A comprehensive systematic literature review of the burden of illness of Lennox–Gastaut syndrome on patients, caregivers, and society
Affiliations for Jeannine Roth and Emma Butcher were at the indicated institutions at the time the study was conducted.
Abstract
Fully elucidating the burden that Lennox–Gastaut syndrome (LGS) places on individuals with the disease and their caregivers is critical to improving outcomes and quality of life (QoL). This systematic literature review evaluated the global burden of illness of LGS, including clinical symptom burden, care requirements, QoL, comorbidities, caregiver burden, economic burden, and treatment burden (PROSPERO ID: CRD42022317413). MEDLINE, Embase, and the Cochrane Library were searched for articles that met predetermined criteria. After screening 1442 deduplicated articles and supplementary manual searches, 113 articles were included for review. A high clinical symptom burden of LGS was identified, with high seizure frequency and nonseizure symptoms (including developmental delay and intellectual disability) leading to low QoL and substantial care requirements for individuals with LGS, with the latter including daily function assistance for mobility, eating, and toileting. Multiple comorbidities were identified, with intellectual disorders having the highest prevalence. Although based on few studies, a high caregiver burden was also identified, which was associated with physical problems (including fatigue and sleep disturbances), social isolation, poor mental health, and financial difficulties. Most economic analyses focused on the high direct costs of LGS, which arose predominantly from medically treated seizure events, inpatient costs, and medication requirements. Pharmacoresistance was common, and many individuals required polytherapy and treatment changes over time. Few studies focused on the humanistic burden. Quality concerns were noted for sample representativeness, disease and outcome measures, and reporting clarity. In summary, a high burden of LGS on individuals, caregivers, and health care systems was identified, which may be alleviated by reducing the clinical symptom burden. These findings highlight the need for a greater understanding of and better definitions for the broad spectrum of LGS symptoms and development of treatments to alleviate nonseizure symptoms.
Key points
- Reducing the high clinical symptom burden of LGS could increase quality of life of patients and caregivers
- LGS is associated with high direct costs due to health care resource use and medications; data on indirect costs are lacking
- Pharmacoresistance is common, and most individuals with LGS require polytherapy and changes to treatment over time
- There is an unmet need for treatments that address the nonseizure symptoms of LGS
- There is a need for a greater understanding of and better definitions for the broad spectrum of LGS symptoms
1 INTRODUCTION
Lennox–Gastaut syndrome (LGS) is a rare developmental and epileptic encephalopathy1 that is estimated to account for 1%–10% of childhood epilepsies2 and is characterized by multiple drug-resistant seizure types, specific abnormal electroencephalogram showing bursts of slow spike–wave complexes and generalized paroxysmal fast activity, and intellectual and behavioral impairment.3 Diagnosis of LGS is hindered by multiple factors, including the lack of disease biomarkers, and heterogeneous etiology and presentation.4-6 Seizures associated with LGS are typically not controllable with antiseizure medications (ASMs), and drug resistance is common.5, 7 In addition to the multiple seizure types experienced by patients, LGS is also associated with a range of nonseizure symptoms, including intellectual disability, motor impairments, deficits in communication and sleep, and psychiatric and behavioral issues, for which there are also no effective treatments.6 Although nonseizure symptoms are likely to contribute significantly to the burden of illness of LGS, there has thus far been no thorough analysis of their effects on patients, caregivers, and society. Although individual studies have identified negative physical, social, emotional, and financial impacts of LGS for individuals and their caregivers,8-10 until now, only one systematic literature review (SLR) has evaluated the burden of illness of LGS.11 Compared with single studies, SLRs are able to cover a broader range of subjects, enabling effective evaluation of all available evidence and easier identification of shortcomings or evidence gaps.
To gain a comprehensive understanding of the burden of illness of LGS, the present SLR evaluated data on clinical symptom burden, comorbidities, care requirements, quality of life (QoL), economic burden, caregiver burden, and treatment burden. The aim of the present SLR was to identify evidence gaps to guide further research and inform development of new interventions to improve the lives of patients and their caregivers.
2 MATERIALS AND METHODS
This SLR was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and registered on PROSPERO (ID: CRD42022317413).12
2.1 Objectives
The primary objective was to describe data on the burden of illness of LGS globally, across the domains of clinical symptom burden, comorbidities, care requirements, humanistic burden on individuals and caregivers, economic burden, and treatment burden. The secondary objective was to identify reasons for variations in findings across the included studies, and any current data gaps.
2.2 Outcomes
The main outcomes were clinical symptom burden (types and patterns of seizure and nonseizure symptoms), prevalence of comorbidities, care requirements, humanistic burden on individuals and caregivers, economic burden, and treatment burden.
2.3 Eligibility criteria
Noninterventional studies of any design were eligible for inclusion if they presented original research on one or more outcomes of interest and included primary or subgroup analyses in an LGS population (Table S1–S6). Inclusion was not restricted by population, country of origin, or publication date. Publications written in English and non-English publications with an English abstract were considered for inclusion. Preclinical studies, case reports, reviews, and interventional studies in which individuals were actively assigned to a therapy were excluded. For this review, the use of screening, diagnostic tests, or assessments (including qualitative interviews) was not defined as intervention.
2.4 Data sources
Relevant literature was identified via MEDLINE, Embase, and the Cochrane Library from inception to March 15, 2022. For the period 2017–2022, supplementary manual searches were also performed by one reviewer (E.B.) to identify relevant proceedings from key congresses, websites, and publications listed in bibliographies of review articles (Table S2).
2.5 Search strategy
Two reviewers (A.J and E.B.) used a combination of Medical Subject Headings and free-text terms to identify relevant literature (Table S2). The results from MEDLINE, Embase, and Cochrane Library searches were downloaded via Endnote into a bespoke Microsoft Excel database. After deduplication, titles and abstracts, followed by full texts, were screened in duplicate by independent reviewers (E.B., A.J., and nonauthor reviewers). Discrepancies were resolved by reviewer discussion or escalation to a third independent reviewer (E.B., A.J., and nonauthor reviewers). Articles identified during supplementary manual searches were added to the final included list of publications after full-text screening.
2.6 Data extraction and quality assessment
Data on the study design, population, and outcomes were extracted from publications by a single reviewer for each article. This reviewer also assessed study quality based on a series of “risk of bias” questions adapted from the Strengthening the Reporting of Observational Studies in Epidemiology statement,13 the Appraisal Tool for Cross-Sectional Studies,14 and the Newcastle–Ottawa quality assessment scales.15 Risk of bias was rated as high, medium, or low across eight categories relating to study design, methodology, and reporting, to reflect potential concerns over the reliability and generalizability of the reported results. Seizure types were recorded exactly as they were described in each article, which included seizure type terminology that has since been discontinued.16
2.7 Data synthesis
Owing to heterogeneity and the qualitative nature of some outcomes, no quantitative synthesis or subgroup analyses were performed. Results are presented as narrative and tabulated summaries, with descriptions of themes, variation, and quality concerns. Costs describing the economic burden were standardized to a single currency, when possible, accounting for or noting potential differences by year of publication, inflation, and other economic conditions.
3 RESULTS
3.1 General findings
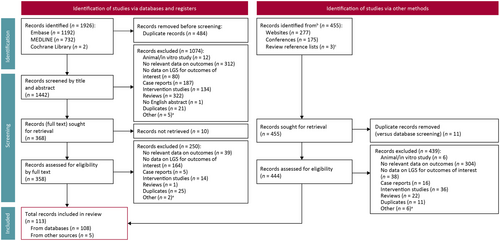
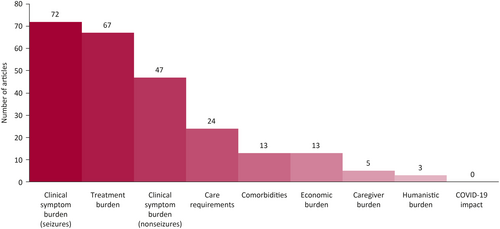
After screening 1442 deduplicated records from databases and registers, 108 were eligible for inclusion in this review (Figure 1). Five additional articles were identified in the supplementary searches, leading to a total of 113 included articles (Figure 1). Most of the included studies reported on the clinical burden of seizure symptoms, treatment burden outcomes, or both (Figure 2). Conversely, there were few reports on caregiver burden or humanistic burden.
North America had the highest representation of studies (n = 39), followed by East Asia (n = 32) and Europe (n = 31) (Table S3). Other regions, including South America (n = 9), western Asia (n = 7), Oceania (n = 3), and Africa (n = 2), were poorly represented.
3.2 Quality assessment findings
A summary of the qualitative assessment results is given in Table S4. Briefly, few quality issues were noted in terms of study design, methodology concerns, and clarity of reporting in the Results and Discussion sections. Quality concerns were noted for sample representativeness (n = 95), disease and outcome measures used (n = 57), and overall reporting clarity (n = 39). These concerns were raised owing to the inclusion of small or selective samples, a lack of clarity on or confirmation of LGS diagnosis, unclear selection criteria, and limited reporting clarity because of the type of publication (i.e., abstract). Although no studies were excluded owing to quality assessment findings, quality concerns were considered when analyzing the extracted data and reporting findings.
3.3 Clinical symptom burden and comorbidities
3.3.1 Seizures
Of the 72 studies reporting the burden of clinical seizure symptoms, six studies (two each in Asia, Europe, and North America) included the age at first seizure, which ranged from 1 month to >9 years. No clear differences by geographical location or publication date were apparent.17-22 Studies including both children and adults (n = 3) reported an average age at first seizure of 1–3 years,20-22 which is older than that reported in the studies that included children only (n = 3).17-19 Across eight studies with relevant data, 0%–60% of individuals with LGS experienced infantile spasms, either before the onset of epilepsy or as the first recorded seizure type.19-26
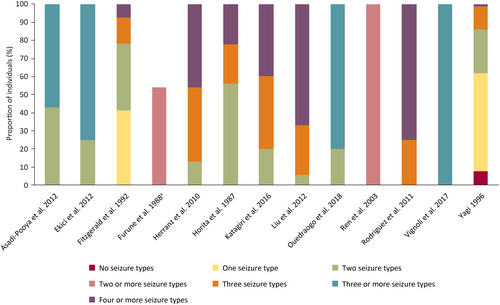
Data from 17 studies showed that individuals with LGS generally experience 2–4 concurrent seizure types, although substantial interindividual heterogeneity existed, with an overall range of 0–7 seizure types per individual.20, 21, 26-40 Five studies reported the mean number of seizure types per individual, which was between 2.6 and 3.6.28, 37-40 These data were generally aligned with those from studies that reported the proportion of individuals experiencing different numbers of seizure types (Figure 3).20, 21, 26-36 One study reported a high prevalence of individuals with one seizure type (38%); however, this study used historical records of seizure types experienced over 14 years.29
Four studies reported a decrease in the number of seizure types experienced per individual during the study, which may reflect changes with age.20, 27, 34, 41 Similarly, a decline in the prevalence of individual seizure types (tonic, tonic–clonic, myoclonic, and atypical absence) over time was reported in multiple studies (Table S5).20, 36, 38, 42, 43 Three studies also noted reductions in the prevalence of atonic seizures over time.20, 36, 43
In all 11 studies that reported continuous measures of seizure frequency, individuals with LGS were found to experience more than one seizure per day.29, 30, 37, 41, 44-50 Seizure frequency ranged widely within and across studies, which may be partly owing to interindividual heterogeneity; in one study, baseline seizure frequency was 15–3000 seizures per month.46, 51 These findings aligned with those from studies in which individuals were categorized by seizure frequency; 50%–100% of individuals were reported to have daily seizures, and those without daily seizures were usually reported to have seizures at least once per week.18, 20, 23, 26, 28, 34-36, 52-58
Several prospective and retrospective studies showed decreases in seizure frequency over time, reporting similar ranges of seizure freedom prevalence at last visit (9%–28% and 4%–28%, respectively).17, 20, 34, 38, 43, 47, 59-67 Although the duration of data collection varied considerably between studies (3–20 years), no correlation between the change in seizure frequency and the duration of data collection was observed.20, 34, 38, 43, 47, 59-64 One study showed that 33.3% of children treated with clobazam had seizure-free status maintained for a period of 5.5 months, 25% of children treated with topiramate had seizure-free periods ranging from 8 months to 2.5 years, and 11% of children treated with lamotrigine had seizure-free periods of 6 months.26 In another study, 70.6% of children had at least one seizure-free period up to their fifth birthday, with a median longest seizure-free period of 13.5 months.17
The prevalence and frequency of seizure types observed in individuals with LGS are summarized in Table S5. Tonic seizures showed the highest prevalence of all reported seizure types, followed by tonic–clonic and myoclonic seizures. Several definitions were used for atonic, astatic (now referred to as atonic),16 and absence seizures, and status epilepticus, precluding a clear conclusion on prevalence. Additionally, some studies reported the prevalence of drop attacks, which can encompass several seizure types, including atonic, tonic, and generalized tonic–clonic seizures.
3.3.2 Nonseizure symptoms and comorbidities
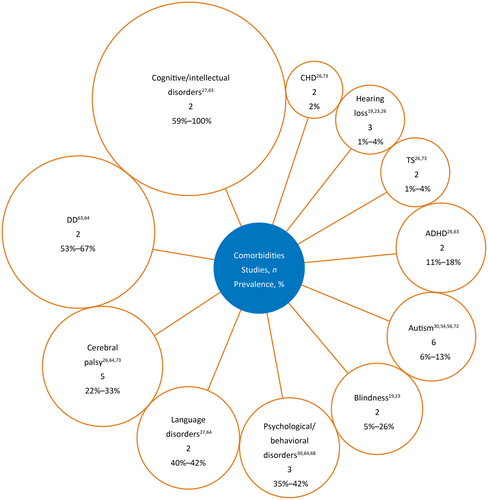
Overall, 59 studies reported on nonseizure symptoms or comorbidities. In the present review, all data are combined and original study terminology (i.e., “nonseizure symptom” or “comorbidity”) is used when relevant. Distinguishing between nonseizure symptoms and comorbidities was difficult given the overlapping definitions across studies, with 47 studies reporting on nonseizure symptoms (Table S6) and 12 studies reporting on comorbidities (Figure 4). Overall, the most common nonseizure symptoms or comorbidities were developmental delay, intellectual disability, and motor impairments.
Developmental delay was common in individuals with LGS, with eight studies reporting a prevalence of 79.6%–100% (Table S6). These delays were often severe or profound (>50% prevalence based on three articles)21, 25, 68 and associated with developmental regression (44% prevalence based on one article).65 Furthermore, based on 19 studies, at least 90% of individuals with LGS had intellectual disability, often of moderate to profound severity (reported by 14 articles to be present in at least 50% of the sample), and four studies noted that severity may worsen over time. Compared with studies in which it was described as a nonseizure symptom, the prevalence of intellectual disability when described as a comorbidity was more varied (59% and 100% in two studies).33, 69
Eleven studies reported on motor impairments, which were frequent in individuals with LGS, with one study reporting difficulty walking in 81% of individuals.70 However, variation in the measures and definitions used prevented definitive conclusions on the prevalence and severity of specific motor problems (e.g., motor handicap, walking/sitting) or symptoms (e.g., ataxia, hypotonia, paresis, spasticity).
Based on six studies, many individuals with LGS showed difficulties with communication, with up to 60% of individuals reported to have speech disorders (including dysarthria) and one study showing 60% of patients to be nonverbal.21, 26, 29, 68, 70, 71 In one study (n = 24 parents), 96% of parents reported that their child could not communicate appropriately for their age.70 Two studies described comorbidities of language disorders, reporting a prevalence of 40% and 42%.33, 72
Autism33, 36, 58, 60, 69, 73 and cerebral palsy25, 32, 72-74 were comorbidities reported in six and five studies, respectively, with prevalence estimates of 6%–13% and 22%–33% of individuals, respectively (Figure 4). The prevalence estimate for autism excluded studies with unreliable estimates, namely one study with only five individuals33 and another study in which “probable” LGS only was identified.69 Although the latter study described patients with “probable” LGS and was excluded from the prevalence estimate for autism, the article did meet criteria for inclusion in the present review, because patients were identified from insurance claims records using a machine-learning model based on treatment- and diagnosis-related data. Identified patients were also reviewed by clinicians to ensure suitability for inclusion. In the present review, the estimate for cerebral palsy excluded studies with small or selective samples, namely a study of vagus nerve stimulation (VNS)73 and another with a small sample size (23 individuals).25 If included, these studies would change the range of prevalence estimates for autism and cerebral palsy to 0%–20% and 22%–56%, respectively.
Other symptoms were grouped into comorbidities defined as psychiatric and behavioral issues, and were reported in five studies24, 26, 68, 75, 76; however, methods and findings were heterogeneous, preventing any clear conclusions regarding prevalence. One study reported that 76% of individuals had behavioral issues68; another study found that 80% of individuals had hyperactivity, aggressiveness, and autistic features.75 Conversely, other studies reported lower prevalence, potentially owing to their focus on specific symptoms (e.g., hyperactivity)75 or behavioral syndromes (e.g., anxiety, attention-deficit/hyperactivity disorder, or psychosis).76 Studies that described psychiatric and behavioral issues as comorbidities generally reported lower prevalence (35%–42%) than those that described them as nonseizure symptoms (35%–50%).36, 72, 77
Other problems included difficulty swallowing (reported in two studies)43, 70 and strabismus (reported in one study with a prevalence of 5.3%).36 Based on five studies, individuals with LGS spent less time in sleep stage 2 and in rapid eye movement sleep than those without LGS.31, 68, 78-80
Prevalence estimates of comorbidities reported in only one study each were overweight/obesity (80%)81; acute upper respiratory tract infections (44%)72; bone fracture (31%)69; postviral encephalitis (6%)74; insomnia (5%)36; Down syndrome (4%)74; hypothyroidism (3%)32; myelomeningocele (2%)74; cancer (2%)32; diabetes, hypopituitarism, and renal failure (1% each)32; and urinary incontinence, osteoporosis, and gastrointestinal conditions (not specified).36
3.4 Care requirements
Overall, 24 studies assessed the care requirements of individuals with LGS, which were found to be considerable owing to the high prevalence of seizure and nonseizure symptoms, as well as frequent comorbidity. In terms of daily assistance, 60.3% of adults (n = 41) in one study lacked independent daily living skills, including bathing, eating, functional mobility, and toileting.82 Thirteen studies reported that individuals with LGS frequently have problems with mobility and eating, including difficulty walking, lack of functional grasp strength, and a requirement for tube feeding (Table 1).10, 21, 26, 28, 45, 49, 58, 68-70, 82-84
| Impact on daily living | |
| Mobility |
|
|
|
| Eating | |
| Other skills and functions |
|
|
|
|
|
| Impact on schooling and living requirements | |
| Schooling |
|
|
|
| Living placement |
|
- Abbreviation: LGS, Lennox–Gastaut syndrome.
The considerable clinical symptom burden associated with LGS means that individuals often require education outside of mainstream settings,30, 59 and few individuals complete high school (5.1% and 22.6% completion rate in two studies) or undertake higher level education (Table 1).22, 32, 38 Additionally, many individuals cannot live independently or support themselves economically, often requiring full-time caregiver support in childhood and residential care in adulthood (Table 1).27, 30, 36, 38, 42, 45, 49, 77, 85
3.5 Humanistic and caregiver burdens
Only three studies provided information on the QoL of individuals with LGS, with sample sizes ranging from 40 to 416.9, 68, 86 Given the absence of validated scales for assessing the QoL of individuals with LGS, this outcome was not measured directly in any of the studies; instead, assessment was reliant on caregiver interviews or surveys. Moreover, no studies directly questioned individuals with LGS, probably owing to the intellectual disability associated with LGS. In one study, a conceptual model was developed to reflect four identified themes around the impact of LGS on QoL (physical, cognitive and behavioral, social, and treatment).9 These themes were consistent with findings from two caregiver surveys, one of which included carers of individuals with other epilepsies (Figure 5).2, 6 Specific aspects of the identified themes could have direct or indirect effects on QoL (e.g., developmental delay has a direct impact and affects the ability to make friends), and certain aspects may have a positive or negative impact, such as treatments (depending on efficacy).9, 68
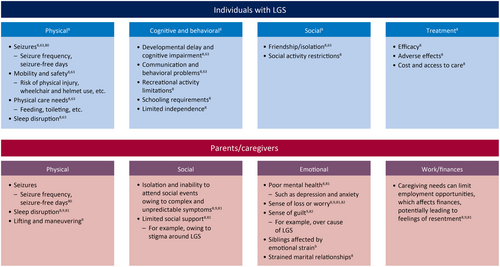
In one study, individuals and caregivers (for Dravet syndrome, LGS, or other epilepsies) provided expected patient QoL scores based on a series of hypothetical vignettes. A strong positive correlation was reported between hypothesized patient QoL scores and the number of seizure-free days per month (p < .001). Another study assessed patient QoL using a mean health utility score, obtained by converting visual analog scale scores to a 0–1 scale by dividing by 100. This study showed that seizure frequency greatly affected patient QoL, with the lowest reported mean health utility score (.14) being associated with the highest frequency of drop seizures (130 seizures and one seizure-free day per month).86
Five studies interviewed parents and caregivers to assess the burden they personally experienced, with multiple countries represented, including Australia, France, Italy, the UK, and the USA.9, 10, 86-88 Effects on caregivers were noted in four domains: physical, social, emotional, and work/finances (Figure 5).9, 87
The reported emotional burden on caregivers was multifaceted; caregivers often went through stages of adjustment after learning that their child had LGS, beginning with disbelief, bargaining, fear, and anger, and followed by acceptance and identity readjustment.10 Although this burden affects all family members, the greatest burden is experienced by the main caregiver, who is usually the mother of the affected child.87 Caregivers experience uncertainty around the diagnosis and cause of LGS, seizure activity, treatment efficacy, and prognosis, but parent support groups can reduce isolation and worry, and provide parents with practical advice.88
3.6 Economic burden
Overall, 13 articles reported on economic burden, with nine studies from the USA and one study each from Germany, Japan, Mexico, and the UK. Of these 13 articles, 11 included information on costs and seven included information on health care usage, with five articles providing information on both (Figure S1). The articles on costs generally focused on direct costs, with little or no information on indirect costs such as lost caregiver productivity and days lost from work.
Direct costs varied considerably across studies, even for studies in the same country. Generally, USA-based studies reported higher per-patient-year costs ($29 911–$80 545) than studies in other countries, including Germany ($24 450.91) and Mexico ($707.34–$2123.25). These costs were often categorized as medical service or pharmacy costs. Medical service costs were generally higher than pharmacy costs; however, there was considerable variation. The main contributor to medical service costs was inpatient costs, which composed 21%–60% of the mean annual health care cost.67, 72, 89
One USA-based study reported a mean (SD) cost of $8147 ($43 218) per medically treated seizure event for Medicaid-insured individuals and $14 759 ($43 600) for commercially insured individuals.67 Another USA-based study showed that costs for LGS were approximately three times higher than for other epilepsies.69, 90 Pharmacy costs ($1592–$24 018) were mainly driven by the high costs of ASMs, with some evidence of rising costs over time.69, 90-92
Across five studies, inpatient admissions ranged from .6 to 3.6 per patient-year,67, 69, 72, 89, 93 with a mean length of each hospital stay of 2–5 days.72, 93 Four studies showed that outpatient visits ranged from 6.3 to 11.8 per patient-year.69, 84, 89, 94
3.7 Treatment burden
Most individuals with LGS receive ASMs to manage their symptoms.23, 26, 28, 30, 34, 36, 42, 53, 55, 60, 69, 78, 80, 82, 83, 88, 91, 95-100 Across 23 studies with information on LGS treatment, 19 reported that all individuals with LGS received ASMs, and the other four studies generally found that more than 80% of individuals used ASMs. However, pharmacoresistance associated with switching of ASMs21, 23, 34, 36, 39, 41, 45, 49, 50, 72, 73, 77, 85, 92, 93, 95, 96, 101-104 and polytherapy51, 105 was common. These findings were consistent for children and adults across several countries, suggesting no difference by demographic or individual characteristics.
Across four studies, 73%–100% of individuals met study-defined criteria for pharmacoresistance.101, 105-107 These four studies covered relatively small samples of children from Italy, Nepal, South Korea, or the USA, but did suggest pharmacoresistance in LGS is not limited to a particular pediatric age group or geographical location. Polytherapy is usually required,26, 30, 60, 97, 105 with concurrent use of 2–4 ASMs per individual with LGS reported across approximately 40 studies.18, 28, 34, 36-39, 42, 44, 48-51, 53-55, 58, 66, 68, 72, 73, 78, 80, 81, 83, 88, 90, 95, 96, 98-100, 102, 104, 108-111 Exceptions to the finding that 2–4 ASMs are used concurrently by individuals with LGS were reported in some studies. For example, a UK-based study in 256 individuals with LGS (110 confirmed, 146 probable) estimated a lower mean number of ASMs used per year (approximately one).93 However, switching of ASMs was common, and the mean total number of ASMs used by individuals with confirmed or probable LGS was 6.7 (SD = 3.4), and 8.5 (SD = 3.5) over a mean follow-up time (averaged across confirmed and probable LGS) of 11.7 (SD = 8.2) years.93 Six studies reported that individuals with LGS generally used more than four ASMs concurrently,65, 69, 84, 85, 112, 113 including one retrospective analysis from the USA (n = 14 712), which used data from the Medicaid multistate database and reported a mean of 5.8 (SD = 2.3) concurrent ASMs after a mean observation period of 11.1 (SD = 4.5) years.69 These data suggest that use of ASMs can vary widely across individuals with LGS.65, 69, 84, 85, 112, 113
The ASM used by the highest proportion of individuals was valproate or valproic acid (30.8%–100%), with upper estimates for the frequency-of-use range being ≤80% for all other ASMs (Table S7).23, 28, 30, 34, 45, 48, 49, 53, 56, 58, 66, 73, 77, 78, 82, 85, 93, 95, 98-100, 108-110, 114 Reported treatment combinations varied, but common combinations across four studies with data were valproate or valproic acid with lamotrigine, clobazam, or levetiracetam.66, 72, 74, 99 Medications reported by fewer than four studies included drugs approved by the US Food and Drug Administration (FDA) and the European Medicines Agency for use in treatment of epilepsy decades ago (acetazolamide, ethosuximide, mesuximide, oxcarbazepine, phenytoin, and primidone), those with more recent FDA approval (lacosamide and midazolam), and those used off-label to treat epilepsy (cannabis-based medicinal products, intravenous gamma globulin, potassium bromide, and sultiame).26, 34, 36, 45, 48, 77, 78, 93, 108, 110 Drug–drug interactions were not discussed in the included articles.
ASM discontinuation rates varied across the included studies from less than 5% up to 50%. There were too few studies of each treatment to evaluate whether particular treatments had higher discontinuation rates than others.39, 45, 53, 73, 85, 95-98, 108, 114 Reasons given for discontinuation were aggravated seizure severity or frequency,95, 97, 108, 114 motor problems,53 vomiting,96 and death,98, 108 as well as reasons not related to safety, including lack or loss of efficacy58, 85, 98, 108 and inability to access the drug.98 No comparisons were made across treatments on rates or reasons for discontinuation given the limited data for each treatment.
Non-ASM treatments for LGS included ketogenic diet, corpus callosotomy, VNS, focal resection, deep brain stimulation, antipsychotics, and selective serotonin reuptake inhibitors.18, 36, 38, 39, 50, 58, 77, 82, 85, 91, 95, 104, 105, 108-110 Additional treatment modalities are commonly used concurrently with ASMs, with one study from South Korea (n = 68) reporting that 61.8% of individuals were receiving non-ASM treatments concurrently with ASMs.82
Adverse events (AEs) were associated with treatment with ASMs, corpus callosotomy, dietary changes, and VNS. Commonly reported AEs for ASM treatment were increased seizure frequency, behavioral problems, somnolence, nausea or vomiting, anorexia or decreased appetite, weight loss, rash, and dizziness.45, 53, 58, 95, 98, 108-110, 114-116
For VNS, AEs tended to occur soon after surgical implantation of the device and to resolve over time.85, 104 Common AEs with VNS included problems related to the respiratory system and speaking, including voice alteration or hoarseness, drooling, and coughing.28, 37, 73, 85, 111 For corpus callosotomy, two studies reported AEs, which related to brain connections (e.g., acute disconnection syndrome, aphasia, ataxia, and paresis).28, 111 AEs related to dietary therapy were reported in two studies, and were mainly gastrointestinal, including vomiting, diarrhea, constipation, and weight loss, as well as metabolic acidosis.56, 96
4 DISCUSSION
Evaluating the literature on the burden of illness of LGS is critical to identifying evidence gaps and guiding further research, which can positively affect individuals, caregivers, health care systems, and society. Overall, the literature reviewed here suggests that the high symptom burden of LGS has wide-ranging negative effects on patients and their caregivers. For example, the high seizure frequency not only decreases patient QoL, but is also associated with a high economic cost and a requirement for assistance with a range of everyday activities that place a substantial burden on caregivers. Severe or profound developmental delay, motor deficits, and problems with communication were present in a large number of patients with LGS. Pharmacoresistance, polytherapy, and treatment-switching are common, with individuals typically taking more than two ASMs at any one time. Although the potential impact of COVID-19 on LGS was captured in the search terms, no studies reported on this domain, and it is therefore not further discussed in this review.
Individuals with LGS experience a high clinical symptom burden from both seizure and nonseizure symptoms. However, more articles focused on clinical symptom burden of seizures and treatment burden (72 and 67 articles, respectively) than on nonseizure symptoms and comorbidities (46 and 12 articles, respectively). Seizure onset in infancy, high seizure frequency, and presentation with multiple seizure types all contribute to substantial care requirements, negative impacts on patient QoL, and a significant humanistic burden for caregivers. Nonseizure symptoms, including developmental delay, difficulties with communication and sleep, impaired motor ability, intellectual disability, and psychiatric and behavioral problems, as well as multiple comorbidities such as autism, blindness, and cerebral palsy, all contribute to a high humanistic burden and lower patient QoL.
Disentangling nonseizure symptoms from comorbidities is often difficult, as evidenced by the overlap of terminology observed in the included articles. When reported as nonseizure symptoms, many individuals with LGS had developmental delay, with eight studies reporting a prevalence of 79.6%–100%. Intellectual disability was also common, with 19 studies reporting a prevalence of at least 90%. In comparison, when described as comorbidities, the range of prevalence estimates reported for developmental delay by two studies was wider (50%–100%), whereas a lower range of prevalence estimates was reported for intellectual disorders, again by two studies (53%–67%). As a result, the true range of prevalence estimates for nonseizure symptoms/comorbidities experienced by individuals with LGS may not be fully captured without considering this overlap in terminology. Additionally, multiple types of motor deficits with varying prevalence were reported, reflecting the complex presentation of LGS. Although a high prevalence of problems with communication and sleep was observed in individuals with LGS, few studies assessed these symptoms in detail.
In general, both nonseizure symptoms and comorbidities were underrepresented in the literature, and accurate assessment was further confounded by overlap in terminologies and the classification of some causes of LGS, such as tuberous sclerosis, as comorbidities. The paucity of information on nonseizure symptoms and comorbidities of LGS make them promising areas for further research, which could inform development of new treatments or help determine when existing treatments may be effective, ultimately improving the QoL of both patients and caregivers.
Individuals with LGS experience a high treatment burden owing to pharmacoresistance,101, 105-107 polytherapy,26, 30, 60, 97, 105 and frequent treatment-switching.21, 23, 34, 36, 39, 41, 45, 49, 50, 72, 73, 77, 85, 92, 93, 95, 96, 101-104 Available treatments are associated with multiple AEs, although these tend to be mild, with few severe or serious AEs reported.45, 53, 58, 95, 98, 108-110, 114-116 Common combinations of ASMs identified included valproate or valproic acid with lamotrigine, clobazam, or levetiracetam.1, 66, 74, 99 Use of cannabis-based medicinal products was reported in one study77; no studies included here reported on cannabidiol use. However, drugs such as cannabidiol, which received FDA approval in 2020, have not been in clinical practice long enough for observational studies to have been published. Nonetheless, most available treatments target the seizure symptoms of LGS and a need remains for treatments that also target nonseizure symptoms, which could potentially alleviate some of the humanistic burden experienced by individuals.
Few studies reported on caregiver burden (n = 5) or humanistic burden (n = 3). The present review identified several studies that reported impact of LGS on patients, with limited independence, restriction in social activities, and lack of friendship/isolation all shown to negatively affect QoL.9, 68 In addition, a similar trend was found for caregivers, with limited employment opportunities, poor mental health, and strained marital relationships being among the negative effects experienced.9, 10, 87 Although three articles in this review reported on QoL for individuals with LGS,9, 68, 86 these studies used questionnaires completed by caregivers rather than validated tools and, given the hypothetical nature of the scenarios, the results may be subject to bias. Development of a reliable disease-specific tool for measuring QoL could enable more effective evaluation of interventions than is possible with existing generic measures.
Although based on limited data, the identified caregiver requirements and burden were substantial, with individuals with LGS requiring assistance with everyday mobility and self-care functions. Moreover, because many individuals with LGS are unable to live independently or to support themselves economically, many caregivers are required to provide lifelong assistance that negatively affects their own employment opportunities and finances, which can lead to feelings of resentment.9, 10 No studies included in this review comprehensively assessed how caring for a patient with LGS can affect workplace productivity, making this an area for potential further research.
Much of the economic burden of LGS derives from the high cost of medications and frequent hospital stays, which contribute to the direct costs for LGS being threefold higher than those for other epilepsies.69, 90 Variation in the direct costs of LGS was observed even when restricting to studies from the same country, and there was no clear trend in change with time, suggesting that more work is needed to determine the true cost of LGS. However, the results presented should be interpreted in the context of potential data-quality concerns regarding sample representativeness, LGS definitions, and the clarity of the reporting. Additionally, the overrepresentation of USA-based sources may decrease the generalizability of the economic burden findings of LGS across countries, because of variation in health care models (e.g., absence of treatment reimbursement systems in some countries). However, it is clear that the economic burden and health care resource use associated with LGS may negatively affect individuals, caregivers, and society.
Comparing findings across studies was often challenging owing to unclear selection criteria, limited details on the case definition for LGS, and lack of confirmation for LGS diagnoses. Moreover, variations in diagnostic criteria for LGS across studies reflect changes to the definition of LGS and associated diagnostic criteria over time, further confounding efforts to compare studies. Consequently, it was difficult to determine whether any identified heterogeneity across studies reflected true heterogeneity in the population or artifactual differences in study methodologies. The LGS electroclinical phenotype shows a high degree of heterogeneity, and LGS diagnoses may encompass multiple separate conditions with different etiologies, genotypes, risks of mortality/sudden unexpected death in epilepsy, and treatment requirements. Additionally, all included studies were published before the introduction in 2022 of updated seizure definitions,3 so the accuracy of distinguishing different seizure types may have been suboptimal.27, 29 This heterogeneity may explain the variation in the findings of this review but hinders a definitive assessment of the true burden of illness of LGS. The introduction of updated diagnostic criteria3 may help to improve the diagnostic framework going forward; however, without a biomarker or genetic cause, the diagnosis of LGS remains differential and, therefore, prone to subjectivity.4
The key strength of this review is the comprehensive analysis of the burden of LGS in several areas, from the perspective of individuals and caregivers. Data were retrieved for all planned domains of burden in LGS, with the exception of the impact of the COVID-19 pandemic on patients and caregivers. The present review also identified areas in which the burden of LGS could be reduced, including development of treatments targeting nonseizure symptoms. Limitations include issues with sample representativeness, disease and outcomes measures, and the clarity of the reporting in some articles. Another limitation of this review is the exclusion of randomized controlled trials from the searches, which were excluded to ensure that the review would provide an overview of the burden of LGS in a real-world setting. Randomized controlled trials could provide more representative samples of patients based on clear diagnostic criteria, as well as a greater insight into the AEs experienced by individuals with LGS, because AEs are usually more thoroughly assessed in these studies.
Future studies should aim to include representative samples of the LGS population and to use clearly defined selection criteria that are generalizable across all individuals with LGS. In addition, use of updated diagnostic criteria would allow accurate identification of individuals with LGS.
5 CONCLUSIONS
LGS is associated with a high burden across several interlinked domains, with factors such as the clinical symptom burden and incidence of nonseizure symptoms and comorbidities resulting in both low QoL for individuals with LGS and considerable burden on caregivers. Key areas for further research are development of treatments that address nonseizure symptoms and overcome the pharmacoresistance commonly observed in LGS, development of a disease-specific tool for measuring QoL of individuals with LGS, and comprehensive assessment of the effect of LGS on caregiver work productivity. Additionally, standardization of LGS definitions and recognition, clear assessment of nonseizure symptoms, and harmonization of high-quality study methods across studies are needed to address the quality concerns identified in this review.
AUTHOR CONTRIBUTIONS
All authors contributed to the study conception and design, interpreted the results, contributed to the writing of the manuscript, and approved the final version of the manuscript for submission.
ACKNOWLEDGMENTS
This review was funded by the sponsor, Takeda Pharmaceutical Company. Under the direction of the authors and funded by Takeda Pharmaceutical Company, William Luderman, PhD, Erin Aldera, PhD, and Adam Hargreaves, PhD of Oxford PharmaGenesis, Oxford, UK, provided writing assistance for this publication, in accordance with current Good Publication Practice guidelines. Editorial assistance in formatting, proofreading, copyediting, and fact-checking was also provided by Oxford PharmaGenesis.
CONFLICT OF INTEREST STATEMENT
A.B., J.S.A., and D.S. are employees of Takeda Pharmaceutical Company and own stock or stock options. J.R. is an employee of Novo Nordisk Healthcare and a former employee of Takeda Pharmaceutical Company. A.J. is an employee of Oxford PharmaGenesis, Oxford, UK. E.B. is a former employee of Oxford PharmaGenesis, Oxford, UK. The following authors have received compensation for serving as consultants or speakers, or they or the institutions they work for have received research support or royalties from the companies or organizations indicated: J.H.C. (Biocodex, Engineering and Physical Sciences Research Council [UK], Epilepsy Research UK, Great Ormond Street Hospital Children's Charity, GW Pharmaceuticals/Jazz Pharmaceuticals, Marinus Pharmaceuticals, National Institute for Health and Care Research, National Institute of Health Research Biomedical Research Centre at Great Ormond Street Hospital, Nutricia, Ovid Therapeutics, Stoke Therapeutics, UCL Great Ormond Street Institute of Child Health, Ultragenyx, Vitaflo, Waterloo Foundation, and Zogenix/UCB) and J.S. (BioPharm Solutions, Bright Minds, CAMP4 Therapeutics, Dravet Syndrome Foundation, Encoded Therapeutics, Epilepsy Study Consortium, Epygenix Therapeutics, Greenwich Biosciences, Longboard Pharmaceuticals, Marinus Pharmaceuticals, Neurocrine Biosciences, PCDH19 Alliance, Praxis, Stoke Therapeutics, Takeda, Xenon Pharmaceuticals, and Zogenix). We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data from the original articles are included in this review, and citations are provided to indicate the original source for all information.




