Gradual evolution towards flightlessness in steamer ducks*
*This article corresponds to Abhimanyu, L. and J. Ottenburghs. 2019. Digest: A single genetic origin and a role for bone development pathways in repeated losses of flight in steamer ducks. Evolution. https://doi.org/10.1111/evo.13827.
Abstract
Flightlessness in birds is the product of changes in suites of characters—including increased body size and reduced anterior limbs—that have evolved repeatedly and independently under similar ecological conditions (generally insularity). It remains unknown whether this phenotypic convergence extends to the genomic level, partially because many losses of flight occurred long ago (such as in penguins or ratites), thus complicating the study of the genetic pathways to flightlessness. Here, we use genome sequencing to study the evolution of flightlessness in a group of ducks that are current and dynamic exemplars of this major functional transition. These recently diverged Tachyeres steamer ducks differ in their ability to fly: one species is predominantly flighted and three are mainly flightless. Through a genome-wide association analysis, we identify two narrow candidate genomic regions implicated in the morphological changes that led to flightlessness, and reconstruct the number of times flightlessness has evolved in Tachyeres. The strongest association is with DYRK1A, a gene that when knocked out in mice leads to alterations in growth and bone morphogenesis. These findings, together with phylogenetic and demographic analyses, imply that the genomic changes leading to flightlessness in Tachyeres may have evolved once, and that this trait remains functionally polymorphic in two species.
The common ancestor of birds could fly, yet flightlessness has evolved repeatedly and independently across the avian tree of life (Roff 1994), and this transition is generally accompanied by changes in suites of associated traits, including increased body size, reduced anterior and increased posterior limb size, and changes in foraging ecology (McNab 1994; Roff 1994; McCall et al. 1998; Wright et al. 2016). The repeated loss of flight under certain ecological conditions, such as release from predation on islands or adaptation to diving as a foraging strategy, suggests that these convergent evolutionary transitions may be predictable (McCall et al. 1998; Wright et al. 2016; Stervander et al. 2019). However, it is unknown whether this general phenotypic convergence extends to the underlying molecular genetic mechanisms.
Although only 1% of extant or recently extinct birds are flightless (∼100 species), the pattern in which these flightless lineages occur on the avian tree of life implies that the number of evolutionary transitions from flighted species to flightless taxa is smaller, and that many took place early in avian evolution (Roff 1994). Speciation and diversification after the loss of flight characterize the penguins (Roff 1994), whereas ratites evolved flightlessness many times independently but long ago (Sackton et al. 2019). Other flightless birds, like the Galápagos cormorant (Phalacrocorax harrisi) or the kakapo (Strigops habroptila), exist as isolated cases within otherwise flighted groups, and many more such species have become recently extinct (Roff 1994). Despite the analytical challenges posed by the accumulation of multiple genetic changes over evolutionary periods of time, several recent genomic studies have identified targets of selection that contributed to flightlessness. For example, both coding and noncoding sequence changes in and around genes that control limb development have led to the loss of flight in the flightless cormorant of the Galápagos (Berger and Bejerano 2017; Burga et al. 2017). Across the much older ratites (the ostriches, emus, rheas, cassowaries, kiwis, moas, tinamous, and their relatives), there is evidence for convergent molecular evolution in conserved noncoding regulatory elements that in some cases also affect limb development (Sackton et al. 2019). A complementary strategy to study the genomic basis of flightlessness involves comparing the rare cases of very closely related living birds that differ in their ability to fly (i.e., polymorphism within species or between incipient species). This strategy avoids the complications of comparing taxa that have been evolving independently for long periods of time, where multiple evolutionary processes, including mutation, drift, and selection, have led to high overall genomic differentiation.
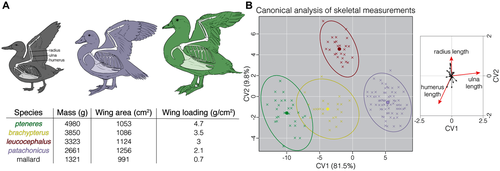
The steamer ducks in the genus Tachyeres represent such a case. These four species diversified during the last 2 million years in southern South America, likely influenced by the different climatic cycles of the Pleistocene (Fulton et al. 2012). Only one species, T. patachonicus, currently includes individuals that are able to fly. The remaining three (T. pteneres, T. leucocephalus, and T. brachypterus) are completely or predominantly flightless. Tachyeres ducks use their wings and feet for locomotion on water, a behavior known as “steaming” owing to its resemblance to the motions of a paddle wheel vessel (Livezey and Humphrey 1983). Steamer ducks differ substantially in their skeletal anatomy and wing loadings (the ratio of the body weight supported by the wing surface area; Livezey and Humphrey 1986; Fig. 1A), representing a continuum between the conditions allowing flight and those resulting in flightlessness. On one side of this continuum is the flying steamer duck (T. patachonicus), where only the heaviest males are flightless (Humphrey and Livezey 1982; Livezey 1986). The species leucocephalus and brachypterus are intermediate, whereas pteneres shows the highest wing loadings (Livezey and Humphrey 1986; Fig. 1A). A multivariate analysis of postcranial skeletal measurements identified the length of the long bones of the wing—the ulna, humerus, and radius—as the most important variables distinguishing the four species (Fig. 1B; Livezey and Humphrey 1986), and was consistent with the continuum of morphological variation from flighted patachonicus to flightless pteneres. Heterochrony during development could explain the morphological variation in the group (Livezey and Humphrey 1986), as a decoupling between wing and body growth would allow individuals to attain larger sizes while wing growth lags behind, leading to flightlessness.
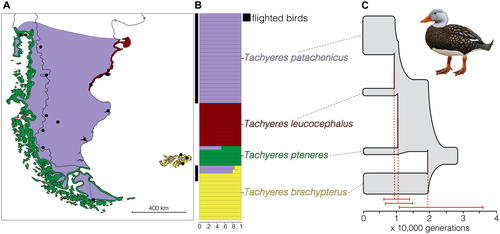
There have been contradicting past reconstructions of the phylogenetic relationships among the four steamer ducks with concomitantly different implications for their evolution of flightlessness (Corbin et al. 1988; Bulgarella et al. 2010), yet the most recent study based on relatively short mitochondrial DNA sequences (Fulton et al. 2012) suggested that the flightless species are not monophyletic, implying that flightlessness evolved multiple times in Tachyeres. The insular brachypterus was identified as sister to a continental clade composed of the remaining species. Within this clade, pteneres was sister to leucocephalus and the flying patachonicus. Although the flightless species are allopatric with respect to each other, the distribution of patachonicus overlaps with the remaining taxa (see map in Fig. 2A). However, the flying individuals from the Malvinas/Falkland islands (hereafter flying MFI), initially identified as patachonicus, were shown to possess the mitochondrial DNA sequences of the resident flightless brachypterus (Fulton et al. 2012). This finding raises the question of whether patachonicus exits at all in the MFI (possibly with the introgressed local mitochondria) or if some brachypterus individuals can still achieve flight. Finally, patachonicus shows morphological (Livezey 1986) and perhaps genetic (Corbin et al. 1988) variation across its range, with an incidence of flightlessness of approximately 25% among males from southern marine localities (Humphrey and Livezey 1982), where it seems flight has become unnecessary. Without resolving the species identity of the flying birds from the MFI or understanding if flightless patachonicus birds (predominantly coastal individuals) are genetically differentiated from those that are capable of flying (inland populations), the number of times flightlessness evolved in the group remains uncertain.
Here we sequence and compare the genomes of multiple individuals from all steamer duck species to explore the genomic basis of the anatomical modifications that led to flightlessness in Tachyeres. We identify narrow genomic regions associated to flightlessness, and combine phylogenomic and population genomic analyses to understand how many times flightlessness evolved in the steamer ducks.
Materials and Methods
SAMPLING, SEQUENCING, AND FILTERING
We used a combination of freshly collected birds, tissues stored in museum collections, and toe pad samples from museum study skins to extract DNA from a total of 74 steamer ducks from the four Tachyeres species (see Table S1 for details). We obtained tissue samples for many of the same individuals that were previously used to assess morphological differentiation in Tachyeres (Livezey and Humphrey 1986). Our sampling included patachonicus individuals from coastal and inland localities and flying birds from the Malvinas/Falkland Islands (originally identified as patachonicus). We extracted DNA using the DNeasy blood and tissue kit (Qiagen) or a phenol/chloroform extraction protocol followed by an ethanol precipitation and magnetic bead clean up. We sequenced whole genomes of 59 individuals (12 leucocephalus, 12 brachypterus, 12 coastal patachonicus, 12 inland patachonicus, six pteneres, and five flying individuals from the MFI), and generated libraries with the TruSeq Nano DNA kit protocol (Illumina), with an insert size of 350 bp. We pooled individual libraries using digital polymerase chain reaction (PCR) into four groups and sequenced each group on an Illumina NextSeq 500 lane at the Cornell Institute for Biotechnology core facility. In total, we obtained 2271 million paired-end reads, 151 bp in length, producing an expected per-individual coverage ranging between 1.4× and 11.6× (average of 4.8×; Table S2). We assessed the sequence quality for each library with fastqc version 0.11.5 (www.bioinformatics.babraham.ac.uk/projects/fastqc). We trimmed, quality filtered sequences, and removed adapters using AdapterRemoval version 2.1.1 (Lindgreen 2012), merging overlapping paired-end reads and increasing the minimum Phred quality score for trimming bases from the default of 2 to 10. The average expected coverage after quality filtering was 3.5× (Table S2).
MITOCHONDRIAL GENOMES AND SINGLE-NUCLEOTIDE POLYMORPHISM DISCOVERY
We extracted and assembled full mitochondrial genomes from the filtered sequences of each individual with MITObim 1.9.1 (Hahn et al. 2013), using the “quick” option and up to 40 iterations with the full mitochondrial genome from the Mallard (Anas platyrhynchos) as a template (GenBank number KJ778676.1). We downloaded the Mallard (version GCF_000355885.1) and Chicken (Gallus gallus, version GCA_000002315.3) full genome assemblies from www.ncbi.nlm.nih.gov. We produced a pseudochromosome level assembly by aligning the more fragmented Mallard genome to the higher quality Chicken genome using the “Chromosemble” function in Satsuma version 3.1.0 (Grabherr et al. 2010) under default settings. We subsequently aligned individual steamer duck filtered reads to the improved Mallard assembly following previously published procedures (Campagna et al. 2017). The average alignment rate was 89 ± 3.5%, leading to a final average coverage of 2.8 ± 1.5× (Table S2). We first produced individual genomic variant call files for each sample with the “Haplotypecaller” module from GATK version 3.8 (McKenna et al. 2010) under default settings, and subsequently used the “GenotypeGVCFs” module to obtain a single variant file for the entire dataset. We retained ∼20 million single-nucleotide polymorphisms (SNPs) that passed the following hard filters: QD < 2, FS > 60.0, MQ < 20.0, MQRankSum < –12.5, and ReadPosRankSum < –8.0. We decided to exclude six samples that showed high levels of missing data (Table S2), leading to an average coverage of the remaining 53 samples of 3 ± 1.5×. Finally, we used vcftools version 0.1.14 (Danecek et al. 2011) to retain 309,864 variant sites with less than 30% missing data, at least 2% minimum allele frequency, and a depth of coverage between 2× and 50×. The depth of coverage of our data could difficult discerning between homozygote and heterozygote calls, especially in the samples or areas of the genome with particularly low coverage. The comparison between Illumina and PCR/Sanger sequenced genotypes at five different candidate loci (see below; Table S4) produced a discrepancy rate of 5.2%, and these were generally cases in which a heterozygote genotype was obtained with PCR/Sanger but recovered as a homozygote with Illumina sequencing (mostly in brachypterus samples). To assess the sensitivity of our results to variations in the filtering parameters, we conducted additional analyses allowing a minimum depth of coverage of between 5× and 50×, eliminating the minor allele frequency filter, and allowing 20% or 10% missing data, at the expense of retaining a smaller number of SNPs.
PHYLOGENETIC TREES AND ASSIGNMENT OF INDIVIDUALS TO GENETIC CLUSTERS
We conducted phylogenetic analyses using the final set of 309,864 filtered SNPs with SVDquartets (Chifman and Kubatko 2014) as implemented in PAUP* version 4a163 (Swofford 2003), and RAxML version 8.2.4 (Stamatakis 2014) with the “ASC_GTRGAMMA” model. We also used SVDquartets to build a species tree and lineage trees for specific regions of interest. We constructed a tree from mitochondrial genome sequences using RAxML and the “GTRGAMMAX” model. Node support was assessed with 500 bootstrap replicates in RAxML and 100 for the SVDquartets trees, with the remaining parameters left as default. Topologies were compared with the “cophylo” function in the R package “phytools” (Revell 2012).
We explored admixture among individuals in Structure version 2.3.4 (Pritchard et al. 2000) by first thinning the dataset to avoid including SNPs in linkage disequilibrium with vcftools. The thinned dataset of 16,543 SNPs excluded genetic markers within 50 kb of each other. We ran structure for 700,000 generations, discarding the initial 200,000 as burn-in, and 10 replicates of K values of 1 through 6. We estimated the allele frequency prior from the data and used the admixture ancestry model with correlated allele frequencies. The most likely value of K was determined in Structure Harvester version 0.6.94 (Earl and vonHoldt 2012); runs were averaged in Clumpp version 1.1.2 (Jakobsson and Rosenberg 2007) and visualized in Structure Plot version 2.0 (Ramasamy et al. 2014). We conducted runs with the individuals from the four species and with patachonicus individuals alone (20,274 SNPs after thinning).
DEMOGRAPHIC MODELLING
We used G-PhoCS version 1.3 (Gronau et al. 2011) to co-estimate effective population sizes, splitting times and bidirectional migration rates based on the topology from the SVDquartets species tree. Because of the computationally intensive nature of this analysis, we only included 14 patachonicus individuals (seven sampled inland and five sampled on the coast). We excluded one pteneres individual of captive origin (Table S1) and included the flying individuals from the MFI as part of brachypterus. For this analysis, we re-exported SNPs without a minor allele frequency filter, and generated sequence files for each individual with the “FastaAlternateReferenceMaker” module in GATK. We subsequently sampled 1 kb sequences in 50 kb intervals from each of the 29 largest chromosomes, generating an alignment of 1021 loci for the G-PhoCS input file. We ran the multithreaded version of the program for 250,000 iterations with a 10% burn-in, and estimated 22 parameters (seven effective population sizes, three splitting times, and 12 migration rates). We visualized traces from the parameter estimates in Tracer version 1.7.1 (Rambaut et al. 2018), and converted the median and 95% Bayesian credible intervals from mutation scale to generations and individuals as described previously (Campagna et al. 2015), assuming a rough mutation rate of 10−9 per bp per generation. We expressed migration as the number of migrants per generation, calculated as the per generation migration rate times a fourth of the theta parameter for the receiving population (ma>b × thetab/4).
GENOME-WIDE ASSOCIATION ANALYSIS AND IDENTIFICATION OF GENES IN DIVERGENT REGIONS
We conducted a phenotype–genotype association analysis using the Wald test implemented in Gemma version 0.96 (Zhou and Stephens 2014). The analysis tests the association between SNP genotypes and a phenotypic variable by fitting univariate linear mixed models, while accounting for population structure by calculating an interindividual relatedness matrix among all samples. We conducted two analyses, one using the average CV1 score as the phenotype (Fig. 1B), and the second using the average wing loading for each species (see values in Fig. 1A). Both analyses aimed to identify genetic effects on interspecies phenotypic differences. Unfortunately, we could not locate the original notes from B.C. Livezey and P. S. Humphrey, which would have allowed us to link genomic sequences to individual CV1 and wing loading scores, as we sequenced many of the same samples measured in Livezey and Humphrey (1986). We therefore used species mean values in our analyses. Because we did not have phenotypic measurements for the flying birds from the MFI, we excluded them from the analyses. To assess statistical significance, we Bonferroni-corrected for multiple comparisons (α = 0.05/309,864 SNPs ∼1.6 × 10−7), and used α = 10−9 as a conservative threshold. To visualize the results, we log-transformed the P-values, changed their sign, and built Manhattan plots with the R package “qqman” (Turner 2018). To test the robustness of our results to the methods employed to correct for population structure, we conducted a genome-wide association analysis (GWAS) using the general linear model implemented in TASSEL version 5 (Bradbury et al. 2007), correcting for population structure using a principal component analysis. We also carried out an FST outlier analyses, scanning the genome in nonoverlapping 25 kb windows with vcftools. We used this strategy to compare between coastal and inland samples of patachonicus and between flightless and flying individuals, regardless of species.
We defined three outlier regions that contained the 24 SNPs with an associated P-value smaller than 10−9 plus 5 kb on either side. We explored patterns of linkage disequilibrium in these regions by calculating the r2 statistic in plink version 1.9 (Purcell et al. 2007). Because our reference genome was improved by aligning to the chicken genome, we were not able to directly use the available annotation for the Mallard. Instead, to search for the genes contained in the outlier regions identified in the GWAS, we extracted these areas of interest and used BLAST (Altschul et al. 1990) with an e-value of 10−10 to align them to the Mallard genome (assembly version GCF_000355885.1). This procedure allowed us to obtain the list of candidate genes and to identify the relative positions of the outlier SNPs with respect to these annotations.
PCR VALIDATION OF OUTLIER SNPS
We selected five SNPs from the 24 showing the highest statistical significance in our GWAS and genotyped a total of 74 Tachyeres individuals at these loci, allowing us to increase our sample size across the full range of morphological variation and to corroborate the genotypes obtained through short-read sequencing. We also genotyped 10 Lophonetta specularoides individuals, the sister species to Tachyeres (Fulton et al. 2012), to evaluate the allele states for these candidate loci in a species capable of flight that is phylogenetically closer to Tachyeres than the Mallard. We designed primers to amplify short segments (250–300 bp) that encompassed the targeted SNP in Geneious version 10.1.3 (Kearse et al. 2012). After PCR amplification, we conducted Sanger sequencing with both the forward and reverse primers at the Cornell Institute for Biotechnology core facility. Details on the selected SNPs are in Tables S3 and S4 and the PCR amplification conditions are in Table S5.
Results
The average FST among the four Tachyeres species, estimated from ∼310,000 genome-wide SNPs, was 0.15 ± 0.1 (range: 0.07–0.25 for patachonicus vs. brachypterus and pteneres vs. leucocephalus, respectively). The mean number of fixed differences (i.e., FST = 1) was 3489 ± 3171, ranging from 300 for the patachonicus/brachypterus comparison to 7505 for pteneres/leucocephalus. Phylogenetic reconstructions did not recover flightless steamer ducks as monophyletic (Fig. 2C; Fig. S1); instead, in the species tree, the MFI endemic brachypterus was sister to a continental clade containing the remaining taxa, which in turn had pteneres as sister to the two remaining species, leucocephalus and the flying steamer duck (patachonicus). The individual-level phylogenetic reconstructions showed patterns consistent with recent speciation. First, leucocephalus was the only species unambiguously recovered as monophyletic, using different tree building methods and data from either the nuclear or mitochondrial genomes (Fig. S1). The remaining three taxa showed some level of paraphyly or polyphyly. Second, the branch lengths within the continental clade were short in the tree derived from mitochondrial genomes (Fig. S1), and the branching order changed depending on the tree building method used to analyze the nuclear genome. Consistent with the phylogenetic uncertainty associated with an episode of recent speciation, the RXML topology contradicted the remaining analyses by recovering leucocephalus as sister to pteneres.
The demographic analysis also supported a scenario of very recent speciation involving small, isolated populations, especially within the continental clade (Fig. 2C; Fig. S2). The splitting time between brachypterus and the continental clade was estimated to have been ∼19,000 generations ago. Within the continental clade, both splitting events were highly concordant in time, dating to approximately 10,000 generations ago. The inferred effective population sizes ranged from 10,000 to 60,000 individuals (for pteneres/leucocephalus and patachonicus, respectively). Finally, the levels of gene flow were below those expected to counteract the effects of drift (one migrant per generation; Wright 1931), with the highest values estimated between patachonicus and brachypterus.
A Structure analysis clearly distinguished the four Tachyeres species, and did not detect population structure within the more widespread patachonicus (Fig. 2B; Figs. S3 and S4). The average FST between coastal and inland patachonicus individuals was 0.03 (with no fixed differences) and homogeneously low across the genome (Fig. S5). Except for one pteneres individual of captive origin, there was little evidence for admixture among species (Fig. 2B; Fig. S3). However, the flying individuals from the MFI (originally identified as patachonicus) either clustered entirely with the brachypterus individuals or were hybrids between brachypterus and patachonicus, and all had brachypterus mitochondrial genomes (Fig. S1).
To search for genomic regions associated with the morphological differences among flighted and flightless steamer ducks, we conducted a GWAS between the genotypes at the ∼310,000 SNPs and the average CV1 scores form each species derived from the multivariate analysis of postcranial skeletal components (Fig. 1B). The flying birds from the MFI were excluded from this analysis because we lacked skeletal measurements for those individuals. We found only two peaks that exceeded the Bonferroni-corrected threshold of significance (P < 10−9; Fig. 3A; Fig. S6), both on chromosome 1. Within these two peaks, there were 24 SNPs with an associated P-value smaller than 10−9 that could be used to delimit three relatively narrow candidate regions. The SNPs showing the highest associations were contained in a 765 kb segment within the first peak and two segments—one 560 kb and the other 373 kb—within the second peak (Fig. 3A).
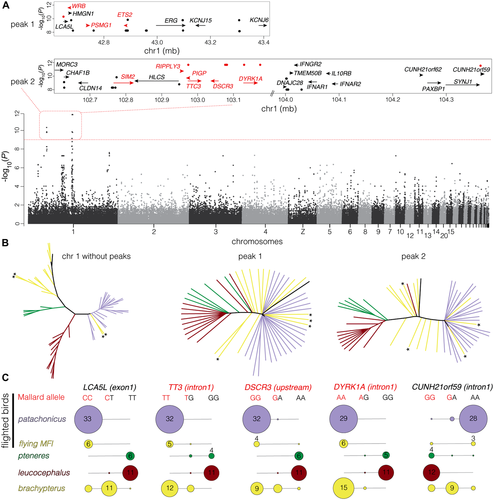
There are 28 genes with known functions annotated in the Mallard genome within these regions (and an additional 18 predicted genes with unknown functions). Nine of these genes contribute to the symptoms associated with Down syndrome (DS) in humans (in red in Fig. 3A; Table S6). The 12 SNPs showing the smallest P-values in the analysis (10−10 for peak 1 and 10−12 for peak 2) clustered within and/or close to five genes: DYRK1A (4–8 SNPs), DSCR3 (up to four SNPs), LCA5L (two), TTC3 (one), and C1H21orf59 (one) (Fig. 3A; Table S3). Thus, two-thirds of the SNPs showing the highest statistical association with variation in skeletal morphology in steamer ducks are located within or near the gene DYRK1A, a candidate gene for DS (Fotaki et al. 2002; Arron et al. 2006; Park et al. 2009). These 12 SNPs showed high values of linkage disequilibrium (average r2 of 0.8; range 0.5–1; Fig. S6), as did many of the SNPs within each peak, suggesting they are inherited together despite being in some cases up to 60 mb apart. Additional association analyses, using either the average wing loading for each species or CV1, or correcting for population structure with a principal components analysis instead of an interindividual genetic distance matrix, produced similar results to those shown in Figure 3A (Fig. S7, Table S7). We also obtained similar results when increasing the stringency of our coverage and missing data filters, and when we eliminated the minimum allele frequency filter (Fig. S8). An FST outlier analysis between flying and flightless individuals also recovered these differentiated regions (Fig. S5).
A phylogenetic tree built using SNPs from chromosome 1 (excluding the SNPs under the peaks) showed a topology consistent with the genome-wide tree (Fig. 3B cf. Fig. 2C; Fig. S1). The two phylogenies built with the SNPs from under the peaks, however, showed a different pattern. Broadly, pteneres and leucocephalus individuals grouped together and were most different from the flighted patachonicus individuals (which in turn grouped together; Fig. 3B). The individuals belonging to brachypterus fell in the middle or associated with patachonicus birds. Finally, the flighted steamer ducks from the MFI, which were either brachypterus or admixed brachypteus/patachonicus in genomic composition (Fig. 2B), grouped with the flighted patachonicus or in an intermediate position, with the brachypterus individuals.
To corroborate the results obtained in the GWAS, we selected five of the SNPs showing the highest association with CV1 to conduct a PCR validation of the genotypes obtained across a broader sample of 74 individuals (21 additional individuals to those used for Illumina sequencing; Tables S3 and S4). The patterns shown by the genotypes at these five loci generally resemble those obtained for the peak-specific phylogenies (Fig. 3C). Flighted individuals (patachonichus and flying MFI birds) were usually homozygous for the Mallard reference allele (“flighted alleles),” whereas flightless pteneres and leucocephalus were homozygous for the alternative allele (“flightless alleles”). With few exceptions, brachypterus birds were either heterozygous or homozygous for the reference allele. The exception to this pattern was the SNP in intron 1 of CUNH21orf59, where the pattern was inverted and the flighted birds were predominantly homozygous for the alternative allele. The sister species to Tachyeres, the flighted Lophonetta specularoides, was homozygous for the Mallard reference allele in the focal SNPs of four out of the five candidate loci, with the exception of six (out of 10) heterozygotes in the DSCR3 focal SNP (see Table S4).
Discussion
Steamer ducks provide an opportunity to study the processes through which birds evolve flightlessness, as they have shallow divergence levels associated with recent speciation and they span a continuum across the morphological traits that allow flight and those that do not. Here, we identified three relatively narrow genomic regions associated with the morphological variation seen in Tachyeres. The first axis of a multivariate analysis of skeletal components, influenced primarily by the length of the ulna and the humerus, produced similar results as the average wing loadings for each species, which also factor in differences in mass. These regions contained multiple genes with well-studied functions because they are implicated in DS, with the majority of the highly statistically significant SNPs in our GWAS clustered around the gene DYRK1A (Fig. 3A). Some of these candidate genes are known to influence morphological aspects in which Tachyeres ducks vary. Several of the human DS symptoms include differences in overall stature, body size, and the length of the long bones, particularly those of the upper extremities (FitzSimmons et al. 1989; Korenberg et al. 1994; Bernstein et al. 2016). Although an increase in the copy number of many genes is involved in DS, the molecular basis of these phenotypic changes is not fully understood (Park et al. 2009; Blazek et al. 2015). In an effort to study genes involved in DS, Fotaki et al. (2002) generated a knock-out mouse for DYRK1A. Although DYRK1A−/− null mutants die during gestation, they present a general growth delay (among many neurological changes), a phenotype that was also apparent in heterozygote DYRK1A+/− mice. Moreover, increased dosage of DYRK1A is thought to lead to the appendicular skeleton abnormalities observed in Ts65Dn mice, a model for DS (Blazek et al. 2015). DYRK1A phosphorylates and prevents the translocation to the nucleus of NFATc transcription factors (Arron et al. 2006), which are regulators of vertebrate development and bone growth (Arron et al. 2006; Winslow et al. 2006; Blazek et al. 2015). Here, we report a strong statistical association between genotypes at SNPs in and around the DYRK1A gene and phenotypes leading to flightlessness in steamer ducks. Copy number variation underlies the effect of DYRK1A on DS phenotypes, yet our data do not allow for a robust test of copy number variation in DYRK1A in Tachyeres. However, we hypothesize that differences in the expression patterns of DYRK1A could lead to the morphological differences observed in steamer ducks.
Despite identifying DYRK1A as the most statistically robust candidate gene in our study, it is likely that the phenotypes contributing to flightlessness in Tachyeres are influenced by many genes. For example, two of the 28 genes located in our candidate regions, LCA5L and C1H21orf59, are involved in ciliary functions, and mutations in the latter gene cause ciliopathies. Skeletal ciliopathies lead to diseases associated with abnormal bone growth in humans, and are also implicated in loss of flight in the Galápagos cormorant (Burga et al. 2017). It is possible that signaling pathways mediated by cilia also regulate changes in bone growth in Tachyeres, yet the specific mechanisms remain to be determined. Further, genomic regions outside of those identified as candidates in our study could also be involved in generating morphological differences among steamer duck species. However, the large number of fixed differences, especially between species with small effective population sizes like leucocephalus or pteneres, makes it challenging to distinguish genomic differentiation associated with species-specific morphological changes from variants that have been fixed by drift. Finally, we cannot rule out that traits that covary with CV1 or wing loading could be responsible for some of the association signals identified in the GWAS.
The phylogenetic relationships inferred by our genome-wide dataset are consistent with a previously published mitochondrial tree (Fulton et al. 2012), where the three species of flightless steamer ducks are not monophyletic. This topology implies that flightlessness evolved independently up to three times (once in each flightless species). A proportion of patachonicus individuals, particularly those from southern marine localities (Humphrey and Livezey 1982; Livezey 1986) is permanently flightless, yet we did not find evidence of population structure within patachonicus, or genomic differentiation among marine and inland individuals. This species presents values of wing loading that are close to the threshold of flightlessness, and most marine individuals were estimated to surpass that threshold and lose their ability to fly, at least temporarily, from weight increase due to food ingestion (Humphrey and Livezey 1982). Taken together, the differences between flying and flightlessness patachonicus individuals do not seem to be determined entirely genetically.
An alternative evolutionary interpretation of the history of this group of species is that flightlessness evolved a single time in Tachyeres. The flightless pteneres and leucocephalus have similar genotypes in our candidate regions (Fig. 3B, C) and we found speciation events within the continental clade to overlap in time (Fig. 2C). If we consider the continental taxa to be involved in a three-way polytomy, then the flightless variants in our candidate regions could have already existed and been polymorphic in the continental ancestor, leading to a single origination, at least from a genomic perspective, of flightlessness in pteneres and leucocephalus. We have also identified the flying birds from the MFI as either pure brachypterus (as suggested by previous mitochondrial analyses; Fulton et al. 2012) or brachypterus/patachonicus hybrids. The flying birds from the MFI generally possess the same genotypes as the flying patachonicus ducks in the candidate regions (Table S3; Fig. 3B, C), whereas brachypterus individuals tend to be heterozygotes for the alleles found in flying ducks (“flighted alleles”) and those in flightless species (“flightless alleles”). Taken together, the Tachyeres tree and the topologies from the candidate regions suggest that there is a low frequency of flighted individuals within brachypterus, and either the “flighted alleles” have been retained and remain segregating at low frequency in this species or they can enter the population after patachonicus individuals that make it to the MFI hybridize with the resident birds. In this latter scenario, either flight was never lost in brachypterus or it has been regained through the introgression of “flighted alleles” from patachonicus. To achieve flight, a brachypterus individual would need to have “flighted alleles” at a sufficient number of loci, and this could account for the low frequency of flighted individuals in the MFI (about 1% of all pairs; Fulton et al. 2012). Whether “flighted alleles” have been retained or regained, brachypterus is one of only three known species where some individuals are able to fly and others cannot (patachonicus and the rail Dryolimnas cuvieri are the other two (Roff 1994; Fulton et al. 2012)).
A third possibility involves flightlessness evolving early in the evolution of Tachyeres, and flight being subsequently regained in patachonicus. Clear examples of transitions from flightless to volant do not exists in birds (Roff 1994; Sackton et al. 2019), indicating that once the adaptations that allow flight are lost, it's unlikely that they will re-evolve. However, flight in steamer ducks is not a dichotomous trait, and instead represents a continuum between flighted and flightless individuals (Livezey and Humphrey 1986). Although regaining flight may not be impossible in steamer ducks (it may indeed be the case in brachypterus), our data do not support this possibility for patachonicus. The genotypes for the SNPs in the candidate regions show that patachonicus tends to be homozygous for the reference Mallard alleles (in 11 out of 12 highly statistically significant SNPs; Table S3; Fig. 3C), whereas flightless pteneres and leucocephalus have derived alleles. This result is consistent with patachonicus retaining the ancestral flighted condition rather than having rederived flight secondarily.
Our findings are consistent with the general scenario outlined by Fulton et al. (2012), where flightlessness could have evolved in an ancestral population that became polymorphic for flight ability. This polymorphism was retained at different levels in the four Tachyeres lineages, with pteneres and leucocephalus losing the ability to fly and brachypterus and patachionicus retaining it at different frequencies. Varying levels of polymorphism in the initial stages of flightlessness could explain how species that eventually became completely flightless have dispersed to occupy distant geographic ranges (e.g., ratites or flightless rails; Roff 1994). Tachyeres ducks may therefore be on a general evolutionary trend toward losing flight. The evolution of flightlessness is especially prevalent among aquatic birds (Roff 1994), and most marine ducks typically withstand long periods of flightlessness during their annual synchronous molt (Guillemette et al. 2007). Furthermore, flightlessness has tended to arise in avian families that already have particularly short wings for their body mass, suggesting that when the cost of flight is already high, it predisposes taxa to transition into flightlessness (McCall et al. 1998). There are a number of reasons why increased body mass and decreased wing length could have adaptive benefits in Tachyeres (see Livezey and Humphrey 1986 for a detailed discussion). Shorter appendages and increased size could assist both in thermoregulation and diving, and in reducing injuries during territorial defense, where males fight each other for territories with food resources using protuberances (i.e., spurs) on their wings (Livezey and Humphrey 1985). Flightlessness has evolved repeatedly on the avian tree of life, and steamer ducks represent a rare case in which this process can be studied while it is still in action, suggesting that the evolution of flightlessness can involve an intermediate state where species are polymorphic for flight.
AUTHOR CONTRIBUTIONS
LC and IJL designed the study, LC and KGM collected data, and LC performed all the analyses. LC wrote this article with help from all coauthors.
ACKNOWLEDGMENTS
This study was possible due to the long-term preservation of scientific collections. We are indebted to the following institutions and curators: Mark Robbins and the University of Kansas Biodiversity Institute (KU) for loaning the original tissue samples collected by Bradley C. Livezey and Philip S. Humphrey and preserved there for approximately 35 years; Mark Adams and the Natural History Museum, London (NHM); Vanya Rohwer and the Cornell University Museum of Vertebrates (CUMV); and the University of Alaska Museum of the North (UAM). The original illustrations in Figure 1A were crafted by Jillian Ditner based on skeletons obtained in loan from KU. The illustration in Figure 2 was obtained with permission from del Hoyo et al. (del Hoyo et al. 2016). This project was funded by National Science Foundation grant DEB 1555754 to IJL. We thank David Toews, David Weisrock, two anonymous referees, and the Fuller Evolutionary Biology Lab group (Cornell Lab of Ornithology) for comments on previous versions of this manuscript.
DATA ARCHIVING
The doi for the data associated with this article is https://doi.org/10.5061/dryad.6n7v8r3.
LITERATURE CITED
Associate Editor: D. Weisrock
Handling Editor: M. Servedio




