Choosing a Mate in a Cocktail Party-Like Situation: The Effect of Call Complexity and Call Timing between Two Rival Males on Female Mating Preferences in the Túngara Frog Physalaemus pustulosus
Abstract
Signal detection, recognition, and localization are hampered when multiple signalers coincide in time and space, a problem known as ‘cocktail party effect’. In many taxa, senders utter complex calls consisting of two or more elements which often vary in the ease with which they can be assessed in different signaling environments. Receivers’ selective attention to different cues may increase the probability of correctly assigning a signal to its source (localization) in face of conspecific interference. Túngara frogs, Physalaemus pustulosus, produce complex calls consisting of an initial whine, followed by zero up to seven broad-banded, amplitude-modulated chucks. Under ideal conditions (without interference or noise), females prefer whines followed by chucks over whines alone, but the preference is not linear; females do not discriminate between whines with one or two chucks. When whines lack chucks, call overlap elicits random responses in females, with no preference for leading calls. In this study, I explored the combined effect of call timing and call complexity on female preferences in a two-choice paradigm—a simplification of the cocktail party scenario. I tested the hypothesis that the effect of call overlap can be reduced when the calls of one of the two rivals have chucks, specifically more chucks than those of the rival. I gave females a choice between whines alone and with chucks (one or two) presented at three time relations (alternated, abutted, and partially overlapped) and two emission orders (whine with less chucks leading and whine with more chucks leading). I found that the preference for one chuck over no chuck was preserved in all the experimental treatments, but when a w + 2chk preceded a w + chk, either overlapped or abutted, a preference existed for the whine with more chucks. Therefore, an interaction between call order and the number of chucks was obtained. The results only partially supported the hypothesis, and call order emerges as an opportunistic component of signaling in P. pustulosus.
Introduction
In many taxa, from insects to mammals, acoustic communication during reproduction occurs in aggregations, single or multispecies, often called choruses, in which the signals of the multiple senders mask and interfere with each other (Klump 1996; Aubin & Jouventin 1998; Hulse 2001; Gerhardt & Huber 2002; Brumm & Slabbekoorn 2005; Greenfield 2005; Langemann & Klump 2005). Acoustic interference and masking hamper the detection, localization, recognition, and discrimination of particular signals and increase the chances of error during communication (Schwartz 1987; Gerhardt & Klump 1988; Wollerman & Wiley 2002). The problem of acoustic interference is particularly important among conspecific signals because of their similarities in amplitude and spectral and temporal characteristics. Consequently, for communally displaying reproductive acoustic animals (Greenfield 2005), finding a mate or effectively competing with a particular rival can pose a difficult task comparable to the human ‘cocktail party problem’ (Cherry 1953). This problem can be solved if animals are capable of segregating sound sources, which involves perceptually separating out behaviorally relevant acoustic signals from the numerous sound sources in the environment (Hulse 2001). Sound segregation (or on the contrary, sound integration) can be achieved by exploiting spectral, temporal, and spatial features of the multiple sound sources in the environment (reviews in Bronkhorst 2000; Bee & Micheyl 2008). Primitive cues (i.e., common to all sounds) for auditory sound integration are as follows: spatial location, temporal cues, spectral cues, and amplitude modulation (Yost & Sheft 1993; Bee & Micheyl 2008). Higher order cues include context-dependent ones such as syntax (specific sequences of the signal components).
The repetitive and multielement calls (complex calls) found in many chorusing frog species have been interpreted as adaptive solutions for communication in chorusing cocktail party environments (Gerhardt & Huber 2002; Candolin 2003; Hebets & Papaj 2005). Different call components often vary in the ease with which they can be assessed at different distances, at different stages of mate attraction, or in different habitats or signaling environments (Richards 1981). Therefore, adjusting the attention paid to different signal cues according to the acoustic landscape may facilitate female choice or reduce errors in cocktail party environments (Candolin 2003; Hebets & Papaj 2005; Wong & Candolin 2005; Akre & Ryan 2010), either by enhancing the detection of message-containing signal components (Richards 1981), the localization or the attractiveness of particular calls. Localization cues are signal components which increase the probability of assigning a signal to its correct source. In humans, and other terrestrial animals, broadband sounds are more accurately located than those with narrowbands as a result of the ability to compare interaural time and intensity differences across multiple frequency channels (reviewed in Warren 1999; see also Konishi 1973 for differences between species). In frogs, insects, and small birds, interaural distance is too small for time and intensity differences to be accurate. In these taxa, sound frequency, duration, repetition, and amplitude modulation play an important role in signal localization (e.g., Wells & Schwartz 1984; Jørgensen & Gerhardt 1991; Michelsen & Löhe 1995; Ronacher et al. 2000). It has also been suggested, but yet poorly tested in frogs, that complex call characters increase signal detectability or locatability (Wells & Schwartz 1984; Pallett & Passmore 1988; Wells 1988; Farris et al. 2002; Gerhardt & Bee 2007). Thus, the same properties that enhance a signal's relative attractiveness to females might also make them easier to localize in a complex chorus. Furthermore, females may be more likely to recognize and respond to complex signals if they are more, rather than less, attractive. A plethora of studies indicate that within the acoustically complex choruses, male frogs do successfully compete vocally with other males, and females do make adaptive mate choice decisions based on males′ calls (reviews in Andersson 1994; Gerhardt & Huber 2002; Gerhardt & Bee 2007; Wells & Schwartz 2007). Thus, both males and females are capable of solving the cocktail party problem.
Besides the problem of locating a male in a chorus, once located, females are challenged to discriminate the differences between the overlapping calls of neighboring males when making adaptive choices. The effect of call timing on female preferences has been analyzed in a number of species (Gerhardt & Huber 2002), and the general result is that preferences based on intrinsic temporal and spectral traits of the calls are reversed or abolished (random response) when calls overlap or abut, typically giving an advantage to the preceding call in the sequence regardless of its characteristics. This behavioral phenomenon, called ‘precedence effect’, may result from the relative importance of the initial transient in signal localization (Zurek 1987) or from suppression of further stimulation by the first stimulus perceived, either peripheral or central (Greenfield et al. 1997). Therefore, the timing of calls is an important component of female choice and male competition in many anurans (Wells & Schwartz 2007). The cues that females use to assign a call to its actual source (localization) and the effect of precedence might interact when females try choose one mate among two or more signaling males.
In this study, I evaluate this interaction in the túngara frog, Physalaemus pustulosus, a chorusing species whose call consists of two elements, the introductory frequency-modulated whine (ca. 300 ms, 900 Hz) and the final chuck (Fig. 1). The chuck is short (ca. 35 ms), amplitude-modulated, non-modulated in frequency, broad-banded, and contains up to 14 harmonics of the fundamental frequency (ca. 215 Hz). The whine and the chuck stimulate different auditory organs in the inner ear (Rand et al. 1992), the amphibian papilla and basilar papilla, respectively. The whine is always present and is necessary and sufficient for female choice and male competition (Ryan 1985); the chuck alone does not elicit female phonotaxis, and it is an opportunistic element that males append at the end of the whine when other males (or females) are present at the chorus; solitary males usually do not utter chucks (see Ryan 1985; Bernal et al. 2009; Akre & Ryan 2010). The attractiveness of the whine is modified by the presence of chucks, with females preferring whines followed by and also those followed by more chucks among the available, although the preference is not linear (Bernal et al. 2009). Specifically, under typical ideal experimental conditions (i.e., without conspecific interference), females prefer one, two, or more chucks over no chuck but do not discriminate between one and two chucks. Therefore, for decades, the role of multichuck whines puzzled researchers (but see recent findings in, Akre & Ryan 2010, 2011; Akre et al. 2011).
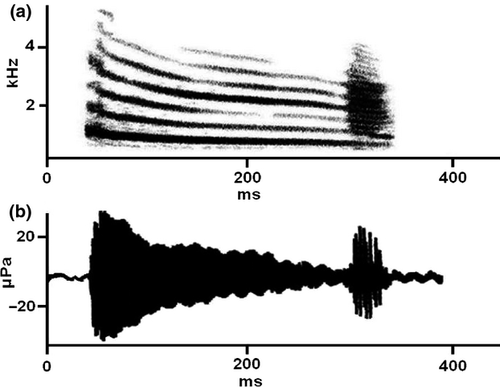
The spectral (broadband, high frequency) and temporal characteristics (short duration, amplitude modulation) of the chuck have led to the hypothesis that it functions as a localization cue (Kime 2001; Farris et al. 2002). Farris et al. (2002) actually demonstrated that spatially separated whines and chucks could be assigned to a same source (auditory grouping) and concluded that the whine and the chuck served different functions which they called ‘what’ (recognition) and ‘where’ (location). In addition, the results of the studies of Wilczynski et al. (1999) and Farris et al. (2005) demonstrated that females are permissive with regard to the natural sequence whine + chuck with the exception of that in which the chuck begins 50 ms after the start of the whine, as this portion is critical to the whine's recognition (Wilczynski et al. 1995).
In this study, I explored the combined effect of call timing and call complexity on female preferences in a two-choice paradigm (only two rivals)—a simplification of the cocktail party scenario. In a previous study, Schwartz & Rand (1991) found that within a pair of identical partially overlapped whines (from 0 to 100 ms onset delay between them), female responses were random, with no preference for the leading whine (i.e., lack of precedence effect). In this study, I tested the hypothesis that the randomizing effect of call overlap can be reduced when the calls of one of the two rivals have chucks, specifically more chucks than those of the rival. I further explored the ideas that the value of multichuck calls vary with the acoustics context (e.g., Akre & Ryan 2010, 2011; Akre et al. 2011). I evaluated whether a two-chuck whine, otherwise as attractive as one-chuck whine, can be chosen when overlapped with a one-chuck whine, and whether the preference for one chuck over no chuck is preserved.
Methods
Study Site and Subject
The study was conducted at Fundo Pecuario Masaguaral (8°34′N, 67°35′W), a cattle ranch located in the central llanos (lowlands) of Venezuela, 50 km south of the town of Calabozo in Guárico State from June to August in 2011–2012. The study site is a seasonal savanna that floods during the rainy season which lasts from mid-May to October. The breeding season of Physalaemus pustulosus coincides with the rainy season and peaks from June to August (pers. observ.).
General Procedure
I collected amplectant pairs of P. pustulosus from choruses between 1900 and 2100 h. Amplexed females are sexually receptive and ready to reproduce. Oviposition usually occurs a few hours after amplexus is achieved; thus, all females had made a choice the same night they were found (pers. observ.). Captured females were separated from males to prevent continued stimulation of oviposition by amplexus; each female was kept in a numbered plastic bag provided with a little water. Males were saved to allow pairs to resume amplexus after the tests and allow oviposition. All pairs resumed amplexus and constructed the foam nest in a plastic bucket. Males and females were released at the site of capture in the next evening. Males and females were placed in a light-safe Styrofoam box and transported to the testing room. Phonotaxis experiments were carried out indoors, between 2200 and 0200 h; thus, most females were tested a few hours after capture. I allowed females an acclimatization period of 30 min to the conditions of the testing room, during which time I broadcast a recording of a natural conspecific chorus.
Female Choice Experiments
I performed phonotaxis experiments following the procedure described in Tárano & Ryan (2002). Briefly, the experimental arena was 2 m in diameter and limited by mattress foam 1.5 cm thick and 80 cm high, supported by a semi-rigid wood frame. I performed the experiments under dim light provided by a 25-W light bulb above the center of the arena. Light intensity was fixed at the minimum necessary to see female movement (≤0.05 μmol/m2/s at the female release point, radiometer LICOR photometer LI-250-S). I placed two speakers (JBL Control Series and flat response 20 Hz–20 kHz) at opposite sides of the arena, facing the center. The speakers were covered by a tissue sheet of the same color of the walls of the arena so that no visual cues were available to females. I broadcast the stimuli from a Toshiba laptop. I set sound intensity at the center of the arena at 85 ± 1 dB SPL (re 20 μPa) with a sound pressure level meter (Radio Shack Digital Meter, flat weighing, fast response). An intensity of 85 dB SPL corresponds to the average intensity of a conspecific call measured at 50 cm of a male (Ryan 1985). The floor of the arena was of clear plastic sheet on which a grid was drawn to allow a precise recording of female movements.
I placed a female at the center of the arena under a plastic funnel that could be lifted by pulling a string. After 3 min of stimulation, I lifted the cone and the female was free to move within the arena. The movements of the female were observed from outside the arena and recorded in sheets. The observer scored a response when the female touched a speaker or remained within a radius of 5 cm around it, provided it had not reached it by following the walls of the arena. A no response was scored when the female remained immobile at the center of the arena for 5 min after raising the funnel, stopped at any site of the arena for more than 5 min, or hopped around the arena without reaching a speaker within 20 min. Thus, each trial lasted 20 min maximum. This time was used typically in phonotaxis experiments with P. pustulosus (Rand et al. 1992; Wilczynski et al. 1999; Kime 2001; Bernal et al. 2009).
Each experimental session consisted of controls and treatment trials arranged as follows: control–trial–trial–control. The maximum number of treatment trials for a female which responded throughout the experimental session was eight. Controls and trials consisted of contrasts between two stimuli (two-choice trials). In controls, I exposed each female to a typical natural conspecific call and to a white noise (considered a biologically neutral stimulus) of the same duration and contour (shape) of the call. The amplitude envelope of the noise was adjusted visually to the envelope of the natural call with which it was tested using a tool in SoundEdit16 (Manugistics Inc.). I used controls to test female responsiveness before and during the experimental session. Females that did not respond to the control preceding a treatment trial were not used. Positive controls (controls in which female chose the conspecific call) also ensured that females were not behaving randomly during the treatment trials. In treatment trials, I exposed females to a conspecific whine with (one or two) or without chucks arranged in different time relations (explained below). I alternated the speaker from which a particular stimulus was broadcast between trials to control for side biases. The time interval between successive trials was that required to place the female under the funnel again and begin to broadcast a different pair of stimuli and was usually approximately 2 min. This resting period is common in experiments with P. pustulosus (Rand et al. 1992; Wilczynski et al. 1999; Kime 2001; Bernal et al. 2009). The experimenter was aware to place the female with her snout perpendicular to the speakers because the direction of the first move was recorded; this caution was necessary not to bias this behavioral variable. The sequence of trials on the session was randomized for each female (lottery method) to control for any order effect. Each female was used in several treatment trials, but never more than once in the same trial, thus avoiding pseudoreplication (McGregor 2000). The sample size for each trial corresponds to the number of different females that responded on it.
Preparation of Stimuli
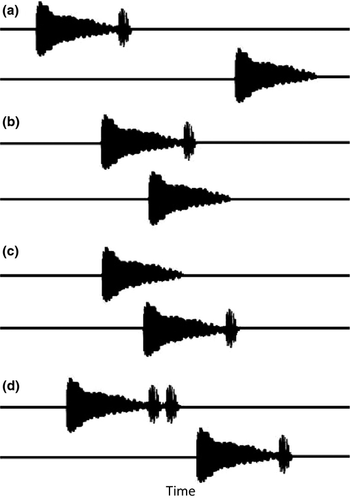
I prepared the stimuli with Raven Pro 1.4 (Cornel Lab Ornithology). Each sound of a treatment trial was placed on a separate track on a sound file, either perfectly alternated (phase opposed), overlapped (50%), or abutting (Fig. 2). Thus, in controls, one speaker broadcast a natural call and the other broadcast the white noise, and in experimental trials, for instance, a whine alone and the other a whine + chuck (w + chk). Each sound was repeated at 1.5-s intervals, close to the typical intercall interval of the species (Ryan 1985). The combinations tested (number of chucks, call order, and timing) are shown in Table 1 (each combination of stimuli in relation to chuck number is called a trial type, that is, whine vs. w + chk or w + chk vs. w + 2chk). I used natural whines and chucks to prepare the stimuli following the procedures described in Bernal et al. (2009) and Tárano & Fuenmayor (2013). The whines contrasted on each trial were identical (as in Schwartz & Rand 1991) but differed in the number of chucks; different whines were used with different females and on the replicates of each treatment trial (Gerhardt 1995; McGregor 2000). I had an acoustic library of at least 100 calls from the study population to choose the experimental stimuli. The selection was based on several criteria, but basically, the calls used were as similar as possible in gross spectral and temporal characters. Each call was digitally modified to append the desired number of chucks (zero, one, or two), by removing or duplicating the original chuck of the call (Bernal et al. 2009).
| Trial Type | Emission phase | Stimulus order | |
|---|---|---|---|
| Leading | Following | ||
| W + CHK vs. W | Alternated | ||
| Overlapped | W + CHK | W | |
| W | W + CHK | ||
| W + 2CHK vs. W+CHK | Alternated | ||
| Overlapped | W + 2CHK | W + CHK | |
| W + CHK | W + 2CHK | ||
| Abut | W + 2CHK | W + CHK | |
| W + CHK | W + 2CHK | ||
Variables and Statistical Analyses
I used several measures to analyze female preferences: the number of females choosing each stimulus, the response time (time elapsed since the funnel was lifted until the female made a choice), the orientation of the first jump in a categorical scale (+1: toward the side of the final choice, −1: opposite to the side of the final choice, or 0: perpendicular to either side), and the number of approaches or visits (within 10–20 cm) to each speaker before making a choice. The number of females choosing each stimulus in a trial was compared within trials (i.e., for each trial separately). The response time was compared across trials of a type, while the orientation of the first jump and the number of approaches to each speaker were compared both within trials and across trials of a type.
I used a Bayesian analysis to evaluate population-level preferences (through the number of females choosing each stimulus) in each trial because the power of the binomial two-tailed test, typically used in this type of studies, is low for small sample sizes and highly sensitive to variations in sample size. The binomial probability was also calculated for comparison. In addition, the Bayesian analysis allowed me to compare several alternative hypotheses (defined beforehand), rather than to simply reject a single null hypothesis (Quinn & Keough 2002; see examples in frogs in Gerhardt 1992; Wilczynski et al. 1999; Kime 2001). Here, I used three possible theoretical choice distributions following Kime (2001): a preference for stimulus X of 75% (P1 = 0.75), no preference for either stimulus (P2 = 0.5), and a preference for stimulus Y of 75% (thus, a preference for X equal to P3 = 0.25). Wilczynski et al. (1999) indicated that 75% choices for one speaker represent the standard difference threshold (just noticeable difference) in two-choice psychophysical discrimination tasks; therefore, it can be used as reference probability in Bayesian analysis of female preferences. In addition, each pre-defined alternative hypothesis was assigned the same theoretical (expected) prior probability of 0.333. The results of the Bayesian analysis are the posterior probabilities that an observed choice distribution actually represents each of the theoretical distributions. These probabilities were calculated using the Bayes’ theorem (Quinn & Keough 2002). Female mating preferences (or lack of preferences) were defined as the theoretical choice distribution with the highest posterior probability. Preferences (or lack of preferences) were considered marginal when the posterior probabilities of two or more theoretical choice distributions differed by <0.2. This convention has been used by other authors (Wilczynski et al. 1999; Kime 2001).
The response time and the number of approaches to each speaker before making a choice reflect the number of repetitions of the stimuli necessary to make a decision, or the motivation of the female. I expect that the harder the choice task (ordered by increasing difficulty: alternated, abutting, and overlapping), the more the approaches to either sound source before making a choice and/or the longer the time to choose a sound. The response time among trials of a type was compared with a Friedman ANOVA, with females as blocks. As the same females were not always involved in all the trials to be compared, I excluded the data from females which were not used in all of them. The direction of the first jump or crawl and the number of approaches to each sound before making a choice were compared within trials with a multinomial test (first jump: p (0) = 0.5; p (−1) = p (+1) = 0.25; number of approaches: p (1) >p (2) >p (≥3)) and between trials of a type with a chi-square test.
Ethical Note
The study adhered to the laws of animal use in biological research of Venezuela and had the legal permission given by the former Oficina de Biodiversidad del Ministerio de los Recursos Naturales (scientific license MARN Memo No. 01-11.0764). The proposal was also revised by the government agency, Fondo Nacional para Ciencia y Tecnología (FONACIT), which certified that it was ethically correct. I certify that no female died or suffered any visible effect during the 24 h of restrain. Each female was allowed to resume amplexus with the male of her original choice, and all the females successfully oviposited in the laboratory. Foam nests were kept in the laboratory until the froglets emerged (usually within 3 days after fertilization) and thereafter released at the site of capture provided it had water. Each female was released at the site of capture after oviposition.
Results
I tested 54 females in total and at least 19 were tested in each trial. As previously demonstrated by other authors, when calls were alternated, females preferred the w + chk over the whine alone but did not discriminate between the w + 2chk and the w + chk (Table 2, Bayesian probability 0.976). When the whine and the w + chk were overlapped, females preferred the w + chk over the whine alone regardless of the order of presentation, but the preference was stronger when the w + chk was the leading call (Table 2, Bayesian probabilies 0.992 and 0.695, respectively). In the w + chk vs. w + 2chk trials with abutting and overlapped calls, the females preferred the w + 2chk only when it was the leading stimulus (Table 2, Bayesian probabilies 1 and 0.979, respectively); when the order of emission was reversed, the response was random (Table 2). These results indicate that the number of chucks interact with call order irrespective of whether the calls actually interfere and that there is not a precedence effect.
| Stimulus Emission | Stimuli | Choice | Bayesian Posterior Probability | P Binomial | Response Time (s) | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | Prefer A | No Preference | Prefer B | |||
| ALTERNATED | W + CHK | W | 16 | 4 | 0.976 | 0.024 | 0.000 | 0.003 | 147.4 ± 161.4 |
| OVERLAPPED | W + CHKa | W | 17 | 3 | 0.992 | 0.008 | 0.000 | 0.000 | 158.2 ± 160.7 |
| W + CHK | Wa | 14 | 7 | 0.695 | 0.305 | 0.000 | 0.08 | 164 ± 195 | |
| ALTERNATED | W + 2CHK | W + CHK | 11 | 11 | 0.039 | 0.922 | 0.039 | ns | 145.1 ± 142.3 |
| OVERLAPPED | W + 2CHKa | W + CHK | 18 | 5 | 0.979 | 0.021 | 0.000 | 0.003 | 155.3 ± 134.3 |
| W + 2CHK | W + CHKa | 10 | 14 | 0.003 | 0.776 | 0.221 | ns | 163.8 ± 176.3 | |
| ABUT | W + 2CHKa | W + CHK | 23 | 1 | 1.000 | 0.000 | 0.000 | 0.000 | 122.5 ± 94.6 |
| W + 2CHK | W + CHKa | 11 | 12 | 0.019 | 0.922 | 0.058 | ns | 170 ± 167.4 | |
- Bayesian posterior probabilities are shown for three alternative preference hypotheses. Posterior probabilities above 0.6 are boldfaced. Posterior probabilities below 0.2 are arbitrarily considered as marginal. Prefer A: 0.75. No preference: 0.50. Prefer B: 0.75 (Prefer A: 0.25). The theoretical a priori probability for each was 0.3333. Binomial probabilities are shown only for reference. Fisher's probabilities correspond to comparisons between the alternated trial and the other trial. Response time: mean ± SD.
- a The leading call when the stimuli were abutted or overlapped.
Within the trials, there was a significant tendency to perform the first move toward the side with the speaker a female eventually chose (Table 3, multinomial test), and a significant proportion of females (>60%) made a choice in their first approach (Table 3, multinomial test). These results indicate that most females had already made a decision when they began to move. In the whine vs. w + chk trials (all the timings), most females did not visit the non-choice speaker (Table 3, multinomial test, p < 0.01 in all tests), while in the w + chk vs. w + 2chk trials (all the timings), all females visited the non-choice speaker at least once, although repeated visits were rare (Table 3, multinomial test). Notice that the probability that a female visits both sound sources before making a choice is higher when both whines have chucks (approx. 75–90% of females approached the unpreferred sound once) than when only one whine has a chuck (most females never approached the unpreferred sound), but did not vary with the timing of these calls.
| Stimulus Emission | Stimuli | Direction of first jump | Multinomial | Approaches to the chosen speaker | Multinomial | Approaches to the rejected speaker | Multinomial | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | 1 | 0 | 1 | 1 | 2 | ≥3 | 0 | 1 | 2 | 3 | ||||
| ALTERNATED | W + CHK | W | 11 | 5 | 4 | 0.0006 | 13 | 5 | 2 | 0.019 | 14 | 4 | 1 | 1 | 0.004 |
| OVERLAPPED | W + CHKa | W | 10 | 7 | 4 | 0.0007 | 14 | 2 | 4 | 0.005 | 17 | 2 | 0 | 1 | 0.0002 |
| W + CHK | Wa | 11 | 7 | 2 | 0.0034 | 14 | 5 | 2 | 0.014 | 20 | 1 | 0 | 0 | 0.00001 | |
| ALTERNATED | W + 2CHK | W + CHK | 12 | 5 | 5 | 0.0001 | 16 | 5 | 1 | 0.003 | 0 | 20 | 1 | 1 | 0.00000 |
| OVERLAPPED | W + 2CHKa | W + CHK | 13 | 3 | 7 | 0.0000 | 13 | 3 | 7 | 0.006 | 0 | 20 | 3 | 0 | 0.00000 |
| W + 2CHK | W + CHKa | 13 | 6 | 5 | 0.0003 | 13 | 6 | 5 | 0.021 | 0 | 22 | 1 | 1 | 0.00000 | |
| ABUT | W + 2CHKa | W + CHK | 16 | 2 | 5 | 0.0000 | 16 | 6 | 2 | 0.009 | 0 | 18 | 4 | 2 | 0.00000 |
| W + 2CHK | W + CHKa | 14 | 5 | 5 | 0.0001 | 16 | 4 | 3 | 0.008 | 0 | 21 | 1 | 1 | 0.00000 | |
- Figures indicate the number of females performing each behavior. The multinomial random probabilities for the direction of the first move are p (+1) = p (−1) = 0.25, p (0) = 0.5. The multinomial random probabilities for the number of approaches to the chosen speaker are p (1) = 0.5, p (2) = 0.30, p (≥3) = 0.20. The multinomial random probabilities for the number of approaches to the rejected speaker are p (0) = 0.5, p (1) = 0.25, p (2) = 0.15, p (≥3) = 0.10. The results do not change when the multinomial probabilities are identical: p (0) = p (1) = p (2) = p (≥3).
- a The leading call when the stimuli were abutted or overlapped.
Among trials of a type, there was no significant difference in response time (Table 2, Friedman ANOVA, w vs. w + chk: H(19,2) = 0.76, p = ns; w + chk vs. w + 2chk: H (19,4) = 0.32, p = ns), in the direction of the first move (w vs. w + chk: χ2 = 1.26, df = 4, p = 0.87; w + chk vs. w + 2chk, χ2 = 3.45, df = 8, p = 0.75), in the number of visits to the choice speaker (w vs. w + chk: χ2 = 3.83, df = 6, p = 0.70; w + chk vs. w + 2chk, χ2 = 6.74, df = 12, p = 0.66), or in the number of visits to the non-choice speaker (w vs. w + chk: χ2 = 5.95, df = 6, p = 0.43; w + chk vs. w + 2chk, χ2 = 5.34, df = 12, p = 1). Altogether, these results suggest that the difficulty of the task was not associated with the behavior of the females during the choice.
Discussion
Acoustical complexity, specifically the production of spectrally and temporally different signal components, is an adaptive solution to the communication problems arising in cocktail party-like conditions (Gerhardt & Huber 2002; Candolin 2003; Hebets & Papaj 2005). Signal components with different salience under different conditions may help receivers segregate sounds produced by different senders (object formation), thus facilitating localization and discrimination of signals (Richards 1981). In the present study, the hypothesis of the chuck being a salient cue which favors segregation of sound sources when signals interference was partially supported by the results. While in the absence of chucks, the response to overlapping whines is random (Schwartz & Rand 1991), the addition of one chuck to a whine confers an advantage over whines without a chuck. There is also an advantage of adding multiple chucks (i.e., two chucks over one chuck), but only when the multichuck whine precedes the less complex one, an advantage that does not occur when the whines with one and two chucks are uttered antiphonally. The results of the present study add to our understanding on the production of multichuck whines because there is ample evidence that females only discriminate between two-chuck and one-chuck whines under certain conditions (see Rand et al. 1992; Bernal et al. 2009; Akre & Ryan 2010, 2011; Goutte et al. 2010). The results also show that the interaction between call timing and complexity affects female decisions in the túngara frog, a behavior not previously demonstrated in this species.
At the proximate level, two mechanisms could be responsible for the preferences observed for two-chuck leading whines: peripheral masking and higher order selective attention. In the present study, the chucks of leading overlapping calls always overlapped with the second half of the following whine (Fig. 2). This half is the lowest in amplitude in the whine and also lower in amplitude than the chuck; therefore, it is always masked by the overlapping chuck. Wilczynski et al. (1999) postulated that the stimulation of the basilar papilla (the organ tuned to the chuck) by this higher amplitude sound would interfere with the stimulation of the amphibian papilla (the organ tuned to the whine) by the lower amplitude whine. In this case, when a leading call has chucks (one or two), the perception of the last half of the following whine would be impaired, and the female should still prefer the more complex leading call. When the w + chk led and overlapped with the whine alone, the preference for the former was preserved but reduced with regard to that when the calls were alternated. Therefore, in this situation, a fraction of the population seems to group the chuck into the following whine instead of into the original preceding whine, contrary to expectation if peripheral masking were the only mechanism involved in call discrimination and/or location.
Selective attention, a higher order perceptual mechanism, might also explain the results. Kahneman & Triesman (1984) proposed that the perceptual systems of many vertebrates have a limited capacity to process streams of information and that this capacity is not allocated equally among perceptual categories. Once the critical portion of a sound is detected, in this case the initial portion of the whine (Wilczynski et al. 1995), attention is focused on its processing to the detriment of other signals. Therefore, when a w + chk and a whine alone (at either order) overlap, attention is selectively focused on the ongoing whine until the critical portion of another is perceived, interfering with the complete processing of the former. If the first whine has a chuck, a probability exists that it is grouped into the second whine (as Farris et al. 2002 postulated), while this would not happen when the first whine lacks a chuck (in this case, the chuck would be always grouped into the following whine). Therefore, in the latter case, the chuck preserves its capacity to enhance the attractiveness of the whine but not necessarily with the same strength when the order of the calls is reversed. According to the hypothesis of Kahneman & Triesman (1984), when the two call components do not overlap, as when the chuck is either at the beginning or at the end of the whine, attention competition would not occur and both call components can be assessed by the frog's call analysis system independently. However, for whines followed by two chucks, neither peripheral masking (Wilczynski et al. 1999) nor selective attention (Kahneman & Triesman 1984) satisfactorily explains the results, because the addition of chucks in a following call would increase the probability that the last chuck in the sequence be perceived without interference. This would lead to preferences for following w + 2chk over preceding w + chk as well as for following w + chk over preceding whine alone, that is, a preference for following calls whose chucks are perceived without interference.
Regardless of the underlying proximal mechanism, the results of the present study show that interference has a partially deleterious effect for males producing only one chuck in a chorus where other males utter either whine alone calls or whines with two chucks. Thus, I propose that males who utter one-chuck calls are expected to time their calls with those of neighbors in ways that avoid interference, either by perfectly alternating their calls with those of other males or by uttering leading abutting calls (see Höbel 2011). On the other hand, males producing two-chuck calls are expected to time their calls in anticipation of those of neighboring males (either abutted or partially overlapped). Thus, call timing would be another form of male competition in this species (Greenfield et al. 1997; Wells & Schwartz 2007) besides the addition of chucks (Ryan & Rand 1998; Bosch et al. 2000, Bernal et al. 2009). Call timing interactions are feasible, regardless of the size of the chorus, because P. pustulosus males pay selective attention only two or three males among all those present in the chorus (see Greenfield & Rand 2000). Call timing interactions have the potential of being less costly than those based in the addition of chucks. In a recent study, Goutte et al. (2010) found that while a male's calling strategy correlates with that of the opponent, the number of chucks added to the whine is, on average, low (1.35–1.53), suggesting that this is a cost-sensitive strategy. Then, call timing adjustments may confer a mating advantage without increasing the cost (in terms of energy consumption or predation risk) of the call.
Female túngara frogs approach several males in a chorus before making a choice (Ryan 1985). Close inspection of males assists females’ mating decisions because multiple signaling cues can be incorporated or assessed at different stages of choice. In the present study, female choice was not typically related to an increase in the evaluation of the subsequently preferred sound because in any trial type, most females chose a call after only one visit. However, in trials involving two-chuck whines, nearly all females approached the rejected sound once. Therefore, the discrimination of whines with more than one chuck requires a close inspection of both sounds. In natural choruses, male competition may affect the choice process at three stages: mate detection, mate evaluation, and actual mating (Wong & Candolin 2005), that should be considered on any future study, in which interactive playbacks should be preferred (i.e., sound sources whose output changes with female proximity). In several species, female approach elicits modifications in male vocal behavior (e.g., Tárano & Herrera 2003; Byrne 2008). Akre & Ryan (2011) also found that female túngara frogs exhibit a repertoire of movements when in close proximity to males which increase the probability that males add complexity to their calls. Interestingly, Akre & Ryan (2010) had found that when females are ‘close’ to males (a situation modeled through increasing call intensity), a stronger preference for more chucks occurs. The results of my study open the issue of whether female preferences for call timing might influence male vocal behavior at close range. The studies of Akre & Ryan (2010, 2011), Goutte et al. (2010), and my own, among others, are providing interesting answers to the old question of ‘Why males produce multichuck whines, as well as opening new lines for research.’
Further research should focus in determining call timing variation in natural choruses and in assessing whether this variation is selectively neutral, or under selection by female choice (see Höbel 2011). The interaction between signal preferences based on intrinsic call traits and relative timing can result in changes in the dynamics of sexual selection and in the maintenance of variation in signal traits (e.g., number of chucks), because males with unattractive signals could gain greater mating success by producing these signals in a leading position, thus weakening or even disrupting feature-based signal selection (Bosch & Márquez 2001, 2002; Höbel 2010, 2011). Mate choice in Alytes cisternasii is affected by call timing patterns: when the call order is random, females fail to choose the otherwise most attractive call, choosing the closest in time to the most attractive (Bosch & Boyero 2006); in consequence, random timing would be the best strategy for less attractive males, while call alternation would be the best for the most attractive ones (Höbel 2010, 2011). Overall, these results indicate that multiple male features interact in male signaling behavior as well as in female preferences (Wong & Candolin 2005; Taylor et al. 2008, 2011).
Acknowledgements
This research was funded by FONACIT S1-2002000276. The field work was conducted in accordance with local wildlife research laws (Scientific License granted to Z. Tárano, MARN Memo No. 01-11.0764). I am grateful to T. Blohm† and J.G. Acosta for their kind support during the many years of research in Masaguaral. G.E. Chacón provided logistic support during my field trips. R. De Nobrega provided assistance with the Bayesian analysis. The comments of M. J. Ryan and of two anonymous reviewers greatly improved the manuscript. I dedicate this study to the memory of Tachi and Lucca Tárano.




