Association of antiseizure medications and adverse cardiovascular events: A global health federated network analysis
Anthony Marson and Gregory Y. H. Lip are joint senior authors.
Abstract
Objective
A diagnosis of epilepsy has been associated with adverse cardiovascular events (CEs), but the extent to which antiseizure medications (ASMs) may contribute to this is not well understood. The aim of this study was to compare the risk of adverse CEs associated with ASM in patients with epilepsy (PWE).
Methods
A retrospective case–control cohort study was conducted using TriNetX, a global health federated network of anonymized patient records. Patients older than 18 years, with a diagnosis of epilepsy (International Classification of Diseases, 10th Revision code G40) and a medication code of carbamazepine, lamotrigine, or valproate were compared. Patients with cardiovascular disease prior to the diagnosis of epilepsy were excluded. Cohorts were 1:1 propensity score matched (PSM) according to age, sex, ethnicity, hypertension, heart failure, atherosclerotic heart disease, atrial and cardiac arrythmias, diabetes, disorders of lipoprotein metabolism, obesity, schizophrenia and bipolar disorder, medications, and epilepsy classification. The primary outcome was a composite of adverse CEs (ischemic stroke, acute ischemic heart disease, and heart failure) at 10 years. Cox regression analyses were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) following 1:1 PSM.
Results
Of 374 950 PWE included; three cohorts were established after PSM: (1) carbamazepine compared to lamotrigine, n = 4722, mean age 37.4 years; (2) valproate compared to lamotrigine, n = 5478, mean age 33.9 years; and (3) valproate compared to carbamazepine, n = 4544, mean age 37.0 years. Carbamazepine and valproate use were associated with significantly higher risk of composite cardiovascular outcome compared to lamotrigine (HR = 1.390, 95% CI = 1.160–1.665 and HR = 1.264, 95% CI = 1.050–1.521, respectively). Valproate was associated with a 10-year higher risk of all-cause death than carbamazepine (HR = 1.226, 95% CI = 1.017–1.478), but risk of other events was not significantly different.
Significance
Carbamazepine and valproate were associated with increased CE risks compared to lamotrigine. Cardiovascular risk factor monitoring and careful follow-up should be considered for these patients.
Key points
- Lamotrigine was associated with reduced risk of adverse cardiovascular outcomes compared to carbamazepine and valproate
- Carbamazepine and valproate demonstrated similar cardiovascular event risk
- Prescribers should consider the impact of antiseizure medication choice on cardiovascular health in patients with epilepsy
1 INTRODUCTION
People with epilepsy (PWE) have an increased risk of cardiovascular disease.1-4 There are several mechanisms underlying this relationship, including the causal etiology of epilepsy, direct seizure-related effects on the myocardium,5 poorer lifestyle behaviors,6 and the association of epilepsy with deprivation.7 The long-term implications of antiseizure medications (ASMs) on cardiovascular health are not well understood. In this population of patients where there is high prevalent8, 9 and incident2 cardiovascular events (CEs), knowledge of ASM-associated cardiovascular risks may influence choice and duration of treatment and monitoring.
An international federation of electronic health data was used to compare incident CEs in adult PWE taking lamotrigine, carbamazepine, and sodium valproate (valproate). These medications have been associated with elevated risk of arrythmias (lamotrigine);10 hyperlipidemia,11, 12 and heart failure (carbamazpine);13 and elevated triglycerides and hyperinsulinemia (valproate),14 although evidence of the long-term impact of these on cardiovascular-related events is limited. We sought to determine how adverse CEs compare between ASMs.
2 MATERIALS AND METHODS
2.1 Study design and setting
A retrospective cohort study was undertaken using TriNetX (https://trinetx.com),15, 16 a global research network of electronic health data from ~250 million patients from >120 health care organizations (HCOs) across 19 countries predominantly in North America but also in South America, Europe, Middle East, Africa, and Asia.15, 16
2.2 Participants
Searches of the TriNetX dataset were undertaken on August 2, 2023. We identified people older than 18 years, and a subgroup of people older than 50 years, with a coded diagnosis of epilepsy (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code G40). We recruited those with at least one G40 code recorded within a January 1, 2000 to January 1, 2015 recruitment period, restricting study entry to those who also had at least two prescription codes for carbamazepine, lamotrigine, or valproate within 6 months of the first G40 code to appear within the recruitment period. The latter age structure was chosen to allow focused assessment of those at highest risk of adverse CEs.
Patients with diagnoses of cardiovascular diseases before their epilepsy diagnosis were excluded. This included ischemic heart diseases (ICD-10-CM I20–I25), atrial fibrillation and flutter (ICD-10-CM I48), ventricular fibrillation and flutter (ICD-10-CM 149.0), other cardiac arrythmia (ICD-10-CM I49), cardiac arrest (ICD-10-CM I46), ventricular tachycardia (ICD-10-CM I47.2), acute ischemic stroke (ICD-10-CM I63), hemorrhagic intraparenchymal or intraventricular stroke (ICD-10-CM I61), and heart failure (ICD-10-CM I50). For each comparator group (carbamazepine, lamotrigine, and valproate), people coded as taking the opposing ASM at any time during the time period under observation were excluded (e.g., when comparing carbamazepine to lamotrigine, the groups were formed of carbamazepine users excluding those with any medication code for lamotrigine, and vice versa).
2.3 Variables
Groups were 1:1 propensity score matched (PSM) according to age, sex, ethnicity, hypertension, diabetes, disorders of lipoprotein metabolism, obesity, schizophrenia and bipolar disorder, cardiovascular medications, antilipemic agents, ASMs (excluding the comparator medications), and epilepsy classification. As small numbers of patients experienced new CEs between their first diagnostic code of epilepsy and ASM treatment codes, we also matched for heart failure, atherosclerotic heart disease, and atrial and other cardiac arrythmias, to ensure at baseline cohorts were well matched. A full list of codes used in the analysis can be found in the supplementary information Tables S1–S3. Cohorts were deemed to be well matched if the standard difference was <.1.
The observation period for each participant began 1 day after the first co-occurrence of G40 code with ASM of interest. Outcomes were assessed within 10 years of the start of this observation period and in a subgroup analysis, outcomes at 5 years were reviewed.
Our primary outcome was a composite of acute adverse CEs (acute ischemic heart disease including acute myocardial infarction and unstable angina, acute ischemic stroke, and heart failure). Our secondary outcomes were individual incident adverse CEs of heart failure, myocardial infarction, atrial fibrillation or flutter, ventricular tachycardia or fibrillation/flutter, acute ischemic stroke, hemorrhagic intraparenchymal or intraventricular stroke, angina (including chronic and unstable angina), and cardiac arrest. We also reviewed the rates of hyperlipidemia, obesity, and all-cause death.
2.4 Statistical analysis
Cox proportional hazard regression models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Kaplan–Meier plots for 10-year composite outcome were produced and displayed in the results. Analyses were performed within the TriNetX platform, which uses R's survival package (v3.2-3).
As this was a retrospective electronic health care data analysis, there were no missing data.
3 RESULTS
A total of 374 950 adult PWE were included for study. We studied three cohorts of PWE. After propensity score matching, for the carbamazepine compared to lamotrigine analysis, n = 4722, mean age 37.4, 51.2% male; for the valproate compared to lamotrigine analysis, n = 5478, mean age 33.9, 54.1% male; and for the valproate compared to carbamazepine analysis, n = 4544, mean age 37.0, 52.9% male (see Table 1).
| Group 1. Carbamazepine compared to lamotrigine over 18 years | Subgroup 1. Carbamazepine compared to lamotrigine over 50 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Patients (N) | % of cohort | p-Value | Std diff. | Mean ± SD | Patients (N) | % of cohort | p-Value | Std diff. | ||
| CBZ | Age at index | 37.4 ± 18.9 | 2361 | 100% | 0.989 | <0.001 | 53.3 ± 10.8 | 1169 | 100% | 0.845 | 0.008 |
| LTG | 37.4 ± 18.6 | 2361 | 100% | 53.4 ± 10.9 | 1169 | 100% | |||||
| CBZ | White | 1579 | 66.9% | 0.622 | 0.014 | 837 | 71.6% | 0.648 | 0.019 | ||
| LTG | 1563 | 66.2% | 827 | 70.7% | |||||||
| CBZ | Black or African American | 288 | 12.2% | 0.894 | 0.004 | 121 | 10.4% | 0.732 | 0.014 | ||
| LTG | 291 | 12.3% | 116 | 9.9% | |||||||
| CBZ | Male | 1206 | 51.1% | 0.884 | 0.004 | 537 | 45.9% | 0.967 | 0.002 | ||
| LTG | 1211 | 51.3% | 538 | 46.0% | |||||||
| Group 2. Valproate compared to lamotrigine over 18 years | Subgroup 2. Valproate compared to lamotrigine over 50 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Patients (N) | % of cohort | p-Value | Std diff. | Mean ± SD | Patients | % of cohort | p-Value | Std diff. | ||
| VAL | Age at index | 33.8 ± 19.4 | 2739 | 100% | 0.738 | 0.009 | 53.6 ± 11.1 | 1102 | 100% | 0.307 | 0.044 |
| LTG | 34.0 ± 18.1 | 2739 | 100% | 53.1 ± 11.0 | 1102 | 100% | |||||
| VAL | White | 1865 | 68.1% | 0.794 | 0.007 | 749 | 68.0% | 0.750 | 0.014 | ||
| LTG | 1874 | 68.4% | 742 | 67.3% | |||||||
| VAL | Black or African American | 322 | 11.8% | 0.582 | 0.015 | 129 | 11.7% | 0.694 | 0.017 | ||
| LTG | 309 | 11.3% | 135 | 12.3% | |||||||
| VAL | Male | 1475 | 53.9% | 0.766 | 0.008 | 553 | 50.2% | 0.609 | 0.022 | ||
| LTG | 1486 | 54.3% | 541 | 49.1% | |||||||
| Group 3. Valproate compared to carbamazepine over 18 years | Subgroup 3. Valproate compared to carbamazepine over 50 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Patients (N) | % of cohort | p-Value | Std diff. | Mean ± SD | Patients (N) | % of cohort | p-Value | Std diff. | ||
| VAL | Age at index | 37.0 ± 19.2 | 2272 | 100% | 0.816 | 0.007 | 53.3 ± 10.9 | 1081 | 100% | 0.666 | 0.019 |
| CBZ | 36.9 ± 18.4 | 2272 | 100% | 53.1 ± 11.0 | 1081 | 100% | |||||
| VAL | White | 1490 | 65.6% | 0.950 | 0.002 | 729 | 67.4% | 0.927 | 0.004 | ||
| CBZ | 1492 | 65.7% | 727 | 67.3% | |||||||
| VAL | Black or African American | 294 | 12.9% | 1 | <0.001 | 127 | 11.7% | 0.471 | 0.031 | ||
| CBZ | 294 | 12.9% | 138 | 12.8% | |||||||
| VAL | Male | 1202 | 52.9% | 1 | <0.001 | 553 | 51.2% | 0.667 | 0.019 | ||
| CBZ | 1202 | 52.9% | 563 | 52.1% | |||||||
- Abbreviations: CBZ, carbamazepine; LTG, lamotrigine; Std diff., standardized difference; VAL, valproate.
Additional information on the cohorts before and after propensity score matching can be found in the supplementary material (Tables S1 and S2). After propensity score matching, all cohorts were well balanced, with standard differences < .1.
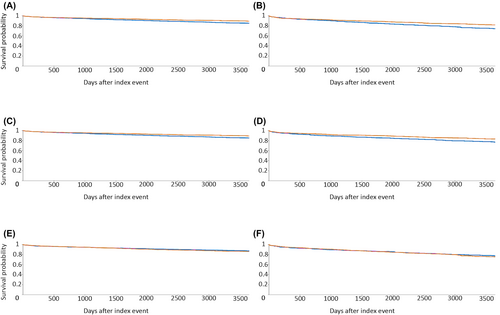
Results for the carbamazepine and lamotrigine comparisons are outlined in Table 2. Kaplan–Meier curves of composite adverse CEs up to 10-year follow-up for patients >18 years of age and the subgroup >50 years of age are depicted in Figure 1. Compared to lamotrigine, carbamazepine was associated with significantly higher risk of composite adverse CEs, with an HR of 1.351 (95% CI = 1.086–1.682) and 1.390 (95% CI = 1.160–1.665) in patients >18 years old up to 5 and 10 years of follow-up, respectively. This was also statistically significant in the age > 50 years subgroup at 10 years of follow-up (HR = 1.405, 95% CI = 1.155–1.708). Risk of heart failure was significantly higher in users of carbamazepine at 5 years (HR = 1.453, 95% CI = 1.051–2.008) and 10 years of follow-up (HR = 1.316, 95% CI = 1.02–1.698). Risk of ischemic stroke was significantly higher in the 10-year follow-up groups for patients aged >18 years and the subgroup aged >50 years (HR = 1.482, 95% CI = 1.144–1.919 and HR = 1.494, 95% CI = 1.21–1.990, respectively). We found no increased risk in all-cause death in patients on carbamazepine compared to lamotrigine.
| CBZ vs. LTG, patients >18 years old (5 years of follow-up) | CBZ vs. LTG, patients >18 years old (10 years of follow-up) | CBZ vs. LTG, patients >50 years old (10 years of follow-up) | |||||
|---|---|---|---|---|---|---|---|
| CBZ | LTG | CBZ | LTG | CBZ | LTG | ||
| n | 2361 | 2361 | 2361 | 2361 | 1169 | 1169 | |
| Age, years, mean ± SD | 37.4 ± 18.9 | 37.4 ± 18.6 | 37.4 ± 18.9 | 37.4 ± 18.6 | 53.3 ± 10.8 | 53.4 ± 10.9 | |
| Composite outcome | n | 189 (8.0%) | 140 (5.9%) | 280 (11.9%) | 202 (8.6%) | 239 (20.4%) | 173 (14.8%) |
| HR (95% CI) | 1.351 (1.086–1.682) p = .007 | 1.390 (1.160–1.665) p = .000 | 1.405 (1.155–1.708) p = .001 | ||||
| Cerebral infarction | n | 95 (4.0%) | 73 (3.1%) | 143 (6.1%) | 96 (4.1%) | 116 (9.9%) | 78 (6.7%) |
| HR (95% CI) | 1.297 (.956–1.760) p = .093 | 1.482 (1.144–1.919) p = .003 | 1.494 (1.21–1.990) p = .006 | ||||
| Nontraumatic ICH | n | 18 (.8%) | 25 (1.1%) | 29(1.2%) | 37 (1.6%) | 16 (1.4%) | 29 (2.5%) |
| HR (95% CI) | .716 (.391–1.312) p = .827 | .774 (.476–1.259) p = .302 | .546 (.296–1.004) p = .048 | ||||
| Acute ischemic heart disease | n | 36 (1.5%) | 22 (.9%) | 64 (2.7%) | 44 (1.9%) | 56 (4.7%) | 45 (3.8%) |
| HR (95% CI) | 1.630 (.959–2.770) p = .068 | 1.436 (.978–2.108) p = .063 | 1.236 (.835–1.830) p = .289 | ||||
| Angina | n | 32 (1.4%) | 23 (1.0%) | 51 (2.2%) | 31 (1.3%) | 46 (3.9%) | 35 (3.0) |
| HR (95% CI) | 1.387 (.812–2.370) p = .229 | 1.633 (1.045–2.551) p = .030 | 1.307 (.842–2.028) p = .232 | ||||
| AF/flutter | n | 46 (1.9%) | 48 (2.0%) | 79 (3.3%) | 70 (3.0%) | 78 (6.7%) | 65 (5.6%) |
| HR (95% CI) | .954 (.636–1.429) p = .818 | 1.115 (.808–1.538) p = .508 | 1.200 (.963–1.668) p = .277 | ||||
| VF/VT | n | 10 (.4%) | 12 (.5%) | 21 (.9%) | 21 (.9%) | 18 (1.5%) | 16 (1.4%) |
| HR (95% CI) | .830 (.359–1.921) p = .663 | .989 (.540–1.810) p = .971 | 1.211 (.572–2.198) p = .740 | ||||
| Heart failure | n | 90 (3.8%) | 62 (2.6%) | 137 (5.8%) | 104 (4.4%) | 126 (10.8%) | 90 (7.7%) |
| HR (95% CI) | 1.453 (1.051–2.008) p = .023 | 1.316 (1.020–1.698) p = .034 | 1.417 (1.081–1.857) p = .011 | ||||
| Cardiac arrest | n | 10 (.4%) | 10 (.4%) | 22 (.9%) | 14 (.6%) | 13 (1.1%) | 10 (.9%) |
| HR (95% CI) | 1.243 (.491–3.151) p = .057 | 1.548 (.792–3.025) p = .198 | 1.292 (.567–2.947) p = .541 | ||||
| Hyperlipidemia | n | 497 (21.1%) | 368 (15.6%) | 607 (25.7%) | 478 (20.2%) | 488 (4.17%) | 405 (3.46%) |
| HR (95% CI) | 1.395 (1.219–1.596) p = .00 | 1.324 (1.174–1.492) p = .000 | 1.292 (1.132–1.474) p = .000 | ||||
| Obesity | n | 330 (14%) | 274 (11.6%) | 426 (18.0%) | 328 (15.6%) | 249 (21.3%) | 215 (18.4%) |
| HR (95% CI) | 1.214 (1.035–1.425) p = .017 | 1.173 (1.020–1.348) p = .025 | 1.185 (.987–1.422) p = .068 | ||||
| All-cause death | n | 108 (4.6%) | 118 (5.0%) | 195 (8.3%) | 198 (8.4%) | 158 (13.5%) | 146 (12.5%) |
| HR (95% CI) | .909 (.700–1.180) p = .474 | .967 (.794–1.179) p =.740 | 1.076 (.859–1.348) p = .522 | ||||
- Note: Bold denotes significant results.
- Abbreviations: AF, atrial fibrillation; CBZ, carbamazepine; CI, confidence interval; HR, hazard ratio; ICH, intracerebral hemorrhage; LTG, lamotrigine; VF/VT, ventricular fibrillation/tachycardia.
Results for the valproate and lamotrigine comparison are outlined in Table 3. Compared to lamotrigine, valproate was associated with higher risk of composite adverse cardiovascular outcome in patients aged >18 years at 5- and 10-year follow-up (HR = 1.271, 95% CI = 1.018–1.586 and HR = 1.264, 95% CI = 1.050–1.521, respectively). This was also statistically significant in the subgroup aged >50 years (HR = 1.399, 95% CI = 1.131–1.729, respectively). There was a significant increased risk of atrial fibrillation/flutter in patients treated with valproate (HR = 1.576, 95% CI = 1.157–2.146). Risks of acute ischemic stroke and all-cause death were higher in the valproate group in patients aged >18 years up to 5-year follow-up (HR = 1.380, 95% CI = 1.013–1.880) and in the subgroup analysis of patients aged >50 years at 10-year follow-up (HR = 1.399, 95% CI = 1.131–1.729). Risk of acute ischemic heart disease events were higher in the valproate cohort compared with lamotrigine up to 5 years of follow-up. Risk of heart failure was significantly higher in users of valproate compared to lamotrigine in patients >50 years of age at 10 years of follow-up (HR = 1.392, 95% CI = 1.025–1.890).
| VAL vs. LTG, patients >18 years old (5 years of follow-up) | VAL vs. LTG, patients >18 years old (10 years of follow-up) | VAL vs. LTG, patients >50 years old (10 years of follow-up) | |||||
|---|---|---|---|---|---|---|---|
| VAL | LTG | VAL | LTG | VAL | LTG | ||
| n | 2739 | 2739 | 2739 | 2739 | 1102 | 1102 | |
| Mean age ± SD | 33.8 ± 19.4 | 34.0 ± 18.1 | 33.8 ± 19.4 | 34.0 ± 18.1 | 53.6 ± 11.1 | 53.1 ± 11.0 | |
| Composite outcome | n | 175 (6.4%) | 142 (5.2%) | 248 (9.1%) | 205 (7.5%) | 196 (17.8%) | 152 (13.8%) |
| HR (95% CI) | 1.271 (1.018–1.586) p = .033 | 1.264 (1.050–1.521) p = .013 | 1.399 (1.131–1.729) p = .002 | ||||
| Cerebral infarction | n | 94 (3.4%) | 70 (2.6%) | 120 (4.4%) | 99 (3.6%) | 93 (8.4%) | 72 (6.5%) |
| HR (95% CI) | 1.380 (1.013–1.880) p = .041 | 1.258 (.964–1.641) p = .091 | 1.379 (1.013–1.876) p = .040 | ||||
| Nontraumatic ICH | n | 27 (1.0%) | 25 (.9%) | 34 (1.2%) | 38 (1.4%) | 27 (2.5%) | 22 (2.0%) |
| HR (95% CI) | 1.100 (.638–1.895) p = .732 | .922 (.580–1.464) p = .730 | 1.287 (.733–2.259) p = .379 | ||||
| Acute ischemic heart disease | n | 32 (1.2%) | 17 (.6%) | 51 (1.9%) | 38 (1.4%) | 45 (4.1%) | 34 (3.1%) |
| HR (95% CI) | 1.942 (1.079–3.498) p = .024 | 1.404 (.923–2.137) p = .111 | 1.439 (.922–2.247) p = .107 | ||||
| Angina | n | 22 (.8%) | 20 (.7%) | 33 (1.2%) | 32 (1.2%) | 31 (2.8%) | 32 (2.9%) |
| HR (95% CI) | 1.133 (.619–2.077) p = .685 | 1.074 (.660–1.746) p = .775 | 1.029 (.628–1.687) p = .909 | ||||
| AF/flutter | n | 69 (2.5%) | 44 (1.6%) | 101 (3.7%) | 67 (2.4%) | 78 (7.1%) | 59 (5.4%) |
| HR (95% CI) | 1.619 (1.109–2.363) p = .012 | 1.576 (1.157–2.146) p = .004 | 1.416 (1.009–1.986) p = .043 | ||||
| VF/VT | n | 16 (.6%) | 12 (.4%) | 26 (.9%) | 25 (.9%) | 20 (1.8%) | 15 (1.4%) |
| HR (95% CI) | 1.363 (.645–2.881) p = .416 | 1.081 (.624–1.872) p = .780 | 1.439 (.737–2.812) p = .284 | ||||
| Heart failure | n | 73 (2.7%) | 68 (2.5%) | 117 (4.3%) | 102 (3.7%) | 94 (8.5%) | 73 (6.6%) |
| HR (95% CI) | 1.107 (.795–1.540) p = .548 | 1.198 (.919–1.563) p = .181 | 1.392 (1.025–1.890) p = .033 | ||||
| Cardiac arrest | n | 18 (.007) | 11 (.004) | 33 (1.2%) | 16 (.6%) | 16 (1.5%) | 10 (.9%) |
| HR (95% CI) | 1.685 (.796–3.567) p = .168 | 2.153 (1.185–3.911) p = .010 | 2.149 (.919–5.023) p = .070 | ||||
| Hyperlipidemia | n | 443 (16.2%) | 393 (14.3%) | 562 (20.5%) | 493 (18.0%) | 412 (37.4%) | 382 (34.7%) |
| HR (95% CI) | 1.165 (1.017–1.335) p = .027 | 1.195 (1.059–1.348) p = .004 | 1.167 (1.015–1.341) p = .030 | ||||
| Obesity | n | 347 (12.7%) | 322 (11.8%) | 457 (16.7%) | 441 (16.1%) | 228 (20.7%) | 216 (19.6%) |
| HR (95% CI) | 1.119 (.961–1.302) p = .147 | 1.089 (.956–1.242) p = .200 | 1.155 (.959–1.392) p = .128 | ||||
| All-cause death | n | 164 (6.0%) | 140 (5.1%) | 260 (9.5%) | 217 (7.9%) | 187 (17.0%) | 146 (13.2%) |
| HR (95% CI) | 1.213 (.968–1.519) p = .093 | 1.252 (1.045–1.499) p = .014 | 1.386 (1.117–1.722) p = .003 | ||||
- Note: Bold denotes significant results.
- Abbreviations: AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; ICH, intracerebral hemorrhage; LTG, lamotrigine; VAL, sodium valproate; VF/VT, ventricular fibrillation/tachycardia.
Results of the valproate and carbamazepine comparison are summarized in Table 4. Compared to carbamazepine, valproate was associated with increased risk of all-cause death in all groups >18 years of age with 10 years of follow-up (HR = 1.226, 95% CI = 1.017–1.478), but risk of other CEs was comparable.
| VAL vs. CBZ, patients >18 years old (5 years of follow-up) | VAL vs. CBZ, patients >18 years old (10 years of follow-up) | VAL vs. CBZ, patients >50 years old (10 years of follow-up) | |||||
|---|---|---|---|---|---|---|---|
| VAL | CBZ | VAL | CBZ | VAL | CBZ | ||
| n | 2272 | 2272 | 2272 | 2272 | 1081 | 1081 | |
| Mean age ± SD | 37.0 ± 19.2 | 36.9 ± 18.4 | 37.0 ± 19.2 | 36.9 ± 18.4 | 53.3 ± 10.9 | 53.1 ± 11.0 | |
| Composite outcome | n | 164 (7.2%) | 180 (7.9%) | 227 (10.0%) | 256 (11.3%) | 189 (17.5%) | 213 (19.7%) |
| HR (95% CI) | .931 (.753–1.150) p = .507 | .918 (.768–1.098) p = .348 | .945 (.777–1.150) p = .572 | ||||
| Cerebral infarction | n | 81 (3.6%) | 92 (4.0%) | 107 (4.7%) | 133 (5.9%) | 88 (8.1%) | 111 (10.3%) |
| HR (95% CI) | .900 (.668–1.213) p = .489 | .835 (.647–1.077) p = .163 | .843 (.637–1.115) p = .231 | ||||
| Nontraumatic ICH | n | 26 (1.1%) | 23 (1.0%) | 32 (1.4%) | 34 (1.5%) | 22 (2.0%) | 19 (1.8%) |
| HR (95% CI) | 1.153 (.658–2.020) p = .619 | .976 (.602–1.582) p = .923 | 1.233 (.667–2.278) p = .503 | ||||
| Acute ischemic heart disease | n | 34 (1.5%) | 37 (1.6%) | 53 (2.3%) | 55 (2.4%) | 46 (4.3%) | 50 (4.6%) |
| HR (95% CI) | .945 (.593–1.506) p = .812 | 1.009 (.692–1.471) p = .965 | .991 (.664–1.480) p = .966 | ||||
| Angina | n | 31 (1.4%) | 27 (1.2%) | 39 (1.7%) | 41 (1.8%) | 27 (2.5%) | 36 (3.3%) |
| HR (95% CI) | 1.178 (.703–1.973) p = .533 | .990 (.639–1.535) p = .964 | .794 (.482–1.309) p = .365 | ||||
| AF/flutter | n | 70 (3.1%) | 50 (2.2%) | 97 (4.3%) | 78 (3.4%) | 81 (7.5%) | 75 (6.9%) |
| HR (95% CI) | 1.438 (1.000–2.067) p = .049 | 1.307 (.970–1.761) | 1.153 (.842–1.579) p = .373 | ||||
| VF/VT | n | 16 (.7%) | 10 (.4%) | 24 (1.1%) | 15 (.7%) | 23 (2.1%) | 16 (1.5%) |
| HR (95% CI) | 2.047 (.876–4.783) p = .091 | 1.680 (.881–3.202) p = .111 | 1.543 (.815–2.922) p = .179 | ||||
| Heart failure | n | 79 (3.5%) | 79 (3.5%) | 112 (4.9%) | 124 (5.5%) | 95 (8.8%) | 105 (9.7%) |
| HR (95% CI) | 1.025 (.751–1.400) p = .875 | .942 (.730–1.216) p = .646 | .968 (.733–1.278) p = .818 | ||||
| Cardiac arrest | n | 19 (.8%) | 11 (.5%) | 30 (1.3%) | 24 (1.1%) | 19 (1.8%) | 14 (1.3%) |
| HR (95% CI) | 1.781 (.848–3.743) p = .122 | 1.325 (.775–2.267) p = .302 | 1.490 (.747–2.972) p = .255 | ||||
| Hyperlipidemia | n | 403 (17.7%) | 470 (20.7%) | 506 (22.3%) | 577 (25.4%) | 405 (37.5%) | 450 (41.6%) |
| HR (95% CI) | .855 (.749–.977) p = .021 | .880 (.781–.991) p = .035 | .902 (.789–1.032) p = .132 | ||||
| Obesity | n | 298 (13.1%) | 328 (14.4%) | 376 (16.5%) | 429 (18.9%) | 221 (20.4%) | 238 (22.0%) |
| HR (95% CI) | .928 (.793–1.085) p = .348 | .904 (.787–1.038) p = .152 | .986 (.821–1.184) p = .876 | ||||
| All-cause death | n | 150 (6.6%) | 119 (5.2%) | 238 (10.5%) | 205 (9.0%) | 179 (16.6%) | 146 (13.5%) |
| HR (95% CI) | 1.302 (1.024–1.656) p = .031 | 1.226 (1.017–1.478) p = .032 | 1.334 (1.072–1.660) p = .010 | ||||
- Note: Bold denotes significant results.
- Abbreviations: AF, atrial fibrillation; CBZ, carbamazepine; CI, confidence interval; HR, hazard ratio; ICH, intracerebral hemorrhage; VAL, sodium valproate; VF/VT, ventricular fibrillation/tachycardia.
Carbamazepine was associated with higher risk of hyperlipidemia compared to lamotrigine and compared to valproate in patients >18 years of age at 10 years of follow-up (HR = 1.324, 95% CI = 1.174–1.492 and HR = .880, 95% CI = .781–.991, respectively). There was higher risk of overweight/obesity in users of carbamazepine compared to lamotrigine in patients >18 years of age at 10 years of follow-up (HR = 1.173, 95% CI = 1.020–1.348), whereas a comparison between carbamazepine and valproate did not demonstrate any significant differences in overweight/obesity (HR = .904, 95% CI = .787–1.038).
4 DISCUSSION
This study demonstrates the following principal findings in PWE: (1) lamotrigine use was associated with fewer incident CEs compared with valproate or carbamazepine, (2) valproate and carbamazepine had comparable cardiovascular risks, and (3) valproate was associated with higher risk of all-cause death compared to lamotrigine or carbamazepine. To our knowledge, this is the largest study comparing CEs on individual ASMs in PWE.
In this study, the focus was on patients without known preexisting cardiovascular disease, as there is prior evidence that in patients with preexisting vascular risk, older generation ASMs such as carbamazepine and valproate are associated with greater cardiovascular disease and mortality.17, 18 For patients with poststroke epilepsy on monotherapy, Larsson et al.17 identified a reduced risk of cardiovascular death with lamotrigine compared to carbamazepine (adjusted HR = .76, 95% CI = .61–.95) and an increased risk of cardiovascular death with valproate compared to carbamazepine (adjusted HR = 1.40, 95% CI = 1.19–1.64). In a Danish registry study of PWE and heart failure, valproate was associated with greater heart failure mortality compared to levetiracetam or lamotrigine (HR = 2.39, 95% CI = 1.02–5.60).18 We also identified a higher all-cause death in users of valproate compared to carbamazepine and lamotrigine, but we were unable to determine cause of death to stratify cardiovascular deaths. In a case–control study of sudden cardiac death (SCD), SCD was associated with current sodium channel-blocking ASM use in PWE.19 In a study of sudden deaths in Finland, ASM use during an acute coronary event for both epilepsy and nonepilepsy indications was associated with an increased risk of SCD.20
Two studies using large UK-based datasets concluded different results when comparing enzyme-inducing ASM (EIASM) to non-EIASMs. Josephson et al.21 demonstrated a significant increased risk of incident composite cardiovascular outcomes in a period-prevalent epilepsy cohort taking EIASMs compared to non-EIASMs (adjusted HR = 1.21, 95% CI = 1.08–1.39) but nonsignificant increases in cardiovascular outcomes in an incident epilepsy cohort. In the Welsh Secure Anonymised Information Linkage (SAIL) dataset analysis, Lee-Lane et al.22 did not identify statistical differences in cardiovascular outcomes between PWE on EIASMs compared to non-EIASMs. The differences described may in part be attributed to a shorter follow-up time in the SAIL study,22 but grouping medications for analysis may have attenuated associations identified with individual ASMs, as seen in this study.
In vitro data have suggested that lamotrigine may exhibit class 1B antiarrhythmic properties, thus raising concerns of an increased risk of arrythmia in patients with premorbid cardiac disease.23, 24 Although our study excluded patients with prior cardiovascular disorders, there was no indication that there was an increased risk of arrythmias in users of lamotrigine compared to carbamazepine or valproate. This supports other work that has failed to determine lamotrigine to be associated with cardiac conduction disorders in populations with23 and without comorbid cardiac disease.24, 25 Christensen et al.24 examined 91 949 new users of lamotrigine in a population-based cohort study and found no increased risk of cardiac conduction disorders in patients with and without cardiac morbidity, with a risk of new arrythmia compared to past users of lamotrigine of HR = 1.03 (95% CI = .76–1.40).24 In a pharmacovigilance study, Aboukaoud et al.26 failed to identify a significant increase in the reports of arrythmias in PWE using lamotrigine. A high risk of cardiac arrest was seen in psychiatric patients prescribed lamotrigine, but this was confounded by overdose, suicide attempts, and coadministration of other arrhythmogenic medications.26
This study outlines important associations but does not prove causation. Despite differing mechanisms of action, both carbamazepine and valproate, but not lamotrigine, have been associated with increased common carotid intima media thickness correlated with duration of ASM therapy.27 In this study, we demonstrated higher rates of lipidemia disorders in the carbamazepine group, which may infer factors underlying the mechanisms for elevated rates of atherosclerosis-related CEs. These findings support historical reports of carbamazepine-related rises in lipids, including total cholesterol, high-density lipoprotein and low-density lipoprotein concentration in both the short and long term,11, 28, 29 which are reversible on switching to a nonEIASM.11 Hyperlipidemia may be the result of both direct drug-related effects and drug–drug interactions that may attenuate lipid-lowering therapies.11, 30, 31 Despite the established associations of carbamazepine, lipid elevation, and problematic interactions with statin therapy attenuating lipid-lowering effects being well reported,31, 32 there is no existing guidance on how to address this in clinical practice. In incident epilepsy, initial ASM choice accounting for vascular risk may be easily accommodated, but in patients for whom changing ASM could lead to deterioration in seizure control, guidance is urgently needed to manage complications such as hyperlipidemia.
The strengths of this study include the large cohort numbers after propensity score matching. Our case-ascertainment strategy combining diagnostic epilepsy codes with ASM prescription is shown to be an accurate way to identify PWE within electronic health datasets.33 We were able to demonstrate significant trends across all groups. We were able to exclude most patients with prior cardiovascular disease to focus on incident events. Further work should investigate mechanisms behind the increased cardiovascular risk and improving guidance on potentially modifiable factors of antiseizure treatment.
4.1 Limitations
This study utilizes anonymized electronic health care record data. As such, small numbers (<10) are rounded to preserve anonymity, and therefore outcomes with low numbers are less well studied.
Due to the nature of the platform used, we are unable to ascertain granularity of detail with regard to epilepsy etiology and how long patients were taking medication for. Data cannot be checked, and electronic health care record data may be subject to erroneous entries or gaps in data. This study was unable to stratify the study group according to whether patients' epilepsy diagnosis was new within the recruitment period or historic with coded epilepsy diagnosed prior to the recruitment period. This may have been a confounder, as duration of epilepsy is likely to have influenced outcomes. Furthermore, the PSM methods used in TriNetX do not account for data clustering within HCOs; however, as the data are largely derived from the USA, this may attenuate the effect of this limitation. Due to limitations in the data availability of the platform, we cannot extract median follow-up time data from the analysis. There are unknown lifestyle and social factors, such as smoking and access to health care, that may have influenced ASM choice and CEs. Further work is needed to study incident and prevalent epilepsy cohorts separately, and to clarify mechanisms and relationships of social factors relating to ASM use and cardiovascular outcomes.
5 CONCLUSIONS
In PWE, carbamazepine and valproate use was associated with higher risk of incident adverse CEs compared to lamotrigine. Practitioners prescribing ASM or managing PWE should consider the implications different ASMs have on cardiovascular risk. Cardiovascular risk factor monitoring and careful follow-up should be considered for these patients. Health care datasets provide an opportunity to investigate long-term outcomes of patients with the required large sample sizes, and further work should be undertaken to analyze other ASMs and associated cardiovascular morbidity and mortality.
AUTHOR CONTRIBUTIONS
All authors were involved in the design, data acquisition, analysis of the results, and writing of the manuscript for this study.
ACKNOWLEDGMENTS
J.M. receives funding from the Association of British Neurologists, the Stroke Association, and Epilepsy Research Institute UK as part of the Association of British Neurologists Clinical Research Fellowship scheme. G.K.M. is funded by a National Institute for Health Research (NIHR) Clinical Lectureship (CL-2022-07-002). A.M. is an NIHR Senior Investigator and also partly funded by NIHR ARC North West Coast. We thank Oliver O'Neill for his assistance in figure editing. The funders played no role in the design or conduct of this protocol. For the purpose of open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising.
CONFLICT OF INTEREST STATEMENT
A.M. is an NIHR Senior Investigator and also partly funded by NIHR ARC North West Coast. The views expressed in this article are those of the authors and not necessarily those of the NIHR, or the Department of Health and Social Care. G.Y.H.L. is a consultant and speaker for BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi-Sankyo. No fees are directly received personally. G.Y.H.L. is an NIHR Senior Investigator and coprincipal investigator of the AFFIRMO project on multimorbidity in atrial fibrillation, which has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement 899871. None of the other authors has any conflict of interest to disclose.
ETHICS STATEMENT
The TriNetX research network is compliant with the security of health care-related data and protects the privacy and security of deidentified health care data as per the Health Insurance Portability and Accountability Act (HIPAA) and US Federal Law. Data are deidentified as per the deidentification standard that is defined in the HIPAA Privacy Rule. All data used in the study are fully anonymized, and therefore ethical approval or patient consent is not required. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Open Research
DATA AVAILABILITY STATEMENT
Data may be accessed by contacting TriNetX (https://live.trinetx.com); a data-sharing agreement is required.




