Short-Term Nutritional Limitation Affects Mating Behaviour and Reproductive Output in Dwarf Spiders
Abstract
Mating decisions can vary considerably depending on individual experience, mate availability and nutritional status. Here, we applied short-term dietary restrictions to adult female spiders that were well fed during the juvenile stage in an effort to understand whether and how brief periods of food shortage can influence male and female mating decisions and mating behaviour. To assess whether responses vary between closely related species, we conducted the same experiment on the dwarf spiders Oedothorax retusus and O. apicatus. During courtship and mating, males of both species offer secretion to females from glandular tissue in their prosoma. Females were subject to food shortage over a period of 3 wks (‘low-diet’ treatment, LD) or fed regularly (‘high-diet’ treatment, HD). We compared courtship probability, mating probability/behaviour, and reproductive output between dietary groups and species. In both Oedothorax species, females in the LD treatment were less likely to mate and more aggressive towards males. Furthermore, LD females produced egg sacs that were significantly lighter than were those of the HD females. Effects of food deprivation on copulation duration, gustatory behaviour and oviposition latency differed between species. Our study shows that short periods of dietary restriction during the adult stage can strongly affect mating behaviour and reproductive output with differences between closely related species.
Introduction
Mating behaviour is influenced by many factors like individual experience and condition, age, or environmental conditions such as food availability and foraging history (Gray 1999; Ortigosa & Rowe 2002; Hebets et al. 2008; Eraly et al. 2009; Immonen et al. 2009; Adams & Morse 2014). Mating behaviour further depends on local mate availability and the quality of the available mating partners resulting in choosiness of either sex (Andersson & Iwasa 1996). Several studies emphasize that mating preferences of both sexes vary during a lifetime (Howard & Young 1998; Brown & Kuns 2000; Burley & Foster 2006; Fisher & Rosenthal 2006) and change depending on an individual's own condition (Cotton et al. 2006; Holveck & Riebel 2010). Consequently, optimal mate choice in both sexes is a compromise between mate qualities, cost of mating and risk of losing an opportunity to mate with another partner (Elgar & Nash 1988; Bonduriansky 2001; Bleu et al. 2012).
Nutritional condition of both mating partners plays an important role in mate choice decisions (Schneider & Elgar 2002; Engqvist & Sauer 2003; Arnqvist & Rowe 2005; Fisher & Rosenthal 2006; Garbutt & Little 2014). Mating decisions of males and females may be influenced on a fine temporal scale depending on momentary food abundance, which may intensify conflicts between the sexes over mating in a particular time window (Fisher & Rosenthal 2006). Generally, well-fed individuals profit in several respects compared with food-limited individuals: higher mating probability, higher number of matings, longer copulation durations and higher reproductive output (Engqvist & Sauer 2003; Hebets et al. 2008; Eraly et al. 2009; Albo et al. 2012). In species such as crickets, scorpionflies, beetles and spiders, male body condition affects male courtship, female mate choice and mating success (Gwynne 1993; Mappes et al. 1996; Andrade & Mason 2000; Ahtiainen et al. 2002; Kotiaho 2002; Hunt et al. 2005; Hoefler et al. 2008; Engqvist 2009; Lomborg & Toft 2009; Albo et al. 2012) and impacts the male's susceptibility to diseases and his sperm quality and quantity (Giaquinto et al. 2010).
Resource limitation will cause mates to discriminate among potential partners based on condition (Gwynne 1993; Byrne & Rice 2006). Males may assess a female's fecundity directly through visual or tactile mechanisms and perform mate choice via proxies of female fecundity, for example condition, size or weight (Gwynne 1993; Bonduriansky 2001; Byrne & Rice 2006; Barry 2010). Female mating decisions can be affected not only by the male phenotype but also may considerably depend on her own feeding history (Engqvist & Sauer 2003; Fisher & Rosenthal 2006; Engqvist 2009). For example, females in poor condition can increase their fecundity by preferentially mating with males that offer nuptial gifts (Fox & Moya-Laraño 2009; Immonen et al. 2009) or by simply consuming their mating partner (Andrade 1998; Herberstein et al. 2002; Barry et al. 2008, 2010; Barry 2010; Berning et al. 2012). In the praying mantid Pseudomantis albofimbriata, females in low body condition are more likely to perform pre-copulatory sexual cannibalism (Barry 2010). However, in spiders even hungry females exhibit mate choice: they cannibalize low-quality males before or after copulation but spare high-quality males (Elgar & Nash 1988).
Here, we investigate how a brief period of food shortage in adult females affects courtship activity, mating probability, mating behaviour and reproductive output. We further analysed whether males differentiate between females with short-term differences in feeding history. We compare these effects between the dwarf spiders Oedothorax retusus and O. apicatus that are considered sister species in a recent phylogenetic analysis (Lopardo & Uhl 2014) in order to assess tentatively to what degree responses to short-term food limitations are generalizable. In both species, the male prosoma is elevated in a species-specific way and possesses glandular tissue, which produces secretions that are taken up by the female during courtship and mating (Michalik & Uhl 2011; Kunz et al. 2013). In O. retusus, the secretion was found to increase mating probability and to stimulate egg production (Kunz et al. 2012). Consequently, we expect that food-limited females would be more prone to forage for these secretory nuptial gifts.
Methods
Study Species
Oedothorax retusus and O. apicatus are European dwarf spiders (Linyphiidae, Erigoninae). O. retusus occurs in wet habitats (salt marshes, riverbanks), whereas O. apicatus is common on cultivated, agricultural areas. For this study, adult females of O. retusus were collected from the banks of the River Rhine, near Bonn, Germany (50°43′00.26″N, 7°08′25.11″E – 50°42′57.40″N, 7°08′36.70″E) in April/September 2010–2012. Adult O. apicatus females were collected from an agricultural field situated between Bonn and Siegburg, Germany (50°45′19.16″N, 7°09′11.11″E – 50°45′17.76″N, 7°09′07.20″E) in September 2011 and April/September 2012. Offspring were individually reared in the laboratory under controlled conditions (23/17°C (d/night), 70% humidity) in 25-ml plastic containers equipped with a moist layer of gypsum. Spiderlings were raised separately from egg sacs and fed ad libitum on springtails (Sinella curviseta) until the subadult stage (Kunz et al. 2012). Subadults were fed on four to six fruit flies (Drosophila melanogaster) per week and were checked weekly for the final moult to adulthood. Males were fed four to six fruit flies per week. Mating experiments were performed between September 2010 and November 2012.
Experimental Set-Up
We randomly assigned freshly moulted, virgin females to ‘high-diet’ (HD) or ‘low-diet’ (LD) treatments. During 3 wks after the final moult, HD females were fed four to six fruit flies per week, while LD females were fed a maximum of three flies in 3 wks. In the laboratory, females of O. retusus and O. apicatus live up to 12 mo after their final moult, while males have a lifespan of up to 8 mo. Thus, 3 wks food limitation is a short time period in those species. Males were kept on a standardized HD like females. Mating trials with a total of 371 virgin females and 371 virgin males (O. retusus: HD: n = 116, LD: n = 78; O. apicatus: HD: n = 91, LD: n = 86) were staged. On the day of the mating experiment, females were on average 21.6 ± 3.8 d (O. retusus: HD 22.4 ± 4.7, LD 22.1 ± 2.6; O. apicatus: HD 21.3 ± 4.1, LD 20.3 ± 2.3), and males 22.0 ± 3.9 d old (O. retusus: HD 22.8 ± 5.0, LD 21.8 ± 2.7; O. apicatus: HD 22.3 ± 4.1, LD 20.9 ± 2.7). We used female weight on the day of the mating trial as a proxy for female fecundity (Uhl et al. 2004; Moya-Laraño et al. 2008). LD females were significantly lighter than HD females in O. retusus (weight (mg): HD 3.62 ± 0.46, n = 21; LD 1.86 ± 0.25, n = 25; T-test: T = 15.72, p < 0.001) and O. apicatus (HD 3.84 ± 0.57, n = 13; LD 1.90 ± 0.20, n = 48; T-test: T = 12.04, p < 0.001). Mating trials started by gently transferring a male into the container of the female. Mating partners were continuously observed until the end of mating or a maximum of 30-min observation time. We compared male courtship probability, probability of female aggressive behaviour (attacking and chasing the male), insertion probability (insertion of one pedipalp into one female genital opening), duration of pedipalp insertion, whether the female salivated onto the male's prosoma for uptake of secretions, and who ended the insertion (see Kunz et al. 2012 for more detailed definitions of parameters). After the mating trials, all females were fed on a regular, weekly HD feeding schedule with four to six fruit flies until oviposition. As parameters of reproductive output, we recorded oviposition latency, weight of the egg sac (Sartorius ME5 micro scale: 5 g capacity, 1 μg readability, ± 1 μg reproducibility), egg number, egg mass (weight of the egg sac/number of eggs) and hatching success (percentage of hatched spiderlings). We compared parameters between HD and LD mating trials within and between species.
Statistical Analysis
Statistical analyses were performed with IBM SPSS Statistics 22.0 using chi-square test, Mann–Whitney U-test, and T-test. Data are given as arithmetic 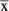 ± SD for normally distributed data or
± SD for normally distributed data or 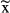 [interquartile range] for non-normally distributed data. All tests were performed two-tailed.
[interquartile range] for non-normally distributed data. All tests were performed two-tailed.
Results
Courtship and Mating Probability
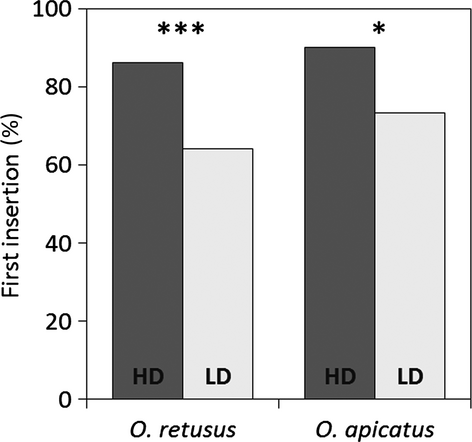
Male courtship probability in O. retusus males was not significantly different between HD and LD trials (Table 1). In O. apicatus, however, males were significantly more reluctant to court LD females (Table 1). Differences in courtship probability between species were not significant (χ² tests: HD: n = 207, χ² = 0.60, p = 0.440; LD: n = 164, χ² = 0.02, p = 0.885). In both species, LD females were significantly more likely to show aggressive behaviour towards the male than HD females (Table 1), but cannibalization did not occur. Mating probability (insertion probability of a pedipalp) depended significantly on female feeding regime in both species and was less likely with LD females (Table 1, Fig. 1). Mating probabilities were not significantly different between species (χ² tests: HD: n = 207, χ² = 0.73, p = 0.392; LD: n = 164, χ² = 1.60, p = 0.206). In both species, it was generally the males that were reluctant to mate; however, the difference between treatments was not significant (Table 1) and most likely a consequence of female aggressive behaviour. Female aggressive behaviour significantly influenced insertion probabilities in both HD and LD treatments. First insertions were less likely if females behaved aggressively in HD and LD treatments of both species (insertion probability: O. retusus HD: aggressive females 50%, 2 of 4; non-aggressive females 87.5%, 98 of 112; χ² = 4.57, p = 0.033; O. apicatus HD: no cases of aggressive behaviour, n = 91; O. retusus LD: aggressive females 28.6%, 4 of 14; non-aggressive females 71.9%, 46 of 64; χ² = 9.36, p = 0.002; O. apicatus LD: aggressive females 55%, 11 of 20; non-aggressive females 78.8%, 52 of 66; χ² = 4.43, p = 0.035). In O. retusus, the duration of the first insertion was significantly reduced with LD females, and LD females applied salvia onto the male′s prosoma with significantly higher probability compared to HD females (Table 1). None of these parameters was significantly different between LD and HD groups in O. apicatus.
| O. retusus | O. apicatus | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘High-diet’ | ‘Low-diet’ | Za, Tb, χ² | p | ‘High-diet’ | ‘Low-diet’ | Za, Tb, χ² | p | |||||
| N | Events 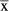 / /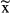 |
N | Events 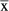 / /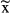 |
N | Events 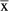 / /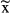 |
N | Events 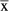 / /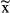 |
|||||
| Male courts (yes:no) | 116 | 113:3 (97.4%) | 78 | 73:5 (93.6%) | 1.73 | 0.189 | 91 | 90:1 (98.9%) | 86 | 80:6 (93.0%) | 4.02 | 0.045 |
| First insertion | ||||||||||||
| Female aggressive (yes:no) | 116 | 4:112 (3.5%) | 78 | 14:64 (20.6%) | 11.65 | 0.001 | 91 | 0:91 (0.0%) | 86 | 20:66 (23.3%) | 23.86 | <0.001 |
| Insertion probability (yes:no) | 116 | 100:16 (86.2%) | 78 | 50:28 (64.1%) | 13.00 | <0.001 | 91 | 82:9 (90.1%) | 86 | 63:23 (73.3%) | 8.48 | 0.004 |
| Insertion duration (s) | 100 | 206.5 ± 64.0 | 50 | 168.8 ± 64.5 | 3.39b | 0.001 | 82 | 369 [265] | 63 | 365 [129] | −0.03a | 0.975 |
| Female salivates (yes:no) | 75 | 24:51 (32.0%) | 46 | 24:22 (52.2%) | 4.85 | 0.028 | 70 | 39:31 (55.7%) | 60 | 38:22 (63.3%) | 0.78 | 0.378 |
| Insertion ended by (male:female) | 100 | 90:10 (90.0%) | 50 | 44:6 (88.0%) | 0.14 | 0.708 | 82 | 77:5 (93.9%) | 63 | 60:3 (95.2%) | 0.12 | 0.727 |
| Second insertion | ||||||||||||
| Female aggressive (yes:no) | 100 | 1:99 (1.0%) | 49c | 2:47 (4.1%) | 1.58 | 0.208 | 82 | 3:79 (3.7%) | 63 | 5:58 (7.9%) | 1.25 | 0.263 |
| Insertion probability (yes:no) | 100 | 92:8 (92.0%) | 49c | 47:2 (95.9%) | 0.20 | 0.658 | 82 | 76:6 (92.7%) | 63 | 60:3 (95.2%) | 0.40 | 0.527 |
| Insertion duration (s) | 92 | 195.0 ± 71.7 | 47 | 189.2 ± 65.3 | 0.47b | 0.641 | 76 | 391 [351] | 60 | 371 [224] | −1.00a | 0.318 |
| Female salivates (yes:no) | 72 | 24:48 (33.3%) | 41 | 17:24 (41.5%) | 0.75 | 0.387 | 64 | 43:21 (67.2%) | 49 | 37:12 (75.5%) | 0.93 | 0.335 |
| Insertion ended by (male:female) | 92 | 87:5 (94.6%) | 47 | 44:3 (93.6%) | 0.05 | 0.820 | 76 | 69:7 (90.8%) | 60 | 59:1 (98.3%) | 3.45 | 0.063 |
| Reproductive success | ||||||||||||
| Oviposition latency (s) | 79 | 9 [30] | 19 | 32 [38] | −3.60a | <0.001 | 68 | 31 [31] | 35 | 57 [38] | −4.75a | <0.001 |
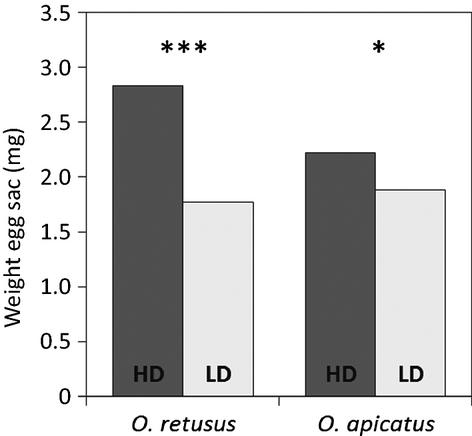
| Weight egg sac (mg) | 76 | 2.83 ± 0.83 | 17 | 1.77 ± 0.83 | 4.72b | <0.001 | 68 | 2.22 ± 0.56 | 36 | 1.88 ± 0.65 | 2.81b | 0.006 |
| Egg mass (mg) | 66 | 0.13 [0.04] | 5 | 0.15 [0.09] | 1.79a | 0.074 | 51 | 0.14 [0.05] | 24 | 0.12 [0.05] | −0.83a | 0.407 |
| Egg number | 66 | 22.9 ± 8.6 | 5 | 10.2 ± 6.2 | 3.24b | 0.002 | 51 | 16.1 ± 5.8 | 24 | 14.6 ± 5.6 | 1.04b | 0.302 |
| Hatching success (%) | 66 | 54 [95] | 5 | 80 [92] | −0.29a | 0.773 | 51 | 100 [77] | 24 | 0 [88] | −3.33a | 0.001 |
-
Data are given as events (proportions),
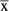 ± standard deviation and
± standard deviation and 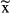 [interquartile range].
[interquartile range].
- Statistical analysis was performed using chi-square test, aMann–Whitney U-test and bT-test.
- Significant values are marked in bold, cmarks a missing case in the O. retusus LD treatment because of a malformed male pedipalp.
Most of the matings entailed a further insertion with the other pedipalp. However, the probability of a second insertion to occur did not differ significantly between feeding regimes (Table 1). Aggressive behaviour by the female rarely occurred prior to a second insertion (1–8%) and was not significantly different between feeding regimes of both species (Table 1). In both species, the duration of the second insertion was not significantly different between feeding regimes (Table 1). In O. retusus, duration of the second insertion was significantly different from the first insertion duration in the LD treatment, but not in the HD treatment (paired t-test: HD: n = 92, T = 1.25, p = 0.215, LD: n = 47, T = −2.06, p = 0.045; see Table 1 for data). In O. apicatus, duration of the second insertion was not significantly different from first insertion duration in both treatments (Wilcoxon test: HD: n = 76, Z = −1.10, p = 0.271, LD: n = 60, Z = −0.11, p = 0.909; see Table 1 for data).
Reproductive Output
Oviposition latency was significantly different between female feeding regimes in both species. In O. retusus, LD females took on average 23 d longer than HD females and in O. apicatus LD females took 26 d longer than HD females (Table 1). Furthermore, female feeding status had a significant effect on the weight of the egg sac with egg sacs of LD females being significantly lighter than those of HD females in both species (O. retusus: 37.5% lighter, O. apicatus 15.3% lighter, Table 1, Fig. 2). However, single egg mass did not differ significantly between feeding regimes of O. retusus or O. apicatus females (Table 1). Lower egg sac weight resulted from significantly fewer egg numbers (−55.5%) in LD O. retusus females, whereas the 15.3% reduction in egg number in O. apicatus females is not statistically significant (Table 1). In O. retusus, the percentage of hatched spiderlings was not significantly different between female feeding regimes, but in O apicatus, LD females suffered from a significant decrease in hatching success (Table 1).
Discussion
We analysed the effect of short-term feeding limitation in the female on mating behaviour and reproductive output in two closely related dwarf spider species. In both species, mating was less likely with females of the ‘low-diet’ treatment (LD) compared to the ‘high-diet’ treatment (HD) which seems to be due to increased aggression by LD females towards their potential mates and partly to male reluctance to court LD females. Short-term feeding limitation resulted in postponed oviposition and reduced weight of the egg sacs in both species. The two species, however, differed with respect to effects of feeding treatment on copulation duration, gustatorial courtship behaviour and reproductive output.
When potential mating partners meet, mating probability depends on female receptivity and on mate preferences of both sexes that often vary greatly (Howard & Young 1998; Brown & Kuns 2000; Burley & Foster 2006; Adams & Morse 2014). Under food shortage, female receptivity can be reduced to avoid costly courtship and mating activity (Perry et al. 2009). In carnivorous species such as spiders, females may consume the male before or after mating depending on their hunger level (feeding opportunism hypothesis, Andrade 1998). In the praying mantid Pseudomantis albofimbriata, cannibalistic females improve their body condition and produce heavier egg sacs compared to non-cannibalistic females (Barry et al. 2008). In both Oedothorax species investigated here, female aggression before mating increased approximately 20-fold in food-limited females, suggesting that females may have tried to cannibalize the male. Deadly attacks, however, did not take place despite the small mating arena. Low cannibalism probability may be due to the fact that males and females in Oedothorax are of similar size in contrast to most other sexually cannibalistic species in which males are much smaller and thus more easily overcome by the female (Andrade 1998). Possibly, LD Oedothorax females react faster to any movement in their webs and thereby discriminate mates from potential prey later than HD females.
Interestingly, despite an increase in aggressive behaviour, a high percentage of females nevertheless engaged in matings even under the food-deprived condition (64% O. retusus, 73% O. apicatus). Consuming nuptial gifts that increase female condition may compensate for costly matings or food deprivation (reviewed in Vahed 2007). In a study on the effect of male cephalic secretions in O. retusus, females that received secretions from the males produced more offspring (Kunz et al. 2012). As LD females were significantly more likely to salivate onto the gift-producing male cephalic structures in the present study, we assume that these females tried to forage for male cephalic secretions. Increasing gustatorial activity was not present in O. apicatus for which the function of the cephalic secretions remains to be investigated. However, our study suggests that the functions of the gustatorial secretions may differ even between closely related species.
Although mate choice has mainly been attributed to females, variation in female quality, low mate search costs and sperm limitation can select for the evolution of male mate choice, which seems to be more common than previously assumed (Bonduriansky 2001; Bateman & Fleming 2006; Barry 2010; Barry & Kokko 2010; Edwards & Chapman 2011; Lombardo et al. 2012; Adams & Morse 2014). In the praying mantid Pseudomantis albofimbriata, it has been shown that the attractiveness of females to males depends on the females’ condition, allowing males to choose mates that provide the highest potential reproductive success (Barry & Kokko 2010; Barry et al. 2010; for other insect species see: Bonduriansky 2001). In our comparative study, males were generally the sex that was reluctant to mate, however, only in O. apicatus, male courtship probability was significantly less likely when the males were confronted with LD females. However, rejecting a female can reduce male reproductive success considerably, which is one of the main reasons why theoretically male mate choice should not necessarily evolve (Barry & Kokko 2010; Barry et al. 2010). In our study, however, O. apicatus male investment in mating activity including gustatorial courtship is very likely lost with short-term food deprived females since the majority of these females failed to produce viable offspring. Consequently, nutritional limitation has a particularly strong impact on female reproductive output in O. apicatus despite the fact that food limitation was only brief and occurred before copulation. Why LD O. apicatus females cannot compensate short-term nutritional limitation during the 2 mo post-mating period remains to be investigated. However, our finding corresponds with theoretical models demonstrating that male choosiness is expected to evolve particularly in species with high variation in female quality as well as high mate availability (Barry & Kokko 2010).
It is known from many arthropod species that a low female nutritional status can affect reproduction through an increase in oviposition latency or a decrease in egg number or egg mass (Gwynne 1988; Vahed 1998, 2007; Simmons et al. 1999; Simmons 2001; Bergström & Wiklund 2002; Engqvist 2007). As food limitation in arthropods is assumed to occur frequently under natural conditions (Denno & Fagan 2003; Fagan & Denno 2004), such effects are expected to strongly shape reproductive behaviour. However, the degree to which short-term resource limitation affects reproductive output in both Oedothorax species is unexpectedly high. In our study, LD females that were food limited for 3 wks before mating, postponed oviposition for about the same time. More importantly, regular feeding after mating could not compensate for the phase of food stress prior to mating. LD females suffered from severe long-term costs in several ways: in both species, females produced egg sacs that were significantly lighter, compared to the HD treatments. O. retusus LD females produced only half the number of eggs and offspring compared to HD females, while in O. apicatus, LD females reduced the number of eggs laid only slightly but suffered from an extremely low hatching success.
Our study demonstrates that short phases of food deprivation – as regularly occur in nature – can have unexpectedly strong immediate effects on mating behaviour and subsequent effects on reproductive output. Overall, our study on the dwarf spider species O. retusus and O. apicatus shows that females in low condition are more aggressive towards courting males and that males are more likely to decide against females with expected low reproductive output at least in O. apicatus. Reproductive success with short-term food deprived females was low in both species, which demonstrates the severe long-term impact of food limitation on fecundity. Our study further demonstrates that effects of short-term feeding limitations cannot easily be generalized even between closely related species.
Acknowledgements
We thank Rainer Jahnke and Anja Röw (both Greifswald) for rearing the Drosophila spider food. Helpful comments on the manuscript were given by Michael Schmitt (Greifswald). The authors declare that there is no conflict of interest.




