would speak of a duplicated leg rather than a beetle horn or an eyespot (i.e., there is a novel character identity). In this new context, the co-opted GRN is modified and expressed such that it governs a different phenotypic outcome than in the original developmental context, such as the partial redeployment of the spiracle network in the origin of the posterior lobe of male genitalia (Glassford et al. 2015).
WHAT IS THE COST OF CO-OPTION?
Contemplating the consequences of co-option immediately leads to a consideration of costs. The cost of co-option is the set of detrimental side effects of the co-opted process. For example, the claim that embryo implantation in mammals evolved through the co-option of a mucosal inflammatory reaction in response to embryo attachment (Chavan et al. 2017; Griffith et al. 2017; Stadtmauer and Wagner 2020) involves several, hypothesized beneficial consequences, such as the induction of vascularization and vascular permeability, which might enhance nutrient transfer to the embryo and fetus. However, the same explanatory hypothesis of co-option must address the fact that a generic inflammatory process would not only be potentially beneficial, but also likely harmful, such as via neutrophil recruitment that would attack the embryo. The generic inflammatory process must be modified at the site of embryo attachment and implantation to suppress these negative effects associated with inflammatory processes. This could be addressed in terms of an associated suppression of both IL-17, a key factor in recruiting neutrophils that can cause extensive tissue damage, and PGF1a signaling, which contributes to smooth muscle contractions that can lead to fetal extrusion (Chavan et al. 2021). Considering the costs is necessary to securing an explanatory notion of co-option.
WHAT OPPORTUNITIES DOES CO-OPTION AFFORD?
Opportunities are mechanistic aspects of the co-opted process that do not derive directly from the ancestral causal capacities of the GRN, but rather became possible after the co-option event. Even though these mechanistic aspects were not initially operative, their evolution is enabled or facilitated by the co-opted mechanisms operating in the new structure (DiFrisco et al. 2020). These opportunities from co-opted causal capacities can arise both in space (e.g., different corporeal locations) or time (e.g., at different life history stages). For example, in Gonium (a multicellular volvocale green alga), the retinoblastoma gene (RB) and cyclinD (cycD) cooperate to regulate gene expression during cell division, but in postmitotic cells these proteins also regulate the expression of cell adhesion genes, which are necessary for maintaining cell aggregation (Olson and Nedelcu 2016). These cell adhesion genes are not regulated by RB/cycD in single-celled, algal relatives, such as Chlamydomonas. Hence, the co-option of cell cycle genes in the origin of multicellularity afforded the opportunity for those genes to acquire control over new target genes after the co-option event.
Therefore, to move beyond the descriptive sense of co-option to an explanatory notion, the requisite mechanistic details for why the genetic co-option of network components and their organization explains the origin of a new character can be gleaned from explicit answers to: (a) the developmental, mechanistic consequences of the co-option, (b) the associated costs arising from detrimental side effects to those consequences, and (c) the identification of opportunities afforded by the causal capacities in new locations or at different times, elaborated from those present in the co-opted GRN or character-identity mechanisms (DiFrisco et al. 2020).
Examples of Evolution via the Co-option of Stress Response Mechanisms
CELL TYPES AND CELL TYPE DIFFERENTIATION
The best documented examples of SIEI pertain to the evolutionary origination of novel cell types. Cell types are distinct phenotypic units that are subject to evolutionary change and typically specialized for a functional role within a multicellular organism (Arendt et al. 2016). Increase in the number of discernible cell types is a major mode of body plan evolution. Extant animals vary in the number of cell types from 5 to 30 in Trichoplax (Grell and Benwitz 1971; Smith et al. 2014) to over 500 in mammals (Vickaryous and Hall 2006). For a handful of cases, there is evidence for the co-option of cellular stress mechanisms in their evolutionary origin.
Soma-germline differentiation in volvocine algae
Among the 27 independent cases of the evolution of multicellularity, the origin of soma-germline differentiation in volvocine algae is arguably the best understood example (Davison and Michod 2021). Within this group, soma-germline differentiation has evolved repeatedly and relatively recently (Umen 2020). Two model species represent the opposite extremes along an axis between unicellular and differentiated multicellularity: Chlamydomonas reinhardtii and Volvox carteri (hereafter, Chlamydomonas and Volvox). Between these extremes are multicellular species without stable soma-germline differentiation, such as Gonium pectorale and Eudorina elegans (Fig. 3A).
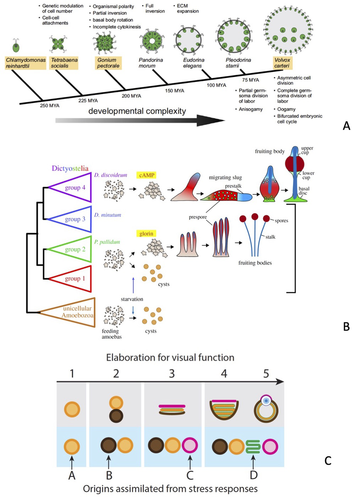
Two populations of cells are produced during Volvox development: a large number of small cells (<8 μm in diameter) and a smaller number of large cells (Kirk et al. 1993). Somatic cells differentiate exclusively from small cells, whereas large cells form the proliferative germline. Somatic differentiation requires the transcription factor regA, which represses the expression of nuclear encoded proteins of the chloroplast and thus inhibits parts of the photosynthetic pathway (König and Nedelcu 2020). The closest homolog of regA in the single-celled Chlamydomonas is RLS1, which is a component of the cellular stress response pathway under nutrient limitation. Besides sequence similarity, the homology of regA and RSL1 is supported by the fact that regA is also activated in Volvox under stress conditions, such as light exposure after extended darkness.
A variety of experimental evidence (e.g., Kirk et al. 1999; summarized in König and Nedelcu 2016) implies that the somatic differentiation pathway evolved by the co-option of an ancestral cellular stress response into the control of somatic cell type differentiation (Nedelcu and Michod 2006, 2020). The critical role of cell size in somatic cell differentiation might explain this connection; small cells with large chloroplasts are vulnerable to oxidative stress because the volume of the chloroplast stroma decreases faster than the surface area. The chloroplast stroma is the site that consumes the metabolites produced at the chloroplast membranes, which makes a metabolic imbalance in smaller cells more likely. Overall, these data suggest that soma-germline differentiation is homologous to switching between proliferative phases of the life cycle to a survival stage in response to unfavorable conditions (i.e., the cellular stress response) in single-celled algae. The logic of somatic cell type origin involves a temporal alternation in cell state (proliferative vs. dormant) becoming transformed into a spatial, synchronous differentiation—the simultaneous presence of germline and somatic cells in the organism.
Fruiting structures in the slime mold Dictyostelium
Members of the slime mold clade Dictyostelia occupy a middle ground between single-celled and multicellular organisms (Kin and Schaap 2021; Schaap 2021). When food and moisture are abundant, they live and forage as unicellular organisms; when moisture evaporates or food becomes scarce, cells form cooperative aggregates (“slugs”) and later a stalk capped by a fruiting body of spores ready to disperse. The slug is a worm-like cluster of cells that crawls toward light and higher temperature, seeking to reach the soil surface. The stalk and fruiting body consist of different cell populations with the former comprising a support disc and a stalk, whereas the latter is a cluster of pre-spore cells that accumulate at the stalk tip. Species that form fruiting bodies are related to various clades that show different levels of elaboration for this system. This permits a detailed reconstruction of its evolutionary history and the mechanistic innovations that led to the semi-multicellular form of life exemplified in Dictyostelium discoideum. The complex behavior and morphogenetic development of its multicellular life stage appear to have evolved from the basic stress response found in most amoeba (Schaap 2011, 2021; Kawabe et al. 2019).
Slime molds belong to the clade Amoebozoa that consists mostly of single-celled protists. All amoebas can form a dormant state in response to starvation or environmental stressors, such as high osmolarity indicating habitat desiccation (Fig. 3B). The dormant cell encapsulated within a two-layered cell wall is called a “cyst.” Encystation is controlled by a highly conserved mechanism, the activation of the protein kinase A (PKA) pathway, which is triggered by the intracellular synthesis of cAMP by AMP cyclase A (Ritchie et al. 2008). The second major evolutionary stage—the origin of aggregation—began from a behavior in service of food exploitation. Organisms that aggregate for food, rather than in response to starvation, use small molecule signals (e.g., glorin). When cells aggregate, the passive leakage of cAMP produced by cells can reach high concentrations (the μM range). The abundance of extracellular cAMP triggers an alternative pathway to the dormant cell state that yields a spore with a three-layered cell wall. This is accomplished by a simultaneous activation of PKA via intracellular cAMP and activation of the cAMP receptor, which together control spore formation. Species that have evolved this combination form cysts when alone and spores when aggregated. Once the cAMP receptor was functionally linked to adenylate cyclase, a feedback loop was created between extracellular cAMP concentrations and the internal production of cAMP. This, together with other changes in cAMP metabolism, enabled the formation of cAMP oscillations that produce spatial pattern formation and the evolution of stalks and fruiting bodies. The last evolutionary step, represented by the situation found in D. discoideum, was an increased sensitivity of phosphodiesterase A (pdsA), an enzyme that breaks down cAMP. This increased sensitivity is an essential element of the mechanism underlying cAMP oscillations. Higher sensitivity of pdsA means lower concentrations of cAMP can be used to cause oscillations, which makes it possible to initiate aggregation under starvation conditions.
Aggregative multicellularity in Dictyostelia is triggered in response to starvation; it is an elaborated stress response leading to the formation of a resistant dispersal stage (the spore). Thus, it is not surprising that these mechanisms evolved from the modification of an ancestral stress reaction—the formation of cysts in unicellular amoebae. Nevertheless, it illustrates how an ancestral cellular stress pathway can be modified to control sophisticated multicellular behavior. The basis for this behavior is the sequential rearrangement of a limited number of elements (adenylyl cyclase, pdsA, cAMP-receptor, PKA, etc.), all belonging to the cAMP signaling and metabolism pathways; additional evidence for other stress-response signaling having been co-opted for differentiation in D. discoideum continues to accumulate (e.g., Kelly et al. 2021). Under food deprivation, the stress pathway will be active. Thus, any innovations, such as the formation of a soil-surface seeking slug or a stalk for dispersion, will most likely be realized by modifications of mechanisms that are activated by the stressor. This connection is less obvious in cases where the stressor has, over evolutionary time, been replaced by a physiological signal.
Decidual stromal cell from endometrial stromal cell
Cell type origination is difficult to study in generic cell types and cell type families such as neurons and muscle cells. These cell types are evolutionarily ancient, and thus much of the information about their origins is inaccessible. However, the details of origination events for recently evolved cell types are more accessible, in part because they have evolved in concert with novel processes or body plan features. A good example is the evolution of placental mammalian (eutherian) pregnancy, which originated sometime between 100 and 65 mya (Mess and Carter 2006; Wildman et al. 2006). Although this was a long time ago, it is nowhere near the >500 mya of evolution for other vertebrate body plan innovations. One innovation associated with the origin of eutherian pregnancy is a novel tissue type, the decidua, which is composed of cells that form the maternal part of the fetal-maternal interface. Within the human decidua, one finds decidual stromal cells (DSCs) along with specialized (uterine) natural killer cells and macrophages.
The development of DSCs in humans and mice has been investigated intensely because of their importance for the establishment and maintenance of pregnancy (Gellersen and Brosens 2014). DSCs develop from a fibroblast-like cell present in the nonpregnant uterus of all mammals, the endometrial stromal fibroblast (ESF). Among mammals, DSCs only exist in eutherian mammals. However, ESFs also exist in marsupials, the sister group of eutherian mammals, yet do not form DSCs (Kin et al. 2014; Erkenbrack et al. 2018). A comparison of gene expression of ESFs in opossum, a marsupial, and humans showed that many transcription factors that are important in human DSC differentiation are also expressed in opossum ESF and respond to the decidualizing signals, such as progesterone and PGE2 (Erkenbrack et al. 2018). However, these genes are not regulating typical decidual effector genes in ESFs but stress response genes (related to inflammation). We thus inferred that DSCs evolved from the stress reaction of ancestral ESF cell types by co-opting the regulatory network to control genes responsible for their function as DSCs at the fetal-maternal interface (Wagner et al. 2019).
Metazoan mesenchymal cells
Animals have two basic types of tissue organization: epithelium and mesenchyme. Epithelia are organized in rigid, two-dimensional sheets of cells polarized between an apical and basal side that yields parallel polarity and is supported by a basal lamina. Mesenchyme, in contrast, consists of cells that are not clearly polarized but oriented quasi-randomly in three dimensions with amoeboid mobility. For a long time, it was unclear how the mesenchymal mode of tissue organization originated (Fritz-Laylin 2020). Choanoflagellates, the closest extant relatives of animals, have strongly polarized cells that can form colonies with quasi-epithelial arrangement (King et al. 2008; Ruiz-Trillo et al. 2008). They can assume an amoeboid phenotype when cells are confined to a narrow space of 2 μm (Brunet et al. 2021). The authors argue that this transformation, analogous to an epithelial-mesenchymal transition in animals, is induced because of stress caused by cell deformation. Their model posits that mesenchymal cell types arose through a co-option of stress-induced amoeboid transformation; the stress that cells experience inside multicellular aggregates is likely the ancestral form of multicellular organization (see also Jacobeen et al. 2018 for the physics of cellular packing at the origin of animals).
DEVELOPMENTAL PATHWAYS, TISSUES, AND ORGANS
Stress mechanisms have not only been detected in connection with the evolutionary origin of cell types; whole tissues and organs have been proposed to carry the imprint of a stress-induced origin. Two that have received substantial attention are the origins of eyes in animals and the process of dorsal closure during insect development. We also highlight cuticle differentiation in Arabidopsis and cetacean epidermis origination.
Eyes and photooxidative stress
Standard accounts of the evolution of animal eyes presume a trajectory of functional improvement from patches of photosensitive skin to pits shielded by pigment cells, which allow a degree of directional vision, to image-forming eyes aided by lenses and other parts of the dioptric apparatus. However, these accounts are based on the availability of potentially beneficial variants that facilitate adaptation. “Attempting to justify the origin of eyes based solely on selection for improved visual acuity creates circular reasoning, leaving no obvious evolutionary starting point for eyes or parts of eyes to independently evolve” (Swafford and Oakley 2019, p. 746). Oakley and collaborators have developed and documented a complementary narrative whereby the main building blocks of complex eyes originated from elements of the light and UV stress response (Oakley and Speiser 2015; Swafford and Oakley 2019) (Fig. 3C). Here, we briefly highlight their argument regarding the evolution of photoreceptor cells.
In extant animal eyes, the most important photopigments are opsins that bind tightly to the chromophore retinal (retinaldehyde). Retinal is a toxic by-product of UV exposure and opsins outside the eye act as sensors for UV stress by monitoring the level of retinal present in a cell. Active opsins then regulate stress-compensatory pathways that protect cells from the effects of photooxidative stress. The latter activity is considered the ancestral function of opsins with their light sensing function evolving secondarily. Thus, the capacity of cells exposed to UV radiation to monitor retinal levels and coordinate the stress response is a consequence of co-option that also affords an evolutionary opportunity to evolve photoreception and thus photoreceptor cells. More generally, there is a repeated pattern of essential physiological functions of the eye being molecularly realized by mechanisms that show strong evidence of homology to stress response pathways, which supports a SIEI model that derives the building blocks of eye development and function from preexisting stress mechanisms (Swafford and Oakley 2019).
Dorsal closure from wound healing
During the early stages of Drosophila embryogenesis, a gap at the dorsal midline of the embryo closes by the convergence and subsequent fusion of epithelial cell sheets on either side from anterior to posterior. The contracting mechanism involves four stages (initiation, epithelial sweeping, zippering, and termination) that bring the lateral epithelial sheets over the underlying squamous epithelial cells of amnioserosal tissue and strongly resembles wound healing (Jacinto et al. 2002). In particular, there are shared molecular components (e.g., Jun N-terminal Kinase signaling pathway), cellular behaviors (e.g., transitions from disorganized arrays to tightly organized, regular rows of cells), and morphology (e.g., intrinsic tension leading to tissue stiffening) between “purse string” wound response and epithelial sheet migration like dorsal closure, which suggests that the latter was derived from the former: “co-option of this mechanism for morphogenesis would then entail a switch from damage-induced signaling to developmentally regulated signaling” (Sonnemann and Bement 2011, pp. 255–256).
Dorsal closure has been observed in other, more representative holometabolous insects that retain distinct extraembryonic tissues (i.e., amnion and serosa) (Panfilio et al. 2013). These and other differences (e.g., cellular architecture and zipper method) point to variation in dorsal closure processes across insects and offer a clue to the rarity of “strict” dorsal closure processes in morphogenesis despite the ubiquity of wound repair. Many morphogenetic movements involving cognate cell shape changes and signaling pathways exhibit comparable features (i.e., “epithelial gap” closure) in tunicates and vertebrates, such as neural tube closure or epiboly (Kiehart et al. 2017; Jain et al. 2020). The possible co-option of injury responses such as wound healing to explain the origin of these morphogenetic processes requires further empirical testing at multiple levels of organization (Horn et al. 2015).
Arabidopsis cuticle differentiation
In many seed plants, the embryos are protected against environmental stresses, such as desiccation, by a tight cuticle within the seed. This cuticle is formed by the endosperm in response to signals from the embryo. Creff et al. (2019) have shown that the development of this protective cuticle is controlled by the activation of MPK6, which is part of the MAPK pathway and a mediator of the stress pathway that is downstream of the receptor kinases GSO1 and GSO2. The authors argue that the intercompartmental signaling between the embryo and endosperm, which is responsible for the integrity of the cuticle covering the embryo, arose by a co-option of the generic stress pathway. Although this finding has not been integrated into an evolutionary scenario with an explicit phylogenetic framework, it is intriguing because of the possible role of stress pathways in contributing to or enabling the evolution of novel tissue components, such as the embryonic cuticle.
Whale skin
In terrestrial mammals, the epidermis consists of a basal layer of proliferating cells and various layers of postmitotic cells. The latter eventually die and leave behind keratin that forms the outer protective layer of the body epidermis. Basal and postmitotic cells express different keratin genes. The basal layer expresses K5 and K14, whereas the postmitotic cells express K1 and K10. After a wound compromises the integrity of the skin, postmitotic epidermal cells in upper layers of the epidermis initiate gene expression commensurate with an alternative identity, switching from K1 and K10 to the expression of K6/K17 or K16. This new regulatory state, triggered by the stress of a wound, is proliferative and involves secreting pro-inflammatory cytokines. Under normal conditions, the stress-related regulatory state of K6/K17 or K16 expression is transient and disappears after wound healing is complete.
However, in permanently aquatic mammals such as whales and sirenians, the epidermis is 50 times thicker and the genes for K1 and K10 have been lost (Ehrlich et al. 2019). Instead, the upper layers of the epidermis express K6/17 and are more proliferative, paralleling activated epidermal cells in terrestrial mammals during wound healing. In contrast to healing skin wounds, the whale epidermis is not inflammatory. It likely arose by the co-option of this epidermal stress reaction into a permanent tissue state, while suppressing the damaging inflammatory signals (Eckhart et al. 2019). Thus, the loss of inflammatory signaling is an example of the elimination of a co-option cost. The transition from amphibian skin to the keratinized amniote epidermis also could have arisen from a co-opted stress reaction in response to skin desiccation in terrestrial environments.
One might be tempted to interpret this as a plasticity-first scenario because the phenotype of the derived epidermis is foreshadowed by the phenotype of the stressed cells of terrestrial mammals. Nevertheless, whale skin involved the evolution of permanent anticipatory allostatic mechanisms independent of a transitory injury and was integrated into normal physiology by the elimination of pro-inflammatory signaling characteristic of epidermal cells during wound healing. Furthermore, it is a qualitative change in the character of the epidermal cells, not just a quantitative modification, such as we see in the increased thickness of keratinization for congenital calluses—the regulatory state of the stress response itself has been evolutionarily transformed into a novel character. Although whale skin may be best understood as a new tissue type, it does not lead to an increase in the number of cell and tissue types in the derived lineage, in contrast to other cell and tissue type innovations, and thus qualifies as a radical transformation akin to Type II innovations sensu Wagner (2014).
Testing SIEI
To secure an explanatory sense of co-option, as is required by the SIEI model, the mechanistic consequences, associated costs due to side effects, and the opportunities afforded all need to be addressed. Although biologists are not in a position to document answers to all three of these questions for each of the above examples, it is possible to derive generalized answers that can translate into testing the SIEI specifically in different contexts. For example, the fact that stress pathways bring with them mitotic quiescence and survival mechanisms represents the primary consequence of co-option: direct functional benefits of using the stress pathway in a new context. The cost of co-option is the fact that cells under stress are also poised for apoptosis or other forms of programmed cell death (PCD). A novel cell type that arises via SIEI must evolve mechanisms that block access to these PCD pathways. We therefore predict that a terminal differentiation pathway derived from a stress mechanism will include an evolutionarily derived mechanism that decreases the likelihood of activating PCD. These anti-apoptotic mechanisms likely evolve early in the history of a new cell type. This suggests another prediction: the existence and phylogenetic age of these mechanisms should provide a signature that the differentiation mechanism evolved through the modification of stress pathways. A good candidate that fulfills these predictions is the DSC (Decidual stromal cell from endometrial stromal cell). Decidual cell differentiation depends on activation of part of the MAPK pathway, but specifically suppresses the activity of the JNK protein by the constitutive expression of a phosphatase (DUSP1). The activated JNK protein is a potent inducer of apoptosis and is suppressed in DSC (Leitao et al. 2010).
Finally, opportunities afforded by co-option also can be used to test the hypothesis that a differentiation pathway is derived from a stress pathway. The SIEI model implies that the new, functionally specific phenotype of the derived cell type is controlled by regulatory pathways that are ancestrally part of the stress pathway. Thus, SIEI also implies that the expression of functionally specific effector genes for a novel cell type should be downstream of the transcription factors that control the effector genes of the ancestral stress reaction. This is because the new functional phenotype of a novel cell type is expected to evolve after the initial co-option of the stress pathway. Hence, regulatory control over these effector genes is likely to evolve through the origin of cis-regulatory elements at the effector genes responsive to trans-regulatory factors expressed in stressed cells. The tripartite scheme of consequences, costs, and opportunities provides a template for empirically testing the validity of the SIEI model by the comparative study of developmental processes in different contexts and yielding a mechanistic explanation of the architecture of stress-derived differentiation mechanisms.
A Principle of Evolutionary Origination?
Assuming that these tests of the SIEI model are being undertaken, can we learn anything more generally from these diverse examples that appear to involve the co-option of stress response mechanisms? We think so and here formulate a principle of evolutionary origination—stress-induced state switching—that involves the capacity to switch between binary phenotypic and gene regulatory states related to either (a) reproduction and proliferation or (b) survival and differentiation. We refer to the basis of this capacity as a “generative dyad” because the stabilization of regulatory states available from different instantiations of the same interactive process induced by stressors (i.e., regulatory state switching between reproduction and survival) in diverse contexts and across levels of organization facilitates evolutionary innovation.
Single-celled organisms generically exist in two states (Fig. 4A). When conditions are favorable for growth and reproduction, cells are proliferative; when conditions become unfavorable (low nutrients, desiccation, etc.), the cell assumes a state that is specialized for survival. In the survival state, cell division is turned off and genes that enhance survival by counteracting the consequences of stressors are turned on. Examples include genes that counteract DNA damage, chaperones that refold proteins, and enzymes that neutralize ROS. In single-celled organisms, these two phenotypes—proliferative and survival—are expressed diachronically in response to temporal changes in environmental conditions. In multicellular organisms, proliferative and mitotically quiescent cells are present at the same time in the same body (Fig. 4B, C). We hypothesize that the regulatory state switching process between (a) reproduction and proliferation or (b) survival and differentiation disposes populations to evolutionary origination. Shifting from temporary state changes arising from stress-response mechanisms to stable and permanent, compensatory alterations through a co-option of these mechanisms and assimilation of their signals recurs repeatedly at different levels of organization in the history of life (Fig. 4).
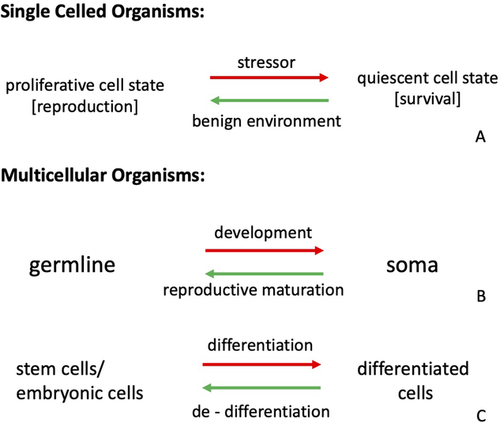
Evidence for this idea is strong for the evolution of multicellularity, where mechanisms underlying the transition from proliferative to quiescent survival states have been co-opted into differentiation of germline and soma (e.g., Nedelcu and Michod 2006, 2020). Within the framework of our hypothesis, one element of the generative dyad is the germline, representing the proliferative cell state, and the other is somatic cells, representing the quiescent, survival state. Both the cases of multicellular volvocine algae and a multicellular stage for cellular slime molds support this interpretation. The core structure of this generative dyad can be recognized throughout development in multicellular organisms, where proliferative processes that add cells to the body, and differentiation events, where cells specialize for somatic functions, alternate in forming the body of an individual organism. Proliferation is performed by embryonic stem cells during development, the re-entry of cells into a proliferative mode during wound healing and regeneration via dedifferentiation, or by set-aside stem cells (e.g., the germline or i-cells in hydra). Differentiated cells are usually postmitotic (or at least temporarily nonproliferative) and functionally specialized, such as neurons, liver cells, or muscle cells. There is evidence for the origin of a few cases of differentiated cell types from stress reactions, including the DSC of the eutherian endometrium, various cell types of eyes, the specialized epidermis of whales, and mesenchymal cells. These examples offer further support for an evolutionary link between the stabilization of stress-induced regulatory switching and the origin of cell differentiation pathways. Together, these observations point toward a broad homology of generative dyads, whether it is the polarity between proliferative and survival states in single-celled organisms, germline and soma, or stem cells and differentiated cells in the development of complex multicellular bodies (Fig. 4). The dyads are generative precisely because they continually provide opportunities for origination via stress-induced regulatory switches becoming stabilized as described by the SIEI model.
Another remarkable aspect of stress-derived cell differentiation is the frequent use of a signaling pathway that is highly conserved among eukaryotes in both stress reactions and differentiation—the cAMP-PKA signaling pathway (Shemarova 2009). In animals, the cAMP-PKA signaling pathway is a central player in cell differentiation. It is most often associated with and causally involved in the switch from a proliferative cell state to postmitotic differentiation, such as in Leydig cell differentiation (Chen et al. 2017). Decidual cell differentiation also revolves around the switch from one form of signaling state (PI3K, Akt) to another dominated by PKA signaling (Gellersen and Brosens 2014). Hence, at least part of animal cell differentiation machinery seems to be homologous to an ancestral cellular stress pathway (cAMP-PKA) and, following the SIEI model, evolutionarily derived from it.
How does such an ancient stress-related signaling transduction pathway become co-opted into many independently derived differentiation pathways with different outcomes (i.e., cell types) and triggering signals? On the signaling side, one factor might be the flexible architecture of G-protein-coupled receptor pathways (Rosenbaum et al. 2009). The PKA pathway is activated by a class of G-proteins with ATP cyclase activity. These G-proteins are controlled by G-protein-coupled receptors, which are physically independent proteins that can interact with a variety of G-proteins and are coded for by different genes. Thus, the protein that produces the second messenger (adenosine cyclase) can be linked to a variety of receptors that are responsive to different extracellular signals. The receptor and second messenger transducer are coded for by different genes, which leads to a rich combinatorial set of possible G-protein plus receptor combinations in both development and evolution. Many signals can be linked to the G-proteins able to produce cAMP and thus activate one of the PKA isoforms.
Another possible reason why the stress signaling pathways may be preferred as a starting point for evolving a differentiation cascade are the common biological features shared among stress reactions and terminally differentiation pathways. Both pathways induce mitotic quiescence, and both are committed to cell survival. Recalling the structure of the generative dyad, it is easier to evolve a terminal differentiation pathway for a novel cell type if one starts from a stress-activated cell—some of the objectives of cell differentiation are already provided by the stress pathway (i.e., mitotic quiescence and activation of survival mechanisms). In this way, both mitotic quiescence and survival pathways are already active and benefit the origin and stability of a terminally differentiated cell. Stress-induced state switching is a widespread principle of evolutionary origination because of its ubiquity in organismal physiology.
Considering the widespread recurrence of the generative dyad structure across the tree of life and levels of organization in conjunction with the capacity for origination through permanent stabilization of the regulatory state controlling stress response, we think there is good reason to consider its possible relevance to other origination phenomena in both development and evolution. This suggests empirical tests for a research program on the creative role of stress, which naturally emerges from (i) situating the SIEI model in a conceptual framework of stress research, (ii) explicitly delineating how it relates to extant treatments of co-option, and (iii) recognizing that it is almost universally present across a variety of clades in the history of life. Although our discussion has primarily concentrated on developmental and evolutionary biology, there may be fruitful extensions into the cognitive sciences to explore the role of psychosocial stress in the origin of psychological capacities during cognitive development. Although the fecundity of applying the SIEI model to understand a broad range of origination phenomena remains to be seen, it is clear that we do not know enough about how stressful conditions can facilitate evolutionary change in populations of organisms.
1 AUTHOR CONTRIBUTIONS
ACL and GPW conceived and designed the study, co-drafted the manuscript, and equally contributed to its revisions and final form.
2 ACKNOWLEDGMENTS
We gratefully acknowledge the financial support of the John Templeton Foundation (Grant #61329). The opinions expressed in this article are those of the authors and not those of the JTF. We also appreciate feedback from the following online audiences where earlier versions of this article were presented: University of Minnesota Institute on Child Development Brown Bag Lunch Series (2021), International Society for the History, Philosophy, and Social Studies of Biology (2021), and Society for Integrative and Comparative Biology (2021). We also want to thank many individuals for helpful comments and suggestions on the manuscript: A. Corris, C. de Weerth, M. Del Giudice, M. Dresow, C. Kurth, P. Lyon, M. Maiese, D. Staudtmauer, B. von Dawans, L. Wilson, L. Wu, and Y. Yoshida.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
Associate Editor: T. Flatt
Handling Editor: T. Chapman
- 1 There are some molecular components that organisms cannot produce themselves, such as essential amino acids or vitamins, which must be consumed or otherwise secured. However, most molecular components of organisms are self-produced from the breakdown products of metabolism.
- 2 These three regimes are not exhaustive. We also can speak of a growth regime during embryogenesis or regeneration in which regulatory mechanisms ensure that production exceeds loss, such that the autopoietic equilibrium of an entity is augmented or elaborated.




