An assessment of Putative Sexually Antagonistic Traits in a Freshwater Amphipod Species
Abstract
Sexual conflict can result in an ‘evolutionary arms race’ between males and females, with the evolution of sexual antagonistic traits used to resolve the conflict in favor of one sex over the other. We assessed the resolution of sexual conflict in a Hyalella amphipod species by manipulating putative sexually antagonistic traits in males and females and used mate-guarding duration as our metric of conflict resolution. We discovered that large male posterior gnathopod size increased mate-guarding duration, which suggests that it is a sexually antagonistic trait in this species. In contrast, female and male body size did not significantly affect mate-guarding duration. Given that male posterior gnathopods show heightened condition dependence, future investigations should explore the interactive effects of sexual conflict and ecological context on trait evolution, phenotypic divergence, and speciation to elucidate the complex mechanisms involved in the evolution of biological diversity.
Introduction
Sexual reproduction was once considered a collaborative effort, an act mutually undertaken by two organisms intended for the transmission of the organisms’ genetic material. Although some have argued that conflicting sexual behavior is implicit in Darwin's arguments for natural selection, recent research has explicitly highlighted the fact that each sex can have different reproductive strategies and trait optima (Arnqvist & Rowe 2005). As a result, the evolutionary interests of the sexes may diverge significantly, resulting in a new understanding of male–female interactions and the evolution of mating systems (Parker 2006). The diverging interests of the sexes, and the conflicts that result, have been proposed as a mechanism for the development of sexual dimorphism and a driver of speciation (Gavrilets 2000; Martin & Hosken 2003). This evolutionary arms race between the sexes, known as ‘sexual conflict’, requires that the male and female have different trait optima that cannot be attained concurrently. Such conflict can manifest itself as a behavioral conflict, including a physical struggle over whether to mate (Arnqvist & Rowe 2005; Parker 2006).
Sexual conflict over mating can be an important engine of diversification that results in the evolution of sexually antagonistic traits that give one sex an advantage over the other. For example, there are many examples of male traits that increase male mating success by grasping, stabbing, or physiologically manipulating females (Thornhill & Sauer 1991; Chapman et al. 1995; Sakaluk et al. 1995; Stutt & Siva-Jothy 2001; Arnqvist & Rowe 2002; Miller 2003; Bergsten et al. 2007). However, our knowledge of female traits that determine whether mating will occur is less extensive (but see, Crudgington & Siva-Jothy 2000; Arnqvist & Rowe 2002; Reinhardt et al. 2003; Bergsten et al. 2007; Karlsson Green et al. 2013).
Numerous male and female traits, including both behaviors and morphological traits, are predicted to affect the outcome of sexual conflict (Arnqvist & Rowe 2005). Body size is one such trait, given that it is often predictive of power asymmetries and resource-holding potential (Parker 1974; Thornhill & Alcock 1983). Interestingly, there are relatively few studies on how body size affects sexual conflict. In fruit flies (Drosophila melanogaster) larger males are more harmful to females, whereas female size has no effect on the ability to resist male harm (Pitnick & García-González 2002). In stone leek leafminers (Liriomyza chinensis), there was no effect of male body size on the harm imposed on females (Tran & Takagi 2006). In crustaceans, where males and females often disagree over precopulatory mate-guarding duration (e.g., Jormalainen & Shuster 1999; Sparkes et al. 2000; Takeshita et al. 2011), some studies have found that larger males impose longer mate-guarding durations, but only when females were able to resist male guarding attempts (Jormalainen & Merilaita 1993, 1995). These studies suggest that larger males are often better at overcoming female resistance to pairing than small males. However, we do not know the extent to which female body size affects a female's resistance ability. Studies that consider the role of body size and other sexual traits in resolving conflicts will provide a more complete understanding of the ecological and evolutionary significance of sexual conflict (Fricke et al. 2009).
We explored the role that male and female traits play in sexual conflict in a Hyalella amphipod species. The mating biology of Hyalella amphipods is tightly linked to the female molt cycle (Wellborn & Cothran 2007). A female cannot store sperm and is only receptive to fertilization for a short period after she molts (Sutcliffe 1992). This results in intense competition among males for the few receptive females in the population. As a consequence, males perform precopulatory mate guarding of females as a time investment strategy to secure access to receptive females and minimize missed opportunity costs (Ridley 1983, Jormalainen 1998). In Hyalella, males use claw-like structure called gnathopods in precopulatory mate guarding. The anterior gnathopods (not their enlarged posterior gnathopods) are used to hold onto females during mate guarding (Sutcliffe 1992). The enlarged posterior gnathopods, which are around 15 times larger in males (Wellborn 2000), are used to seize females to initiate mate guarding (Strong 1973; Cothran et al. 2010). Mate-guarding duration in the Hyalella species used in this study averages 3–5 days depending on the population (Wellborn & Cothran 2007). Males prefer longer mate-guarding durations than females, because females incur the costs of lengthy guarding durations (e.g., increased predation risk) without the benefits that males receive in terms of increased mating success (Cothran 2004). Hence, there is behavioral conflict over mate-guarding duration that has been observed as females that are early in their molt cycle thrashing and tightly curling their body to avoid male pairing attempts (RDC personal observation) and as pairing duration increasing when female resistance is blocked by sedating females (Cothran 2008). Further evidence for female resistance playing a role in reducing mate-guarding duration comes from comparisons of female molt intervals and time spent in precopula. While female molt intervals are fairly consistent across species and populations in Hyalella (~10 days), time spent in precopula is variable (Strong 1972; Wellborn & Cothran 2007). Moreover, species that live with visual predators that prefer precopulatory pairs have shorter guarding durations than species that live in habitats that lack these predators (Cothran 2004). Previous amphipod studies have demonstrated large males with larger posterior gnathopods (a large claw-structure) are more likely to be found in mate-guarding pairs (Wellborn 1995, 2000; Wellborn & Bartholf 2005). Removal of the male posterior gnathopods causes a dramatic reduction in mate-guarding duration, which suggests that this trait is used to coerce females into longer guarding durations (Cothran et al. 2010). Although the posterior gnathopod appears to be important in resolving conflicts between the sexes, we have no evidence that it is used in male–female interactions (Cothran et al. 2010). Here, we take a more complete look at putative sexually antagonistic traits by examining how female and male body size and male posterior gnathopod size affect mate-guarding duration. We hypothesized that larger male body size and larger posterior gnathopod size would be associated with longer mate-guarding durations, whereas larger female body size would allow better resistance against males and would be associated with shorter mate-guarding durations.
Methods
Animal Collection and Husbandry
In North America, Hyalella amphipods are represented by many undescribed species that include two ecomorphs (large and small) that are each represented by multiple species (Wellborn & Broughton 2008). Large ecomorph species are found in habitats without predatory fish (especially Lepomis species), and small ecomorphs are found in habitats that contain these predators (Wellborn 1994a; Wellborn et al. 2005). We used laboratory-reared large ecomorph amphipods [from the OK-L clade in Wellborn & Broughton (2008)] that were collected from Lake Thunderbird (Cleveland County, OK, USA) in October 2011. Stock animals were housed in 14-L tubs containing carbon-filtered, UV-irradiated water, sand, waterweed (Elodea sp.), and artificial macrophytes made of polypropylene rope. The tubs were kept in a temperature-controlled room (mean ± 1 SD: 22.2°C ± 0.2) with a 16:8 h day:night cycle. Three times each week, we fed the animals a 3:1 mixture of ground Tetramin® fish flakes (Tetra Werke, Melle, Germany) and alfalfa (Spring Valley, Bohemia, NY, USA). This food was supplemented with 0.5 g of spirulina (Nutrex Hawaii Inc., Kailua-Kona HI, USA) and 20 ml of high-phosphorus Scenedesmus algae suspended in 20 ml Bacto®-agar solution. We used this diet for all animals in the study.
Animal Preparation and Phenotypic Manipulation
We sorted male and female amphipods by sex using a dissecting microscope and then divided each sex into three size classes—small, medium, and large—using a multistep technique.
Animals were initially separated by visual identification into three size classes. Animals from each of the three size classes that overlapped with another size class were then removed to create distinct size classes that had no overlap. The distinct size classes of female and male amphipods were kept separately in plastic containers containing carbon-filtered, UV-irradiated water, sand, and waterweed. These animals were on the same feeding schedule as the stock animals. Stock males were added to the female size class quart containers to allow mate guarding to occur.
To investigate the effect of gnathopods on sexual conflict, we needed to weaken the allometric relationship between male gnathopod size and male body size. To do so, we used ablation of the posterior gnathopod followed by a period of regrowth. This manipulation increased the phenotypic space occupied by males in each size class, thus producing groups of males that possessed smaller gnathopods than expected based on body size. We removed the posterior gnathopod (both the propodus and the dactyl were removed, Fig. 1) under a dissecting microscope for half of the males in each size class. To control for the effects of surgery, the remaining males in each size class had two to three of the most distal segments of the 5th walking leg (peraeopod) removed. In two cases when this limb was missing, we removed the distal segments of the 4th peraeopod (Fig. 1). Because peraeopods are serially homologous to the posterior gnathopod, wounds produced by each surgical manipulation were similar in size. Moreover, removal of the peraeopod does not alter mate-guarding duration (Cothran et al. 2010). All animals were anesthetized prior to surgery using a clove oil solution with an ethanol carrier (clove oil concentration: 2.97 × 10−4 ml/ml; Venarsky & Wilhelm 2006). After surgery, males were allowed to recover for 3 wks, which was sufficient time for males to go through at least one molt to regrow their ablated gnathopods and peraeopods. This necessary 3-wk recovery period and the fact that growth rates decrease with size and age in amphipods (Wellborn 1994b) allowed males from the medium size class to grow to a similar size as those males assigned to the large size class (male size (mean ± SD): small size class: 0.68 ± 0.03; medium size class: 0.75 ± 0.05; large size class: 0.75 ± 0.03). However, our use of size classes achieved the goal of covering a wide range of male sizes in the experiment (see 3).

Males from each ablation group were held separately in 14-L plastic tubs containing carbon-filtered and UV-irradiated water, sand, and waterweed. For each body size class-by-ablation group, the plastic tub contained around 40 males. These animals were kept on the same feeding schedule as the stock animals.
To ensure that the behavioral trials were conducted across an entire female molt cycle, females from each size class found in mate-guarding pairs with stock males (again, size classed females were housed with males that were haphazardly selected from the stock population) were isolated in individual 48-ml plastic cups that were filled with water and sand. We provided these animals ad libitum food; old food was removed and replaced at least three times per week. After the female oviposited, which indicates that she had completed a molt cycle, she was transferred to a new 48-ml cup containing water and sand, which served as our experimental arena for observing male and female mating behavior. In the experimental arena, the isolated female was then presented with three males from a given body size-by-appendage ablation combination and food was provided ad libitum. Females in each size class were evenly distributed among male body size class-by-ablation groups. In Hyalella, female molt cycles are asynchronous, so we had to stagger the setup of experimental arenas between 18 and 28 March. By adding three males to each experimental arena, we ensured that females experienced male encounter rates that were more typical of those experienced in nature (population density is typically in the thousands per m2; Wellborn 1994a). Evaporated water was replaced to ensure consistent water levels.
Experimental arenas were checked for mate-guarding pairs three times each day (0800–1000, 1200–1400, and 2000–2200 EST) for the duration of the experiment. During each observation, we noted the presence or absence of mate-guarding pairs and dead animals were removed. Based on the observations of mate-guarding pairs, we estimated mate-guarding duration as the time spent paired between observations until the female either oviposited or died, thus avoiding overestimates of total time spent paired. If a female separated from a male and was found paired again at another observation, her total time paired was estimated as the sum of all pairing events. Dead males were replaced with new males from the same body size class-by-ablation group, until the supply of amphipods that had undergone appendage manipulations was exhausted. Male survival did not differ significantly among male body size classes or between male appendage ablation groups (likelihood ratio χ2 = 3.0, p = 0.7; large male-by-gnathopod ablated: n = 12, large male-by-peraeopod ablated: n = 9, medium male-by-gnathopod ablated: n = 16, medium male-by-peraeopod ablated: n = 12, small male-by-gnathopod ablated: n = 14, small male-by-peraeopod ablated: n = 12). Average male survival across all male size class-by-ablation groups was approximately 57%. Female survival was 63% (details on how female survival responded to male traits are provided in the Results).
When the female oviposited or was found dead, all animals from an experimental arena were preserved in 70% ethanol. Preserved animals were later digitized using an Olympus SZX16 microscope fitted with a DP25 digital camera (Olympus America, Center Valley, PA). After images of the intact individuals were obtained, we dissected each animal and obtained additional images at 20X magnification for the head capsules of both sexes and both posterior gnathopods of males. We used ImageJ software (v.1.42q National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/) to measure head length, which is strongly correlated with body length and dry mass in amphipods (Edwards & Cowell 1992), and posterior gnathopod width.
Statistical Analyses
We used an ANCOVA to test whether males with ablated gnathopods had relatively smaller gnathopods than males with ablated segments of their peraeopods (after a period of regrowth). We used head length, an indicator of body size, as a covariate to examine body size-independent differences in gnathopod size.
We then used regressions to assess whether female survival and mate-guarding duration was dependent on female body size, mean male body size, and mean male gnathopod size. The latter two values consist of the mean body size and gnathopod size of all three experimental males in the arenas. We did not size-correct male gnathopod size because there was no evidence of collinearity problems in the regression analyses. For female survival, we used a logistic multiple regression to account for the binary nature of these data (n = 71). For total time spent paired, we used a linear multiple regression and only included cases where females survived to the end of the experiment (n = 47). Finally, we used a second set of regression models to ask whether relative male to female size (i.e., male size divided by female size) and male gnathopod size affected female survival and mate-guarding duration. All statistics were performed using SPSS v.20.
Results
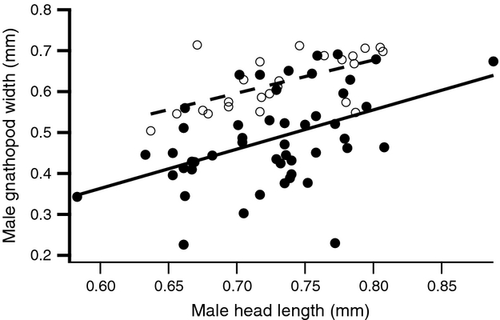
Males that had their gnathopods ablated and regrown had relatively smaller gnathopods than males that had their peraeopod ablated. We found no interaction between body size and the ablation group, which justified the use of the ANCOVA (F1,70 = 0.107, p = 0.744). For a given body size, males in the gnathopod-ablated group had gnathopods that were 31% smaller than males in the peraeopod-ablated group (F1,71 = 103.817, p < 0.001 Fig. 2). As expected, body size also explained a significant amount of variation in gnathopod size (F1,71 = 24.952, p < 0.001). This allowed us to increase the phenotypic space occupied by males in each size class.
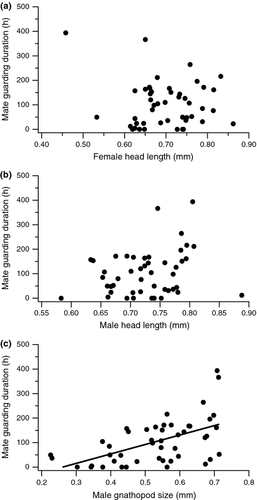
We found that mate-guarding duration was not affected by male body size or female body size, but it did increase with larger male gnathopod size (Table 1, Fig. 3). Specifically, our results suggest that a 0.1-mm increase in gnathopod size (measured as the width of the propodus) results in nearly a 40-h increase in mate-guarding duration. Similar results were obtained when we used the ratio of male:female body size instead of absolute size for each sex (Table 1). This result appears due to longer mate-guarding durations, rather than less discontinuous pairing, by males with large gnathopods (pair separations (mean ± SD): gnathopod-ablated males: 1.65 ± 1.05; peraeopod-ablated males: 1.67 ± 0.832). Inspection of residual plots showed that the assumptions of linear regression were reasonably met.
| Explanatory Variable | B | SE | t | p | Collinearity statistics | |
|---|---|---|---|---|---|---|
| Tolerance | VIF | |||||
| (a) Absolute body size | ||||||
| Male body size | −33.57 | 231.133 | −0.145 | 0.885 | 0.762 | 1.312 |
| Absolute gnathopod size | 393.404 | 100.813 | 3.902 | <0.001 | 0.778 | 1.285 |
| Female body size | −154.708 | 152.83 | −1.012 | 0.317 | 0.972 | 1.029 |
| (b) Male:female body size | ||||||
| Male:female body size | 80.233 | 68.72 | 1.168 | 0.249 | 0.937 | 1.067 |
| Absolute gnathopod size | 359.22 | 90.533 | 3.968 | <0.001 | ||
- For male traits, we analyzed means for the three males that were interacting with the female. Regression coefficients, t statistics, p-values, and collinearity statistics are presented. Collinearity statistics are only listed once in (b) because listing them twice is redundant. n = 47.
We found no evidence that neither male body size, male gnathopod size, nor female body size affected female survival in the experiment (Table 2). Female survival was 63% over the course of the experiment. Similar results were obtained when we used the ratio of male:female body size instead of absolute size for each sex (Table 2).
| Explanatory Variable | B | SE | Wald χ2 | df | p |
|---|---|---|---|---|---|
| (a) Absolute body size | |||||
| Male body size | 0.963 | 5.473 | 0.031 | 1 | 0.860 |
| Absolute gnathopod size | 0.151 | 2.382 | 0.004 | 1 | 0.950 |
| Female body size | 1.652 | 3.350 | 0.243 | 1 | 0.622 |
| (b) Male:female body size | |||||
| Male:female body size | −0.503 | 1.547 | 0.106 | 1 | 0.745 |
| Absolute gnathopod size | 0.544 | 2.127 | 0.065 | 1 | 0.798 |
- For male traits, we analyzed means for the three males that were interacting with the female. Regression coefficients, Wald chi-squared statistics, degrees of freedom, and p-values are presented. n = 71.
Discussion
In our search for sexually antagonistic traits in a Hyalella amphipod, we found that males with larger gnathopods impose longer mate-guarding durations on females. However, contrary to our predictions, male body size and female body size had no effect on mate-guarding duration. These results suggest that body size does not affect a male's ability to overcome female resistance to pairing or a female's ability to resist male pairing attempts. Thus, it appears that of the traits examined here, only the male gnathopod is a putative sexually antagonistic trait in this Hyalella amphipod species.
Our results suggest that sexually antagonistic selection may have contributed to the elaboration of the posterior gnathopod in male Hyalella. In the species studied here, males with larger posterior gnathopods have higher pairing success (Wellborn & Bartholf 2005). Moreover, under the extremely male-biased operational sex ratios observed in Hyalella, males that are able to overcome female resistance and begin mate guarding early in the female's molt cycle will have higher mating success given the low prevalence of takeovers of paired females in this group (i.e., unpaired males usurping a guarding male; Wellborn & Cothran 2007). The degree to which posterior gnathopods enhance male reproductive success, however, may vary across Hyalella species. The posterior gnathopod allometric relationship is steeper in the large ecomorph species used in this study compared with small ecomorph species (Cothran & Jeyasingh 2010). This pattern suggests that the payoffs of having large gnathopods as male amphipods grow may be greater in large ecomorph species than small ecomorph species (Bonduriansky 2007).
Species of amphipods that have evolved the small versus large ecomorphs have contrasting life histories that appear to be driven primarily by the kinds of predators they experience—small ecomorph species live with predatory fish, whereas large ecomorph species live in habitats with weak or no predation by fish (Wellborn 1994a; Wellborn et al. 2005). It is possible that the same aspects of ecology that have fostered the evolution of different body sizes and life-history strategies in these species are also responsible for shaping differences in the value of large gnathopods in males of varying sizes. Although we examined a species that is a large ecomorph, there could be valuable insights gained regarding how ecology intensifies or weakens the strength of sexual conflict by examining whether posterior gnathopods also act as a sexually antagonistic trait in small ecomorph species.
Surprisingly, we found no evidence that body size acts as a sexually antagonistic trait in this Hyalella species. Body size is generally thought to be a good predictor of the outcome of physical contests (Parker 1974; Thornhill & Alcock 1983). In some crustaceans, larger males are more likely to overcome female resistance to pairing compared with smaller males; however, this is not always the case (Jormalainen & Merilaita 1993, 1995; Jormalainen 1998). Male size may not be correlated with pairing duration for a variety of reasons including that males are not interested in guarding females for long periods, other male traits are the primary determinants of whether a male succeeds in pairing with females, or females possess ways of resisting males that ‘neutralize’ a male's ability to use his body size to some advantage. We have evidence that males do pair with females for long periods in this species, especially when female resistance is removed (Cothran 2008). In the current study, we found that gnathopod size is important in determining a male's ability to impose longer mate-guarding durations on females, so it is possible that this trait alone suffices in overcoming female resistance to pairing. Finally, females have behavioral responses that appear to be effective at preventing pairing very early in their molt cycle (e.g., thrashing and tightly curling their body) that may provide adequate resistance regardless of a male's body size (Cothran 2008; RDC personal observation).
Although the male gnathopod is the only sexually antagonistic trait identified of those investigated in this study, these findings do not preclude the possibility of additional sexually antagonistic traits in females or males in this species. In particular, behavioral traits intended to dislodge or prevent grasping of the male, such as kicking or flexing of the female's body, have been proposed as a trait providing female resistance to male mate-guarding attempts (Jormalainen 1998) including Hyalella amphipods (Wellborn & Cothran 2007). For instance, when female resistance was experimentally reduced by sedation in the same species used in this study, both male grasping (a behavior that precedes mate guarding) and mate-guarding duration increased (Cothran 2008), suggesting that female resistance behavior is important for resolution of sexual conflict in the females’ favor. Other potential behavioral counteradaptations such as female aggregation or avoidance of males (Arnqvist & Rowe 2005) were prevented from being expressed in our experimental design, but they may be important in this system. In fact, the mean mate-guarding duration in this study (6.42 ± 2.6 d) is higher than the estimated mate-guarding duration for this species in nature (3 to 5 d; Wellborn & Cothran 2007). This suggests that the conflict was skewed toward the male optimum in this study and that additional female strategies for resisting long mate-guarding durations may have been prevented. However, as these species exist in natural environments at high densities (700 to 8400 individuals/m2, Wellborn 1994a) where intersexual interactions are frequent, female avoidance of males as a resistance strategy is unlikely. Alternatively, the perceived costs of lengthy precopula, which may depend on predators in the environment (Cothran 2004), may have been low in our experiment resulting in longer mate-guarding durations. However, we still found an effect of male posterior gnathopod size on mate-guarding duration, suggesting that males and females were still in conflict in our study. Further research in this system should explore the relative importance of these and other potential male and female sexually antagonistic traits to fully characterize their role in resolving sexual conflict in this species.
As frequently noted, and recently experimentally validated in Drosophila melanogaster (Arbuthnott et al. 2014), sexually antagonistic trait evolution and development does not occur in an ecological vacuum, but rather these traits evolve within an environmental context. In fact, some studies have found no conflict over precopulatory mate-guarding duration or potential benefits associated with guarding, which is likely contingent on the ecological context in which mating takes place (Galipaud et al. 2011; Benvenuto & Weeks 2012; Cothran et al. 2012a).
For the Hyalella species used in this study, posterior gnathopods are sensitive to the availability of phosphorus in the diet (Cothran et al. 2012b). Phosphorus availability varies widely in freshwater environments (Wetzel 2001), and thus, the strength of sexual conflict may be dependent on the nutrient profile of the habitat in which sexual conflict is occurring (Cothran et al. 2012b). Our gnathopod manipulations artificially increased the phenotypic space occupied by males, perhaps producing males that would be found in environments of low-to-intermediate phosphorus availability (i.e., we produced males with smaller gnathopods than expected based on body size). Under this scenario, mate guarding may be kept to relatively short durations and females may avoid the costs of lengthy precopulatory mate guarding (Jormalainen 1998). On the other hand, the increasing nutrient levels of many freshwater environments due to cultural eutrophication may intensify sexual conflict—male posterior gnathopods may become larger relative to body size. Under this scenario, our results suggest that mate-guarding durations may increase and that females will be exposed to the costs associated with precopulatory mate guarding for longer periods. Such an escalation in the conflict may result in counteradaptations in females. Having robustly identified posterior gnathopod size as a resource-dependent, sexually antagonistic trait allowing males to increase precopulatory mate-guarding duration, future research designed to explore the interactive effects of sexual conflict and ecological context on trait evolution, phenotypic divergence, and speciation will elucidate the complex mechanisms involved in the evolution of biological diversity.
Acknowledgements
We would like to thank Tom Harper for taking the scanning electron micrograph of the male posterior gnathopod.




