Difficulty in identification of patients with active secondary progressive multiple sclerosis by clinical classification tools
Funding information
This study was supported by an unrestricted grant from Novartis Pharma GmbH. Novartis or other commercial entities did not have any influence on any part of the study. The MS Progression Discussion Tool (MSProDiscuss) is funded by Novartis Pharma
Abstract
Background and purpose
The transition from relapsing–remitting to secondary progressive multiple sclerosis (SPMS) is not well defined. Different definitions and tools to identify SPMS have been proposed. Meanwhile, early diagnosis of “active” SPMS is getting progressively more important as pharmaceutical treatment options are developed. In this study, we compared different classification methods regarding their accuracy to reliably identify “active SPMS.”
Methods
Independent from previous diagnostic classification, we descriptively analyzed the disease course (regarding relapses, progression, and magnetic resonance imaging activity) in 208 consecutive multiple sclerosis (MS) patients treated in our MS outpatient clinic in 2018. Patients were reclassified according to different SPMS criteria and tools. Diagnostic accuracy in identifying patients with “active SPMS” was determined.
Results
Comparing the tools to each other, significant variability in the number of patients identified as having SPMS as well as in the proportion of these patients having “active SPMS” was noted. Applying both diagnostic criteria “SPMS” and “active disease” reduced the sensitivity in identifying patients with active progressive disease in all approaches.
Conclusions
We propose lessening the emphasis on the label “SPMS” in favor of the more open term “active progressive disease” to simplify the process of identifying patients who may benefit from immune therapy.
INTRODUCTION
In routine clinical practice, disease phenotype in multiple sclerosis (MS) is classified as either relapsing–remitting (RRMS), primary progressive (PPMS), or secondary progressive (SPMS) [1]. At time of diagnosis, most patients suffer from RRMS, but with time, a significant number develop SPMS with progressive worsening and disability accumulation independent of relapses [2]. This categorization is vitally important for therapeutic decision-making, as the available drugs are approved only for certain disease types. More recently, the label “active MS,” meaning evidence of active inflammation in terms of either new or enlarging lesions on magnetic resonance imaging (MRI) or clinical relapses, has gained significance for all three clinical courses of MS.
At the same time, it has become increasingly evident that labeling MS and particularly the distinction between RRMS and SPMS are oftentimes not as clear-cut as they seem. Especially in the transition phase between RRMS and SPMS, there is an overlap between the two disease courses and classification is arbitrary. In many cases, there still are relapses, but there are also signs of clinical progression, oftentimes viewed as prolonged relapses with insufficient response to steroids at first. In later stages, progression may be the more prominent feature; however, relapses or MRI activity is still present [3]. Even in RRMS, most of the disability accumulation is not caused by relapses but by underlying relapse-independent progression [4].
More options for medical treatment of MS have emerged in recent years [5]. The majority of therapeutics influence relapsing forms of MS. Only recently has medication become available for progressive forms as well, but only as long as disease activity is still present. In the United States and the European Union, siponimod was approved to treat “active SPMS” patients. This label challenges treating physicians, as it requires the appropriate classification of patients as both “active” and “SPMS.” However, a clear definition of SPMS and its distinction from RRMS was always missing, although many attempts have been made, including the one proposed by Lorscheider et al. [6]. Ultimately, based on pathophysiological considerations and the overlap in the clinical course as outlined above, the exact determination of the time point when RRMS transforms into SPMS (i.e., establishing the diagnosis of SPMS) is likely not possible.
The aim of this study was to compare different tools used to classify patients in the transition phase between RRMS and SPMS. We determined whether they are able to reliably identify patients with an active progressive MS for whom medication is still an option.
METHODS
The study was approved by the ethics committee of the University Hospital Frankfurt (20-552). We retrospectively analyzed all patients older than 18 years who were classified as having RRMS during any time in their disease history and who presented to our MS outpatient clinic in 2018. Because the focus was on the transition phase between RRMS and SPMS, only those patients were included who experienced their first symptoms at least 6 months ago. Patients not having a clear clinical diagnosis of MS were excluded.
In a first step—independently from any diagnostic classification obtained so far by previously treating physicians—we retrospectively extracted information on the two key features of active SPMS (i.e., “disease progression” and “disease activity”) from discharge letters and brain imaging, respectively. For doing so, patients were rated as having had “disease progression” if objectifiable (in terms of neurological examination) clinical worsening of symptoms was reported without a relapse over a period of at least 3 months. Clinical worsening had to be confirmed by subsequent neurological examinations (“3-month confirmed disability progression”). A specific Expanded Disability Status Scale (EDSS) step needed to be counted as clinical worsening was not defined. Patients were rated as having had “disease activity” if either at least one relapse or signs of MRI activity (new lesions, lesions gaining in size, or contrast enhancement) occurred in the past 2 years. Patients fulfilling both of the criteria mentioned above were defined as having “active progressive MS.” If time of last relapse or last MRI activity was unknown (n = 49), the patient was grouped as not having had signs of activity within the past 2 years.
In a second step, we collected information on how MS diagnosis was classified for the included patients according to the treating physician at the time of the appointment. Since 2017, important clinical data for each patient treated in our MS outpatient clinic have been summarized in a standardized way at the beginning of each discharge letter [7]. This includes classification of diagnosis into RRMS, SPMS, and PPMS, information on disease activity, EDSS, diagnostic workup, and therapy.
- In 2016, Lorscheider et al. attempted to establish a uniform definition of SPMS. The following criteria were determined to best describe patients with SPMS and applied to our patient cohort [6]:
- Disability progression of 1 EDSS point (EDSS ≤ 5.5) or 0.5 EDSS points (EDSS ≥ 6.0) in the absence of a relapse
- Minimum EDSS = 4.0
- Pyramidal Functional systems score = 2.0
- Confirmed 3 months of progression
- Another tool that has been proposed to identify patients with SPMS is the MS Progression Discussion Tool (MS MSProDiscuss), which can be found at https://msprodiscuss.com [8]. Here, the likelihood of a patient to have progression is calculated based on a reference population and displayed color coded in green (unlikely), yellow (possible), or red (likely). Both “yellow” and “red” results were considered SPMS for the purpose of this study. Within the tool, the following criteria were applied to our patient cohort:
- Age, EDSS, relapses, and MRI activity in the past 6 months
- Signs and symptoms in the past 6 months; relation of the symptoms to a relapse; dynamic of the symptoms
- Impact of the symptoms on the patient's daily life
- The EXPAND study was the pivotal trial for the approval of siponimod in active SPMS patients [9]. We applied the key inclusion criteria of the EXPAND study to our patient cohort:
- Prior history of relapsing remitting MS
- SPMS defined as progressive increase of disability over at least 6 months
- EDSS = 3.0–6.0 (patients with EDSS > 6 were also viewed as having SPMS, despite patients with a higher EDSS being excluded from the study)
Statistical analysis
IBM SPSS (Statistical Package for the Social Sciences) Statistics, version 22 was applied for statistical analyses. Chi-squared statistics were used to compare the proportion of identified SPMS between groups after applying the respective diagnostic tools. Chi-squared statistics were also used for post hoc testing between individual groups. Venn diagrams were used to visualize the composition of the SPMS cohort with respect to key criteria (“disease progression” and “disease activity,” respectively).
RESULTS
In total, 206 patients presenting to our MS outpatient clinic in 2018 fulfilled the inclusion criteria. Baseline variables of this cohort are presented in Table 1. The descriptive evaluation of the disease course independent from any previous diagnosis revealed that 79 patients (38%) had 3-month confirmed disability progression, 81 patients (39%) had MRI activity at any point in the past 2 years, and 121 patients (59%) had at least one relapse within the past 2 years.
| Variable | All patients | Clinical diagnosis (FAST) | MS ProDiscuss | Lorscheider | EXPAND |
|---|---|---|---|---|---|
| n | 206 | 71 | 108 | 63 | 75 |
| Age, years, mean ± SD | 43.3 ± 12.3 | 51.4 ± 10.9 | 49.0 ± 11.0 | 51.3 ± 11.1 | 51.5 ± 11.1 |
| Sex | 141 w, 65 m | 52 w, 19 m | 78 w, 30 m | 43 w, 20 m | 52 w, 24 m |
| EDSS, median [IQR] | 3.5 [2.5–5.125] | 6.0 [4.5–6.5] | 5.0 [4.0–6.0] | 6.0 [5.0–6.5] | 6.0 [4.5–6.5] |
| Disease duration, years, mean ± SD | 12.2 ± 8.9 | 16.7 ± 9.7 | 16.0 ± 9.2 | 16.7 ± 9.6 | 17.3 ± 9.6 |
| Relapses in past 2 years, mean ± SD | 1.2 ± 1.6 | 0.5 ± 1.0 | 0.9 ± 1.2 | 0.6 ± 1.1 | 0.7 ± 1.2 |
| MRI activity in past 2 years | 39.3% | 19.7% | 23.1% | 22.2% | 22.7% |
| Confirmed 3-month progression | 38.3% | 98.9% | 72.2% | 100% | 100% |
| “Active” MSa | 64.6% | 39.4% | 50.0% | 39.6% | 45.3% |
- Abbreviations: EDSS = Expanded Disability Status Scale; FAST = Five-Dimensional Approach for Surveillance and Therapy; IQR = interquartile range; MRI = magnetic resonance imaging; MS = multiple sclerosis; MS ProDiscuss = MS Progression Discussion Tool.
- a Active MS was defined as patients having had relapses or MRI activity within the past 2 years.
Regarding the diagnosis established so far by the treating physicians (according to the standardized diagnosis field in the medical record), there were 71 patients (34%) labeled as having SPMS. Of the SPMS patients, 70 (99%) had confirmed disability progression over a period of at least 3 months, 14 (20%) had MRI activity at any point in the past 2 years, and 16 (23%) had clinical relapses within the past 2 years.
There were notable differences in the proportion of patients identified as having SPMS when applying the three classification methods to the entire patient cohort. Sixty-three patients (31% of all patients) met the SPMS criteria proposed by Lorscheider et al., 108 patients (52%) were identified as having SPMS by the MS Progression Discussion Tool, and 75 patients (36%) met the inclusion criteria of the EXPAND study. These differences were confirmed by applying chi-squared statistics over all tests (p < 0.001). In the post hoc test between the groups, this was shown to be mainly due to the highly significant difference between the MS Progression Discussion Tool and the other groups (p < 0.001 vs. Lorscheider et al., p = 0.001 vs. the EXPAND criteria). There was no significant difference between the criteria of Lorscheider et al. and the EXPAND study (p = 0.125). As expected, with all tools as well as with regard to the clinical diagnosis, patients classified as SPMS were older, had a higher EDSS, and had a longer disease duration compared to the whole group (Table 1). Comparing the three tools to each other, patients identified by the Progression Discussion Tool as having SPMS had lower EDSS values and higher relapse activity.
We then compared the tools using Venn diagrams to evaluate how many patients with confirmed disability progression alone and in combination with MRI activity or relapses (i.e., “disease progression” with and without “disease activity” as defined above), respectively, were identified by each tool (Figure 1). The MS Progression Discussion Tool identified 78 of 79 patients with confirmed disability progression (sensitivity 99%), but also included a relevant number of patients without progression (specificity 80%; Figure 1b). In comparison, Lorscheider criteria showed a reduced sensitivity of 80% (63 of 79 patients) at a specificity of 100% (Figure 1c), and the EXPAND criteria revealed a sensitivity of 95% (75 of 79 patients) at a specificity of 100% (Figure 1d). Sensitivity for detection of patients with both confirmed disability progression and relapses (n = 30) for the three tools was 97%, 76%, and 97%, respectively. Sensitivity for detection of patients with both confirmed disability progression and MRI activity (n = 17) for the tools was 94%, 82%, and 100%, respectively. The MS Progression Discussion Tool identified 11 patients as having SPMS who had had relapses but no progression or MRI activity along with nine more patients who had relapses and MRI activity, but no confirmed progression. In addition, 10 patients were included by using the MS Progression Discussion Tool, who had neither relapses nor MRI activity nor confirmed progression. None of the other tools identified these false-positive patients.
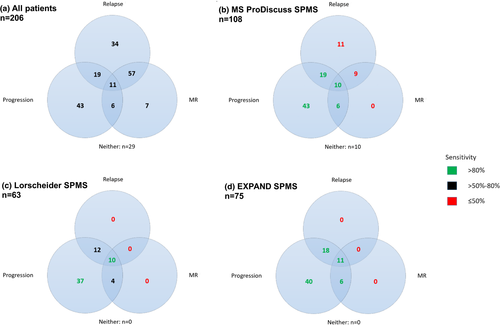
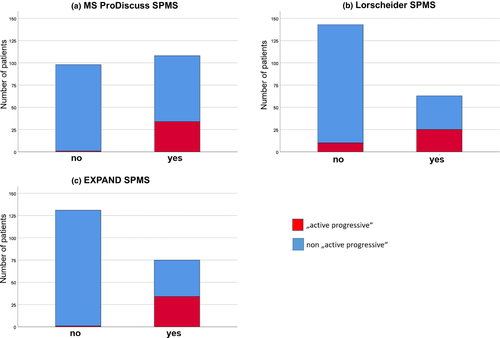
In a final step, we evaluated whether the tools were able to reliably identify patients with active progressive MS (defined as having confirmed 3-month disability progression as well as either a relapse or MRI activity during the past 2 years). As seen in Figure 2a, the most sensitive tool remains the MS Progression Discussion Tool (97%; 35 of 36 patients; identified 108 patients), where, however, also the highest number of patients without active progressive MS were included. On the other end of the spectrum, with the quite restrictive criteria proposed by Lorscheider et al., the highest number of active progressive MS patients were missed (Figure 2b). Results for the EXPAND criteria are shown in Figure 2c.
DISCUSSION
At the time when no therapies for SPMS were available, the labeling of the disease course as SPMS had severe implications for patients, leading to a delay in switching the diagnosis to SPMS by the treating physician [10]. Now, with treatment options starting to expand, patients benefit from an earlier diagnosis of SPMS, especially as the timeframe when SPMS patients benefit from disease-modifying therapy remains short [11].
According to the current EU label, treatment with siponimod requires the diagnosis “active SPMS.” Whereas “active” can be more easily defined based on objective criteria (progression of MRI lesions, new or contrast-enhancing lesions, clinical relapses), the diagnosis of “SPMS” is more critical to establish, as the distinction between RRMS and SPMS is oftentimes not as clear-cut as it seems.
Different methods have been proposed to identify patients with SPMS in a clinical context. We compared those tools and found a high level of heterogeneity regarding the number of patients detected as well as the clinical characteristics of the identified patients. This held true particularly for the identification of patients with active progressive disease, where tools were either less sensitive or less specific in distinguishing them from RRMS. This is partly due to the different goals of the tools and their focus on progression rather than disease activity. Whereas Lorscheider et al. wanted to establish criteria to identify patients already suffering from SPMS most accurately, the MS Progression Discussion Tool is more focused on the risk for progressive disease and detecting possible progression as early as possible. As a caveat, both the “yellow” and “red” labels were treated as producing a result of SPMS in this study. The inclusion criteria for the EXPAND study can be classified as lying somewhere in the middle between the two. Here, because of the missing requirement of a certain pyramidal score in EDSS testing, more patients, especially in the early phase of SPMS, were included. None of the tools used was designed to specifically single out active SPMS. With the Lorscheider criteria, relapses and MRI activity are not considered at all. In the EXPAND trial, patients with relapses in the 3 months before randomization were excluded. Contrary to this, a relevant benefit could only be shown in the subgroup analysis for patients with signs of disease activity in the past 2 years, underlying the importance of the concept. In the MS Progression Discussion Tool, both information regarding relapses and MRI activity are required during the evaluation, but the result given does not answer the question of whether a patient still has signs of active disease.
Our study also demonstrates the challenges of transferring research results to clinical practice. Obviously, when establishing the inclusion criteria for a trial, very strict criteria to define SPMS and progression are needed, as the study population has to be well defined. When these are used in the same way for treatment decisions, however, they may not be sensitive enough, which results in the exclusion of too many patients. For example, applying the Lorscheider criteria in clinical practice would lead to a significant number of patients not being able to receive treatment during the time they still have active disease, indicating that a tool developed for research might not be ideal for treatment decisions.
Given the difficulties in classifying patients as having SPMS demonstrated here, we suggest using “active progressive” as a label for clinical decision-making in the future. This way, patients could be prescribed appropriate medication effective for both progression and relapses as soon as the treating physician has noticed a relapse-free progression over some time. A difficulty arises in defining “relapse-free progression,” similarly to defining SPMS. Using the EDSS alone, where in later stages the score is exclusively determined by the patient's ability to walk, might not be sensitive enough. As Ziemssen et al. pointed out, there is no distinct clinical feature predicting disease progression [8]. Tools like the MS Progression Discussion Tool can be helpful in identifying these patients early, but even here, disease activity was not considered sufficiently. So far, existing tools cannot replace the diagnosis made by the treating neurologist, given their lack of either sensitivity or specificity. In this study, clinical worsening reported by the patient without relapse was considered progression. By using this approach, progression would become much easier to identify, as many of the symptoms suffered by SPMS patients are beyond the reach of objectification in regular clinical practice. To achieve this, patients need to be screened regularly for signs of transitioning to a progressive disease course. These include, but are not limited to, decreasing walking distance, reduced coordination, and increasing fatigue [12]. In addition to regular clinical examination (i.e., every 3 months), this includes taking a thorough history frequently to detect patient-reported worsening independent from a relapse. When combined with evidence for additional clinical relapses or MRI activity, those patients with active progression can receive adequate treatment. Careful history-taking is also needed to distinguish between a relapse with insufficient remission and the beginning of progression.
A limitation to this study is its retrospective approach. Especially for the MS Progression Discussion Tool, information about a patient's daily activities was not always available. Furthermore, the reclassification of cases (i.e., setting the “gold standard”) was only done once and not multiple times by different neurologists. However, as the study focused on evaluating relative differences between the tools rather than on defining their “absolute” accuracy in identifying progression, this seems appropriate. Besides that, a common “gold standard” for defining progression is not available.
To summarize, the efficacy of anti-inflammatory medication can be expected to be greater in the transition phase between RRMS and SPMS than in later phases of SPMS. However, switching from one diagnosis to the other is often delayed, although in those patients, relapse-independent progression will already have a large, if not larger than possibly still occurring relapses, impact on disability accumulation. Here, we propose discontinuing this label in favor of the more general term “active progressive” MS to minimize these delays.
ACKNOWLEDGEMENT
Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
S.K. received research support from Novartis. Y.Y. has been supported by travel grants from Novartis and Sanofi Genzyme, and has received an honorarium for active participation on an advisory board from Sanofi Genzyme as well as speaking honoraria from Roche and Sanofi Genzyme. C.F. reports speaker honoraria and honoraria for participating on advisory boards from Novartis, Teva, Merck, Sanofi Genzyme, Alexion, Bristol-Myers Squibb, and Roche. C.F. has received research support from Sanofi Genzyme and Novartis.
AUTHOR CONTRIBUTIONS
Svenja Klinsing: Conceptualization (equal), data curation (equal), formal analysis (supporting), writing–original draft (lead). Yavor Yalachkov: Conceptualization (supporting), validation (equal), writing–original draft (supporting). Christian Foerch: Conceptualization (equal), formal analysis (lead), funding acquisition (lead), supervision (lead), writing–original draft (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




