Delays in the diagnosis and surgical treatment of drug-resistant epilepsy: A cohort study
Abstract
Objective
Delay in referral for epilepsy surgery of patients with drug-resistant epilepsy (DRE) is associated with decreased quality of life, worse surgical outcomes, and increased risk of sudden unexplained death in epilepsy (SUDEP). Understanding the potential causes of delays in referral and treatment is crucial for optimizing the referral and treatment process. We evaluated the treatment intervals, demographics, and clinical characteristics of patients referred for surgical evaluation at our level 4 epilepsy center in the U.S. Intermountain West.
Methods
We retrospectively reviewed the records of patients who underwent surgery for DRE between 2012 and 2022. Data collected included patient demographics, DRE diagnosis date, clinical characteristics, insurance status, distance from epilepsy center, date of surgical evaluation, surgical procedure, and intervals between different stages of evaluation.
Results
Within our cohort of 185 patients with epilepsy (99 female, 53.5%), the mean ± standard deviation (SD) age at surgery was 38.4 ± 11.9 years. In this cohort, 95.7% of patients had received definitive epilepsy surgery (most frequently neuromodulation procedures) and 4.3% had participated in phase 2 intracranial monitoring but had not yet received definitive surgery. The median (1st–3rd quartile) intervals observed were 10.1 (3.8–21.5) years from epilepsy diagnosis to DRE diagnosis, 16.7 (6.5–28.4) years from epilepsy diagnosis to surgery, and 1.4 (0.6–4.0) years from DRE diagnosis to surgery. We observed significantly shorter median times from epilepsy diagnosis to DRE diagnosis (p < .01) and epilepsy diagnosis to surgery (p < .05) in patients who traveled further for treatment. Patients with public health insurance had a significantly longer time from DRE diagnosis to surgery (p < .001).
Significance
Both shorter distance traveled to our epilepsy center and public health insurance were predictive of delays in diagnosis and treatment intervals. Timely referral of patients with DRE to specialized epilepsy centers for surgery evaluation is crucial, and identifying key factors that may delay referral is paramount to optimizing surgical outcomes.
Key points
- The longest treatment interval spanned the time from initial epilepsy diagnosis to diagnosis of drug-resistant epilepsy (DRE; median 10.5 years), which is influenced by referral practices.
- The median time to surgery following DRE diagnosis was 1.4 years, suggesting that earlier referral to epilepsy centers may facilitate timely surgical treatment.
- Patients with public health insurance are more likely than patients with private insurance to experience delays in receiving surgery for DRE (~3× longer).
- Traveling longer distances to our institution did not introduce delays; patients living further away experienced reduced timelines for DRE diagnosis and surgery.
1 INTRODUCTION
Epilepsy affects roughly 1% of the general population, both in the United States and globally. Among all patients with epilepsy, 20%–40% do not experience adequate seizure control with antiseizure medications alone. This subgroup, commonly referred to as patients with drug-resistant epilepsy (DRE), demonstrates the highest lifetime health care use rates.1 Because of the cost of increased emergency department use, recurrent hospitalizations, and required short-interval outpatient follow-up, patients with DRE account for nearly 80% of all health care costs attributed to epilepsy.2, 3 Despite high-level evidence supporting surgical intervention for DRE,4-6 delays in referral to level 4 epilepsy centers for neurosurgical evaluation remain a significant problem.7, 8 Recent examinations of DRE referral patterns demonstrate that racial, socioeconomic status, and geographic disparities impact rates of surgical evaluation and intervention in the United States.9, 10
Delays to surgical evaluation for DRE come with indelible risks. These delays have been associated with worsened quality of life, higher rates of psychological or mood disorders5 and a further decline in socioeconomic status because of disability or unemployment.9, 11, 12 Furthermore, a Canadian registry study by Burneo et al.13 examining patients with DRE from 2001 to 2010 reported that over 10% of patients died within 2 years of DRE diagnosis. Despite growing numbers of epilepsy centers and the continued innovation of surgical techniques and technologies, surgical intervention rates have seen only marginal increases over the past decade.14-16 Systematic reviews of referral patterns specifically highlight the role geography plays in time to surgical evaluation for DRE.9
Institutional evaluations of referral patterns in the United States primarily describe urban academic teaching centers, often located on the East Coast. However, there has been limited evaluation of DRE referral and surgical patterns in the Intermountain West (the geographic area in the United States generally comprising Colorado, Idaho, Montana, Nevada, New Mexico, Utah, and Wyoming). This area is uniquely predominated by rural communities, which may be at risk of identifiable delays to surgical evaluation. Therefore, this work aims to describe the surgical epilepsy experience and barriers to referral at a level 4 epilepsy center in the Intermountain West.
2 MATERIALS AND METHODS
2.1 Data collection and curation
We performed a retrospective review of all patients with epilepsy who underwent epilepsy surgery between March 2012 and May 2022 at our institution. Patients were included consecutively in an unbiased fashion. Individuals with epilepsy who were not surgical candidates (i.e., did not receive some form of epilepsy surgery or phase 2 intracranial monitoring) were not included in the cohort.
Demographic information (age, sex, and ethnicity), clinical characteristics (surgery performed, pre-operative seizure frequency, seizure frequency at 1-year post-operatively, seizure frequency at last follow-up, age at epilepsy diagnosis, and number of antiseizure medicines at last follow-up), health insurance status, and dates of events related to diagnosis or treatment (date of epilepsy diagnosis, date of DRE diagnosis, and date of epilepsy surgery) were obtained. The date of DRE diagnosis was determined by identifying the first mention of DRE in each patient's medical record. If complete information regarding a particular event was unavailable, we approximated the date; for example, if a chart indicated that the diagnosis of DRE was made in “March 2016,” we assigned the date “3/1/16.” If only the year was available, we assigned the event date as January 1 of that year. Estimates of seizure frequency were either self-reported or pulled from records that automatically log epileptiform events. Given that some patients had multiple seizure types with variable semiology, we reported only the frequency of their dominant seizure type.
We calculated three main intervals of interest related to diagnosis and treatment: the time between epilepsy diagnosis and DRE diagnosis (Dx to DRE), the time between epilepsy diagnosis and epilepsy surgery (Dx to surgery), and the time between DRE diagnosis and epilepsy surgery (DRE to surgery).
The distance traveled to our level 4 epilepsy center was determined using the zip code of each patient's home address and mapping the fastest route to our institution by motor vehicle. Because the data were not normally distributed and nonlinear relationships were likely, distance traveled was split into two groups, ≤3 h traveled (which approximates 180 miles by car) vs >3 h traveled.
2.2 Statistical analysis
All analyses were performed using custom Python scripts. Demographic data and clinical characteristics were summarized using appropriate descriptive statistics. Intervals were calculated by quantifying the number of days between specific evaluation periods (e.g., date of DRE diagnosis and date of epilepsy surgery). We used Spearman correlation, Mann–Whitney U test, and a Kruskal–Wallis test to determine whether the measured treatment intervals were associated with our demographic predictors of interest (age, sex, distance traveled, and health insurance status). We used logistic regression models to test for relationships between potential predictors of delays in diagnosis and surgical treatment of DRE. Specifically, we first applied a median split to each of the three intervals of interest (Dx to DRE, Dx to surgery, DRE to surgery) to identify patients who experienced above-average times between periods of diagnosis and treatment. Relevant pre-operative clinical characteristics (sex, distance traveled, health insurance status, pre-operative seizure frequency) were then evaluated as predictors of the binarized outcomes (i.e., intervals).
3 RESULTS
3.1 Demographics
Our retrospective review identified 185 patients with epilepsy who underwent epilepsy surgery at our institution during a 10-year period spanning 2012–2022. The mean (± standard deviation [SD]) age of our cohort was 38.4 (±11.9) years. Approximately half (53.5%) of our cohort was female, and most individuals were White (92.4%); there was a smaller representation of other ethnic backgrounds, namely Hispanic/Latino (4.3%), American Indian or Alaska Native (1.6%), African American (1.1%), and Asian (.5%). The median (1st–3rd quartile) distance traveled to our level 4 epilepsy center was 54 (25–212) miles, and 28.7% traveled >3 h (180 miles). Most patients in our cohort were insured (96%); of the insured, 62.1% had private insurance (e.g., BlueCross BlueShield, AETNA) and 37.9% had public insurance (e.g., Medicaid, Medicare).
3.2 Clinical characteristics
The mean (±SD) age at epilepsy diagnosis was 16.6 (±12.4) years. The median (1st–3rd quartile) seizure frequency pre-operatively, at 1-year post-operatively, and at the last follow-up was 5 (2–20), 1 (0–10), and 1 (0–5.5) events per month, respectively. Most patients were taking one or more antiseizure medicines (ASMs) at the last follow-up visit (median: 2 ASMs, 1st–3rd quartile: 1–3 ASMs).
3.3 Surgical management of epilepsy
Most patients in our cohort (68.6%) underwent phase 2 intracranial monitoring for seizure localization before epilepsy surgery. At the time of analysis, 95.7% of patients in our cohort had received definitive epilepsy surgery; a small subset of patients (4.3%) participated in phase 2 intracranial monitoring but had not yet received definitive surgery. In the patients who had undergone definitive epilepsy surgery, neuromodulation procedures were most common, followed by resection, ablation, and disconnection (Table 1).
| Surgery type | Procedure | No. performed |
|---|---|---|
| Phase 2 monitoring | 127 | |
| Neuromodulation | ||
| Responsive neurostimulation (RNS) | 73 | |
| Vagus nerve stimulation (VNS) | 6 | |
| Deep brain stimulation (DBS) | 2 | |
| Resectiona | ||
| Temporal | 52 | |
| Frontal | 20 | |
| Parietal | 6 | |
| Occipital | 2 | |
| Ablation | ||
| Laser interstitial thermal therapy (LITT) | 19 | |
| Stereotactic radiosurgery (SRS) | 2 | |
| Disconnection | ||
| Corpus callosotomy | 9 | |
| Combined | 3 | |
| Miscellaneousb | 2 |
- Note: At the time of analysis, 95.7% of patients had undergone definitive epilepsy surgery.
- a Patients may have ≥1 type of resection within a single surgery.
- b Miscellaneous surgeries include RNS battery or pulse generator replacement.
3.4 Predictors of delays in diagnosis and treatment intervals
The median (1st–3rd quartile) intervals related to diagnosis and treatment were 10.1 (3.8–21.5) years from Dx to DRE, 16.7 (6.5–28.4) years from Dx to surgery, and 1.4 (.6–4.0) years from DRE to surgery (Figure 1). Mann–Whitney U tests revealed a statistically significant difference in the median Dx to DRE time between those traveling >3 h (median: 6.0 years) and those traveling ≤3 h (median: 13.9 years) (U = 1642.5, p < .01). In addition, we observed a statistically significant difference in the median Dx to surgery time between those traveling >3 h (median: 10.2 years) and those traveling ≤3 h (median: 18.2 years) (U = 2065, p < .05); we did not observe any significant difference between groups with respect to DRE to surgery time. Given that our cohort had a large fraction of patients who received neuromodulation, we performed a sub-analysis comparing the diagnosis and treatment intervals of those who received neuromodulation against those who received resection or ablation. We observed a greater time from DRE diagnosis to surgery among those who received neuromodulation (median: 1.7 years) than those who received resection or ablation (median: 0.9 years) (U = 3106, p < .01). There were no statistically significant differences in Dx to DRE time and Dx to surgery time between groups (p > .05) (Figure S1).
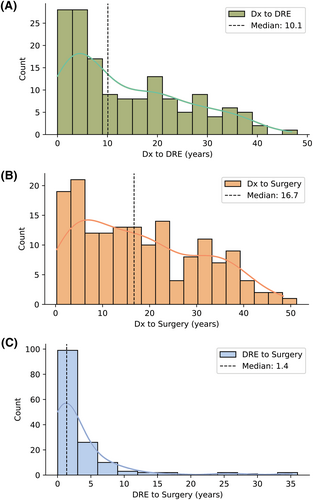
A Kruskal–Wallis test was conducted to determine whether treatment intervals differed depending on insurance status (public, private, or uninsured). The Kruskal–Wallis statistic highlighted a significant difference in the Dx to surgery time when patients were grouped by insurance status (H (2)=16.2, p < .001); a Dunn-Bonferroni post hoc test revealed a robust difference in the median DRE to surgery time between those with private insurance (median: 1.0 years) and those with public insurance (median: 2.9 years). Unsurprisingly, we observed significant, positive associations between patient age and Dx to DRE time (ρ(149) = 0.47, p < .001), as well as Dx to surgery time (ρ(160) = 0.42, p < .001); age was not associated with DRE to surgery time (Table 2).
| Predictor | Median Dx to DRE | Median Dx to surgery | Median DRE to surgery |
|---|---|---|---|
| Health insurance status (private vs public) | 12.0 vs 10.3 years; p = .1656 | 15.7 vs 18.9 years; p = .2098 | 1.0 vs 2.9 years; p = .0003 |
| Distance traveled (≤3 h vs >3 h) | 13.9 vs 6.0 years; p = .0025 | 18.2 vs 10.2 years; p = .0139 | 1.4 vs 1.2 years; p = .8814 |
In the logistic regression analysis predicting above-average Dx to DRE times, traveling >3 h emerged as a strong negative predictor (B = −1.5, SE = 0.5, p < .01), while controlling for other variables. No other variables across the other two models (Dx to surgery, DRE to surgery) were significantly associated with above-average delays.
4 DISCUSSION
We characterized the treatment intervals, demographics, and clinical characteristics of patients diagnosed with DRE who presented at our institution during a 10-year period from 2012 to 2022, to identify potential causes of delays in timely evaluation and surgery. In this retrospective analysis we identified a cohort of 185 patients diagnosed with DRE, 95.7% of whom had received definitive epilepsy surgery, with a mean age at surgery of 38.4 ± 11.9 years. Neuromodulation procedures were most common (responsive neurostimulation, vagus nerve stimulation, or deep brain stimulation), followed by resection, ablation, and disconnection.
In our analysis of referral patterns, we observed that the median intervals of interest were 10.1 (3.8–21.5) years from initial Dx to diagnosis of DRE, 16.7 (6.5–28.4) years from Dx to surgery, and 1.4 (.6–4.0) years from DRE to surgery. This aligns with prior literature from Benbadis et al., which reported that the length of time from the onset of seizures until an epilepsy center visit (corresponding with referral for DRE) averaged 18 years (range 2–58 years).7 It is important to note that the series by Benbadis et al. was published in 2003. Despite growing evidence of the benefits of earlier surgical referral over the last 20 years, the time from epilepsy diagnosis to surgical referral appears to be only marginally shorter. More recently, Blumcke et al. found that the mean duration of epilepsy before surgery was 20.1 years among adults and 5.3 years among children in a large multicenter series (36 centers from 12 European countries over 25 years).17
Notably, most patients in the series by Benbadis et al. and Blumcke et al. were treated with resection, ablation, or disconnection procedures, but in our cohort, neuromodulation procedures were most common. This discrepancy may result from a patient population with increased rates of nonlesional or bitemporal epilepsy. Our institution serves a large catchment area in the Intermountain West spanning Colorado, Montana, Nevada, New Mexico, and Utah. Although not characterized at length, the patients with DRE referred to our level 4 epilepsy center tend to have advanced disease (i.e., high prevalence of multifocal, non-resectable, generalized epilepsy). The increased prevalence of patients receiving neuromodulation highlights important ethical considerations unique to this therapeutic modality, for example, barriers resulting from the need for frequent programming visits or device maintenance.18 Our results suggest that individuals who received neuromodulation surgery experienced a greater time from DRE diagnosis to definitive surgery than those who received resection or ablation. This discrepancy may result from challenges in treating seizures that are difficult to localize or have complex, inconsistent etiologies.
It is likely that attitudes toward resection/ablation/disconnection vs neuromodulation surgery for DRE play an important role in referral patterns for epilepsy surgery. In a secondary analysis, Benbadis et al.7 found that 39% of patients were not sent by their neurologists and had not been given an opportunity to discuss surgical options, with 14% noting that they were explicitly advised against surgery. In a recent systematic review addressing health care professionals’ knowledge, attitudes, and perceptions toward epilepsy surgery, Samanta et al.19 highlighted that insufficient understanding of the risks and benefits of epilepsy surgery and poor identification and referral of patients with DRE were key health care professional-related barriers to epilepsy surgery. In addition, they found that neurologists with surgical exposure during training, formal instruction in epilepsy, participation in high-volume epilepsy practice, or prior experience with surgical referral may refer more patients for surgical evaluation.
Patient demographics were a key focus of our analysis, given that many studies have highlighted disparities in access to epilepsy surgery. For example, Jackson et al.11 described multiple factors, including travel distance, health insurance status, and race, that influenced time to surgery in children with epilepsy—noting that non-White patients were more likely to have a longer duration of epilepsy before surgery. Similarly, Armor et al.20 found that Asian, Hispanic, and African American patients had lower rates of epilepsy surgery than White patients. In the adult population, Thompson et al.21 found that African American and Asian/Pacific Islander patients had significantly longer wait times from presurgical evaluation to surgery. Because our cohort was predominantly White, we could not assess whether race predicted differences in referral or surgical timing.
In addition, we assessed the relationship between rural and urban-based patient populations by estimating the distance traveled to our center (determined using each patient's home zip code). As part of the state's only academic medical center, our institution serves as a major referral center for a significant portion of the rural communities in the Intermountain West. The rural–urban divide is key to understanding referral patterns in epilepsy surgery; Erba et al.22 demonstrated that proximity to surgical centers (most of which are located within cities) was positively associated with parental acceptance of epilepsy surgery in children. Conversely, Rubinger et al.23 showed that longer time to surgery was unrelated to rural or urban residence location and, therefore, not related to distance from tertiary care centers.
We observed significant differences in the Dx to DRE time and Dx to surgery time when patients were grouped by distance traveled to our epilepsy center. Specifically, our results suggest that those traveling >3 h were less likely to experience delays in receiving a diagnosis of DRE and, subsequently, epilepsy surgery. This observation was surprising, given our hypothesis that distance traveled would be associated with delays in referral and treatment. One possible explanation is that medical providers in rural Intermountain West regions (farther from our center, on average) may have a greater tendency to refer patients with epilepsy to our level 4 epilepsy center than those located in suburban areas (closer to our center, on average), which may have greater access to general neurologists. This interpretation, however, cannot be empirically tested with our available data and is therefore speculative.
Our analyses highlighted two potential delays in the surgical treatment of epilepsy. First, the interval between epilepsy diagnosis and DRE diagnosis was far greater than the interval between DRE diagnosis and surgery. Although this interval also captures disease progression, which may unfold over months to years, this period is likely especially susceptible to various health care professional-related barriers that patients may encounter throughout their clinical course (e.g., failure to promptly identify DRE, provider bias against surgical treatment). In addition, we observed that the time to receive definitive surgery after diagnosis of DRE was approximately three times longer for those with public health insurance compared to those with private insurance. Given the retrospective nature of our study design, we were unable to survey patients about any delays experienced, including those related to health insurance coverage or procedure approval. Nonetheless, our results motivate future prospective studies that seek to characterize the specific barriers that patients with DRE experience while undergoing evaluation for epilepsy surgery.
A growing body of evidence has demonstrated that longer epilepsy duration is associated with worse outcomes after resective epilepsy surgery24-26 and that earlier resective surgery soon after diagnosis of DRE is associated with improved seizure outcomes.4 Some groups have developed novel tools and initiatives to address the epilepsy surgery gap. For example, Jette et al.27 developed a web-based decision tool that provides a guide for evaluating candidacy for epilepsy surgery. Other efforts, like those reported by Peterson et al.,28 have highlighted how collaborative agreements between community and tertiary-care epilepsy centers might improve patient completion of the epilepsy surgery referral process; in their analysis, they showed that collaboration led to a 254% increase in epilepsy surgery completion (compared with the pre-collaboration period). Our results highlight the need for novel initiatives that specifically target community providers and facilitate earlier referral of patients with epilepsy; for example, implementing web-based educational tools that may aid in the identification and referral of appropriate surgical candidates.25
4.1 Limitations
We obtained our data by reviewing the charts of patients undergoing surgical evaluation for epilepsy. There are several limitations to this retrospective approach that are important to note. First, clinical details, especially the exact dates of specific events, were frequently missing or incomplete; for example, if a patient had relevant records from a separate institution that were unavailable. Where necessary, we approximated the dates used in our interval calculations with the information that was provided. However, this approach does not entail the level of precision afforded by studies that prospectively track a group of patients throughout their clinical course. In addition, because the information is self-reported, clinical features related to epilepsy severity (e.g., seizure frequency) may be challenging to track quantitatively.
Finally, our analyses characterize the experience of patients from the Intermountain West region of the United States. This is a large catchment area serving predominantly rural communities. Thus our experience will likely differ from other level 4 epilepsy centers that serve large urban populations and potentially those in other countries. Despite the large catchment area, the demographic makeup of our cohort was relatively homogeneous. Accordingly, we were underpowered to determine whether specific characteristics (e.g., ethnicity) were associated with delays in epilepsy diagnosis or treatment.
Although we speculate about how increased out-of-state referrals may bias our sample toward the inclusion of patients with more advanced disease, we have not systematically compared the clinical characteristics of our cohort to those treated at other institutions. Moreover, given that our cohort was predominantly White, it is reasonable to assume that our patients did not encounter significant health care–related barriers related to racial or ethnic disparities throughout their clinical course. The generalizability of our results should, therefore, be interpreted in the context of these distinctions.
5 CONCLUSION
We observed significantly shorter median times from epilepsy diagnosis to DRE diagnosis and epilepsy diagnosis to surgery when patients were grouped by distance traveled. Moreover, patients with public health insurance had a significantly longer time from DRE diagnosis to surgery. Timely referral of patients with DRE to specialized epilepsy centers for surgical evaluation is crucial, and identifying key factors that may delay this is paramount to optimizing epilepsy outcomes. Future studies are needed to better predict which patients are most at risk of encountering health care professional-related barriers related to epilepsy surgery. To this end, subsequent research that seeks to (1) characterize epilepsy referral patterns among neurologists, (2) understand patient experiences and specific barriers encountered throughout their clinical course, and (3) identify areas where targeted interventions (e.g., provider education) may facilitate timely diagnosis and treatment, may be especially informative.
AUTHOR CONTRIBUTIONS
Justin M. Campbell: data curation, methodology, formal analysis, writing–original draft. Samantha Yost: data curation, writing–review & editing. Diwas Gautam: data curation, writing–review & editing. Alysha Herich: data curation. David Botros: data curation, writing–original draft. Mason Slaughter: data curation, writing–review & editing. Michael Chodakiewitz: data curation, writing–original draft. Amir Arain: writing–review & editing. Angela Peters: writing–review & editing. Sindhu Richards: writing–review & editing. Blake Newman: writing–review & editing. Brian Johnson: writing–review & editing. Shervin Rahimpour: conceptualization, methodology, project administration, writing–review & editing. Ben Shofty: conceptualization, methodology, project administration, writing–review & editing.
ACKNOWLEDGMENTS
We thank Cortlynd Olsen and Kristin Kraus for editorial assistance.
FUNDING INFORMATION
J.M.C. received funding from the National Institute of Neurological Disorders and Stroke (NINDS T32 NS115723).
CONFLICT OF INTEREST STATEMENT
None of the authors has any conflict of interest to disclose.
ETHICS STATEMENT
This retrospective review was approved by the University of Utah Institutional Review Board with a waiver of patient consent (protocol #115230). We confirm that we have read the Journal's position on issues involved in ethical publication and that our report is consistent with those guidelines.
PATIENT CONSENT
This study was conducted under a waiver of informed consent from the University of Utah Institutional Review Board.
Open Research
DATA AVAILABILITY STATEMENT
Data will not be made publicly available to protect patient privacy. Further inquiries can be directed to the corresponding author.




