Effect of intravenous alteplase on post-stroke depression in the WAKE UP trial
Funding information
WAKE-UP received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no.278276 (WAKE-UP).
See commentary by M. Diomedi and I. Maestrini on page 1799
Abstract
Background and purpose
The aim was to study the effect of intravenous alteplase on the development of post-stroke depression (PSD) in acute stroke patients, and to identify predictors of PSD.
Methods
This post hoc analysis included patients with unknown onset stroke randomized to treatment with alteplase or placebo in the WAKE-UP trial (ClinicalTrials.gov number, NCT01525290), in whom a composite end-point of PSD was defined as a Beck Depression Inventory ≥10, medication with an antidepressant, or depression recorded as an adverse event. Multiple logistic regression was used to identify predictors of PSD at 90 days. Structural equation modelling was applied to assess the indirect effect of thrombolysis on PSD mediated by the modified Rankin Scale.
Results
Information on the composite end-point was available for 438 of 503 randomized patients. PSD was present in 96 of 224 (42.9%) patients in the alteplase group and 115 of 214 (53.7%) in the placebo group (odds ratio 0.63; 95% confidence interval 0.43–0.94; p = 0.022; adjusted for age and National Institutes of Health Stroke Scale at baseline). Prognostic factors associated with PSD included baseline medication with antidepressants, higher lesion volume, history of depression and assignment to placebo. While 65% of the effect of thrombolysis on PSD were caused directly, 35% were mediated by an improvement of the mRS.
Conclusions
Treatment with alteplase in patients with acute stroke resulted in lower rates of depression at 90 days, which were only partially explained by reduced functional disability. Predictors of PSD including history and clinical characteristics may help in identifying patients at risk of PSD.
Abbreviations
-
- BDI
-
- Beck Depression Inventory
-
- CDE
-
- controlled direct effect
-
- CI
-
- confidence interval
-
- IQR
-
- interquartile range
-
- MRI
-
- magnetic resonance imaging
-
- mRS
-
- modified Rankin Scale
-
- NIE
-
- natural indirect effect
-
- NIHSS
-
- National Institutes of Health Stroke Scale
-
- OR
-
- odds ratio
-
- PSD
-
- post-stroke depression
-
- SAE
-
- serious adverse event
-
- SD
-
- standard deviation
INTRODUCTION
Post-stroke depression (PSD) is a frequent complication after acute stroke with reported prevalence ranging from 29% to 43% [1-4]. PSD impairs recovery from stroke, in particular cognitive and motor function, and negatively affects quality of life after stroke [5]. Several studies have attempted to identify predictive factors of PSD with heterogeneous results. Factors found to be associated with PSD comprise socio-demographic factors such as female sex [2], genetic factors like serotonin transporter gene polymorphisms [6], stroke-associated factors like disability [7], symptom severity or stroke location [8], or history of mood disorders and mild cognitive impairment [9], and depression and cognitive dysfunction at baseline [10].
Intravenous thrombolysis with alteplase is the standard of care for acute ischaemic stroke and improves functional outcome as well as quality of life [11, 12]. The potential effect of thrombolysis on PSD, however, has not yet been investigated in the setting of a randomized controlled trial, and there are only few data from observational studies with heterogeneous findings [7, 13].
The aim was to investigate the effect of intravenous alteplase on PSD 3 months after stroke in the WAKE-UP trial (efficacy and safety of magnetic resonance imaging [MRI] based thrombolysis in wake-up stroke: a randomized, double-blind, placebo-controlled trial), and to test whether there is an effect on PSD not captured by the traditional measurement of functional outcome. Moreover, the aim was to identify predictors of PSD.
METHODS
This is a post hoc analysis of included patients with unknown onset stroke randomized to treatment with alteplase or placebo in the WAKE-UP trial. WAKE-UP was a multicentre, randomized, double-blind, placebo-controlled clinical trial of MRI-based intravenous thrombolysis in unknown onset stroke. The trial protocol and main results have been published previously [14]. Briefly, patients with acute ischaemic stroke of unknown onset that represented with a mismatch between an acute ischaemic lesion visible on diffusion-weighted imaging but no marked parenchymal hyperintensity in the corresponding region on fluid-attenuated inversion recovery, and who otherwise met clinical inclusion and exclusion criteria for intravenous alteplase, were randomized to receive either alteplase or placebo. Primary outcome was a favourable outcome at 90 days after stroke defined as a score of 0 or 1 assessed on the modified Rankin Scale (mRS). Among the secondary outcome measures, depressive symptoms at 90 days after stroke were assessed using the Beck Depression Inventory (BDI), a 21-item scale asking for depressive symptoms with a four-point rating (0–3) for each item based on the severity of each item, adding up to a total score of 0–63. For this secondary analysis, all randomized patients for whom a completed language-specific BDI questionnaire in each participating country at 90 days was available were included.
Study outcomes—post-stroke depression
For the present analysis PSD was investigated as a composite end-point, defined by any depression according to the BDI at 90 days following the established threshold of ≥10 [15-17], depression recorded as a serious adverse event (SAE), or the use of antidepressants in concomitant medication up to 90 days after stroke. As it cannot be ruled out that antidepressants were given to patients for other reasons than depression, an additional sensitivity analysis was performed with the combined end-point of a BDI of ≥10 or depression as an SAE, disregarding the use of antidepressants.
Statistical analysis
Sample characteristics are given as absolute and relative frequencies or mean ± standard deviation as well as median with interquartile range, whichever is appropriate. Statistical analysis of the treatment effect of alteplase on PSD was performed in the intention-to-treat population for all patients with available information for the composite end-point PSD (BDI ≥10, medication with an antidepressant, or depression recorded as an SAE) and a secondary composite end-point composed of BDI ≥10 or SAE.
The effect of treatment on PSD was estimated using a multiple logistic regression model adjusted for age and National Institutes of Health Stroke Scale (NIHSS) at baseline. To identify possible predictors of PSD, three multiple logistic regression models were fitted with the composite end-point of PSD as dependent variable and three sets of baseline variables as predictors. In the first model, only age, NIHSS and treatment (alteplase/placebo) were included as predictors. In a second model, sex, baseline medication (antidepressant/other psychiatric medication), depression in medical history and hemisphere of stroke lesion were added. Finally, in a third model stroke lesion volume after 22–36 h was further added which was included as the base-2 logarithm of its third root due to its skewed distribution. For all models odds ratios (ORs) with corresponding 95% confidence intervals (95% CIs) were reported.
As thrombolysis is known to ameliorate functional outcome after stroke, it was hypothesized that better functional outcome may be the main reason for lower rates of depression in the alteplase group. To evaluate the role of the functional outcome variable mRS as a potential mediator of the relationship between treatment and PSD, a mediation analysis was performed with PSD as dependent variable, mRS as the mediator, treatment with alteplase or placebo as predictor, and age and NIHSS as covariates using structural equation models [18, 19] within Stata 16.0 (StataCorp. 2019, Stata Statistical Software: Release 16, College Station, TX, USA, StataCorp LLC) and the PARAMED package [20]. The results are visualized by forest plots showing effects with corresponding 95% CI.
All of the models present analyses of cases with available information. A two-tailed p < 0.05 was considered to be statistically significant. Nominal p values are reported without correction for multiplicity.
RESULTS
Of 503 randomized patients, 438 (87%) patients had information on PSD available. Of these, 224 were assigned to alteplase and 214 to placebo. Baseline and outcome variables are displayed in Table 1. For 65 patients, no information on depression as an SAE or medication or BDI was available. These patients had higher baseline median NIHSS scores compared to patients with all information available (7 vs. 5, p = 0.023), higher median mRS scores at 90 days (3 vs. 2, p < 0.001) and less often antidepressant medication prescribed at baseline (0% vs. 8.9%, p = 0.009), while other baseline and outcome variables did not differ significantly between the two groups.
| Variables | Patients with available information on the composite end-point PSD (N = 438) | ||
|---|---|---|---|
| Placebo group | Alteplase group | p value | |
| N = 214 | N = 224 | ||
| Age in years, mean ± SD | 65.1 ± 11.6 | 64.7 ± 11.2 | 0.71 |
| Sex = male | 132 (61.7%) | 150 (67.0%) | 0.249 |
| NIHSS, median (IQR) | 5.0 (4.0–9.0) | 5.0 (3.0–9.0) | 0.635 |
| Lesion volume at baseline in ml, median (IQR) | 2.6 (0.8–9.0) | 1.8 (0.7–7.8) | 0.324 |
| Missing | 3 (1.4%) | 6 (2.7%) | |
| Lesion volume at 22–36 h in ml, median (IQR) | 3.4 (1.2–16.8) | 3.0 (1.0–18.2) | 0.401 |
| Missing | 17 (7.9%) | 28 (12.5%) | |
| Lacunar stroke | 44 (20.6%) | 48 (21.4%) | 0.52 |
| Missing | 168 (78.5%) | 175 (78.1%) | |
| Infratentorial stroke | 22 (10.3%) | 25 (11.2%) | 0.601 |
| Missing | 17 (7.9%) | 30(13.4%) | |
| Hemisphere | |||
| Left | 107 (50.0%) | 117 (52.2%) | 0.005 |
| Right | 94 (43.9%) | 76 (33.9%) | |
| Bilateral | 6 (2.8%) | 21 (9.4%) | |
| Missing | 7 (3.3%) | 10 (4.5%) | |
| History of depression | 12 (5.6%) | 14 (6.3%) | 0.785 |
| Missing | 1 (0.5%) | ||
| History of other psychiatric disease | 1 (0.5%) | 5 (2.2%) | 0.097 |
| Missing | 1 (0.5%) | ||
| Baseline medication with antidepressant | 20 (9.3%) | 19 (8.5%) | 0.739 |
| Missing | 1 (0.5%) | ||
| Baseline medication with other psychiatric drug | 10 (4.7%) | 11 (4.9%) | 0.916 |
| Missing | 1 (0.5%) | ||
| At 90 days | |||
| mRS, median (IQR) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.018 |
| Missing | 2 (1.0%) | 1 (0.5%) | |
| BDI at 90 days, median (IQR) | 7.0 (2.0–14.0) | 6.0 (2.0–11.0) | 0.14 |
| Missing | 13 (6.1%) | 11 (4.9%) | |
| Any depression (BDI ≥10) | 79 (30.4%) | 65 (29.0%) | 0.061 |
| Missing | 13 (6.1%) | 11 (4.9%) | |
| Medication with an antidepressant | 68 (31.8%) | 54 (24.1%) | 0.069 |
| Missing | 1 (0.5%) | ||
| SAE depression | 27 (12.6%) | 21 (9.4%) | 0.27 |
| Missing | 1 (0.5%) | ||
| PSD | 115 (53.7%) | 96 (42.9%) | 0.023 |
- Abbreviations: BDI, Beck Depression Inventory; IQR, interquartile range; mRS, modified Rankin Scale; n, number of patients; NIHSS, National Institutes of Health Stroke Scale; PSD, post-stroke depression; SAE, serious adverse event; SD, standard deviation.
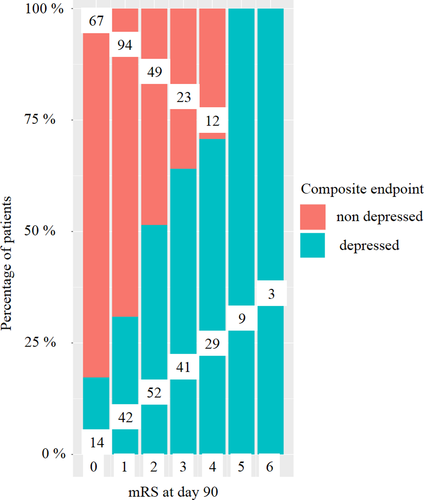
The BDI and mRS correlated moderately with each other (Spearman correlation coefficient 0.42, p < 0.001). The percentage of patients with and without depression according to the composite end-point PSD, stratified by mRS, is depicted in Figure 1.
Effect of alteplase on the post-stroke depression composite end-point
Treatment with alteplase was associated with significantly lower rates of PSD, observed in 96 of 224 (42.9%) patients in the alteplase group compared to 115 of 214 (53.7%) patients in the placebo group (age and NIHSS adjusted OR 0.63; 95% CI 0.43–0.94; p = 0.022) (cf. Figure 2).
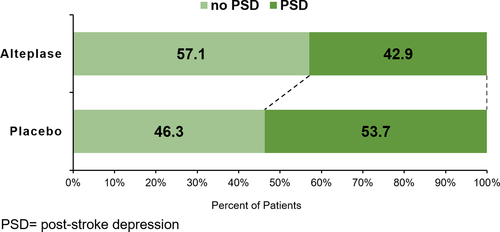
In the sensitivity analysis of the composite end-point, information on depression was available for 424 patients. Also after excluding the intake of antidepressants from the definition, PSD was observed less frequently in the alteplase group with 79 of 219 (36.1%) patients than in the placebo group with 95 of 205 (46.3%) patients (adjusted OR 0.63; 95% CI 0.42–0.93, p = 0.023).
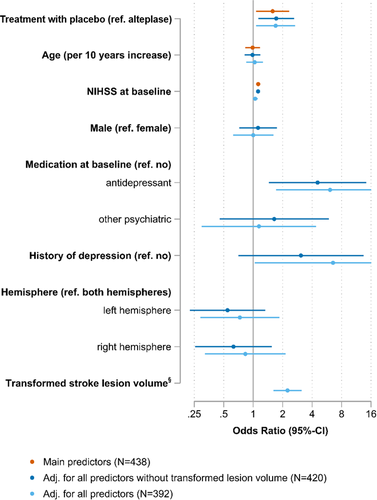
Predictors of post-stroke depression at 90 days
In the simple model, NIHSS at baseline (OR 1.13, 95% CI 1.08–1.18, p < 0.001) and treatment with placebo (OR 1.58, 95% CI 1.07–2.34, p = 0.022) were significant predictors of PSD. In the model with additional baseline parameters but without adjustment for stroke lesion volume, higher NIHSS score at baseline (OR 1.12, 95% CI 1.07–1.17, p < 0.001), baseline medication with antidepressants (OR 4.55, 95% CI 1.45–14.31, p = 0.010) and treatment with placebo (OR 1.72, 95% CI 1.13–2.62, p = 0.012) were significant predictors of PSD. In the full model, stroke lesion volume (OR 2.24, 95% CI 1.62–3.12, p < 0.001), history of depression (OR 6.54, 95% CI 1.04–41.17, p = 0.045), medication with antidepressants (OR 6.10, 95% CI 1.72–21.67, p = 0.005) and treatment with placebo (OR 1.70, 95% CI 1.07–2.68, p = 0.024) were significant predictors of PSD 90 days after stroke (cf. Table 2, Figure 3).
| Main predictors (N = 438) | All predictors without transformed lesion volume (N = 420) | All predictors (N = 392) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Treatment with placebo (ref. alteplase) | 1.58 (1.07, 2.34) | 0.022 | 1.72 (1.13, 2.62) | 0.012 | 1.70 (1.07, 2.68) | 0.024 |
| Age (per 10 years increase) | 0.99 (0.83, 1.17) | 0.882 | 0.99 (0.82, 1.18) | 0.875 | 1.04 (0.85, 1.26) | 0.727 |
| NIHSS at baseline | 1.13 (1.08, 1.18) | <0.001 | 1.12 (1.07, 1.17) | <0.001 | 1.05 (0.99, 1.11) | 0.109 |
| Male (ref. female) | 1.12 (0.72, 1.75) | 0.611 | 1.00 (0.62, 1.61) | 0.995 | ||
| Medication at baseline (ref. no) | ||||||
| Antidepressant | 4.55 (1.45, 14.31) | 0.010 | 6.10 (1.72, 21.67) | 0.005 | ||
| Other psychiatric | 1.64 (0.46, 5.92) | 0.449 | 1.15 (0.30, 4.40) | 0.843 | ||
| History of depression (ref. no) | 3.07 (0.70, 13.42) | 0.135 | 6.54 (1.04, 41.17) | 0.045 | ||
| Hemisphere (ref. both hemispheres) | ||||||
| Left hemisphere | 0.55 (0.23, 1.33) | 0.183 | 0.73 (0.29, 1.86) | 0.511 | ||
| Right hemisphere | 0.63 (0.26, 1.55) | 0.316 | 0.83 (0.32, 2.15) | 0.705 | ||
| Transformed lesion volumea | 2.24 (1.62, 3.12) | <0.001 | ||||
- Abbreviations: CI, confidence interval; OR, odds ratio; NIHSS, National Institutes of Health Stroke Scale.
- a Instead of the stroke lesion volume after 22–36 h, the base-2 logarithm of its third root was included in the model, such that the effect must be interpreted as a 2-fold increase of the stroke lesion length.
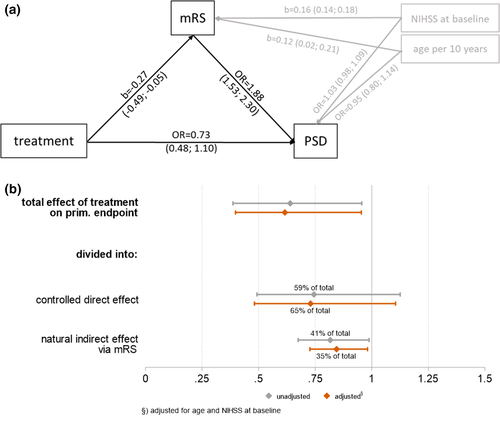
Effects of alteplase on functional outcome and post-stroke depression—structural equation modelling
In structural equation modelling without adjustment for age and NIHSS, the total effect of treatment with alteplase was negatively associated with the probability of PSD (0.61, 95% CI 0.39–0.96, p = 0.031). Of this total effect, a 41% proportion was mediated by the natural indirect effect (NIE), that is, an improvement of the mRS. A higher mRS was significantly associated with a higher probability of PSD (OR 2.0, 95% CI 1.67–2.38, p < 0.001), and treatment with alteplase reduced the mRS significantly by a mean of 0.29 points (95% CI for reduction 0.03–0.56, p = 0.031). Thus, the NIE of treatment with alteplase on PSD was 0.82 (95% CI 0.67–0.99, p = 0.037) and the controlled direct effect (CDE) was 0.74 (95% CI 0.49–1.13, p = 0.162). The effect of treatment on PSD is in our model referred to as the direct effect, in the sense that it is not mediated by the mRS.
After adjusting for age and NIHSS at baseline, the results changed slightly and the mediated path described 35% of the total effect (NIE 0.84, 95% CI 0.73–0.98, p = 0.027 vs. CDE 0.73, 95% CI 0.48–1.10, p = 0.136). Detailed results of the structural equation modelling are presented in Figure 4.
DISCUSSION
In this post hoc analysis of the WAKE-UP trial, treatment with intravenous alteplase was associated with lower rates of PSD assessed at 90 days after stroke than placebo. Moreover, structural equation modelling revealed that the effect of intravenous alteplase on the reduction of depressive symptoms 90 days after stroke was only explained in part by the beneficial effect of alteplase on functional outcome defined by the mRS.
PSD is frequent after ischaemic stroke and has detrimental effects on functional outcome and dependence after stroke [21]. In our trial cohort, 33% of patients overall showed BDI values indicating depression at 90 days after stroke, and 48% of patients met the composite end-point of BDI values indicating depression, depression recorded as an SAE, or use of antidepressants. These numbers are rather high but within the range of reported prevalence rates of PSD 3 months after stroke and underline the importance of screening for PSD after stroke [22-24]. In a sensitivity analysis of an end-point of PSD which did not include medication with antidepressant, 41% of patients met the criteria of PSD, and the observed treatment effect of alteplase in reducing PSD at 90 days after stroke was comparable.
Intravenous thrombolysis with alteplase improves functional outcome after stroke, but the effects on PSD have not been reported in a randomized controlled trial. Given the fact that patients in the thrombolysis group presented with more severe strokes, comparable rates of PSD between patients receiving thrombolysis and those who did not receive thrombolysis were interpreted as possible indirect signs of a positive effect of thrombolysis on patients’ mood after stroke [25]. Previous observational studies have pointed towards a possible beneficial effect of alteplase on PSD [7], but it was assumed that this was mediated by an indirect effect resulting from improved functional outcome with thrombolysis. In support of this, functional impairment was identified as a predictor of PSD in previous studies [26, 27]. On the other hand, depressive symptoms were observed in a relevant proportion of patients with no or only minimal neurological deficit at 90 days after stroke [28], and this has been attributed to possible traumatic effects of stroke beyond resulting symptoms and disability which might not be modulated by thrombolysis [13].
In our study, treatment with intravenous alteplase resulted in an absolute risk reduction of PSD of 10.8% for the composite end-point of depression defined by the BDI, depression recorded as SAE, or the use of antidepressants. In other words, PSD can be averted in about one in 10 patients by treatment with alteplase. By the use of structural equation modelling analysis it was further possible to show that the effect of intravenous alteplase on PSD is not fully captured by the traditional measurement of functional outcome by the mRS. As expected based on the reported association of functional outcome with PSD, a large part of the effect of alteplase treatment on symptoms of depression after 90 days can be explained by mRS values reflecting functional outcome at the time of assessment. However, more than half of the effect of intravenous alteplase on PSD was not captured by the mRS score at 90 days, shown by a direct effect of treatment with alteplase on PSD in our model. This effect was stable with and without adjustment for age and stroke severity. The potential mechanisms underlying this beneficial effect of thrombolysis on depressive symptoms after stroke can only be speculated on. A direct pharmacological effect of short-term acute treatment with recombinant tissue-plasminogen activator on mood 3 months later appears unlikely. So it is more likely to be the biological effects of alteplase, that is, reperfusion leading to salvage of brain tissue from final infarction, that may be responsible for the protective effect against PSD. This may include the prevention of symptoms or social and emotional consequences from stroke beyond those factors captured by the mRS at 90 days. As patients’ symptoms regularly exceed symptoms captured by ‘classical’ outcome measures, patient-reported outcome measures including symptoms of depression should represent an essential part of future studies [29].
Altogether, these results further corroborate the beneficial effects of intravenous thrombolysis as a standard treatment for acute ischaemic stroke, leading to better functional outcome and improved quality of life after stroke [11, 12]. Since wake-up stroke and strokes in a larger time window are treated with thrombolysis as a consequence of recent studies [14, 30], this has gained even more significance.
Given the high prevalence of PSD and its impact on functional outcome and quality of life after stroke, the identification of patients at risk of depressive symptoms after stroke is a clinical need. Previous studies that investigated PSD and its predictors accredited a significant effect to high NIHSS score on admission [31], which was confirmed in the statistical analysis of this study. Only when stroke lesion volume was added to the prediction model, NIHSS no longer predicted PSD, which may be explained by the fact that both parameters, NIHSS at baseline and stroke lesion volume, reflect stroke severity.
Other predictors that were discussed in the literature could be confirmed in this study as well, such as medication with antidepressants [9]. Lesion volume [32] has been identified as a predictor of PSD [33] and was also associated with PSD in our analysis. Recurrently named predictors of PSD include higher disability after stroke, history of depression, low social support, lower education and cognitive impairment [22, 27, 31, 34] of which history of depression was supported by this study. Additionally, higher mRS was associated with higher BDI score, as shown previously [13]. Therefore, screening of stroke patients with regard to these is crucial to identify early patients at risk of PSD.
There are limitations to our study. Assessment of PSD at 3 months was available for 438 of 477 surviving patients (86.58%) available for follow-up at 90 days. It cannot be ruled out that the more severely affected patients in particular did not respond, resulting in a potential bias that underestimated the prevalence of PSD in our sample. Moreover, the assessment of PSD primarily relied on a single, although standardized and well established, rating scale and did not involve psychiatric evaluation. For the assessment of PSD, a follow-up of 3 months may also be rather short. Additionally, depression was not measured at baseline. It was attempted to adjust by adding baseline antidepressant medication to the model but still patients with baseline depression might have been missed. Indications for prescription of baseline medication were not recorded. It cannot be excluded that some of the patients may have received antidepressants for other reasons than depression [35], but in a sensitivity analysis it could be shown that, even when using a composite end-point without including antidepressant medication, there was a significant treatment effect of alteplase on PSD. Also, pre-stroke cognitive status was not recorded and may have accounted for PSD. Finally, due to the upper age limit of 80 years in WAKE-UP the results of this analysis cannot be generalized to populations of very elderly stroke patients.
CONCLUSION
In the randomized controlled WAKE-UP trial, after adjustment for age and stroke severity, intravenous alteplase resulted in a significant reduction of the proportion of patients with PSD 90 days after stroke. While about one-third of the effect of alteplase on PSD was mediated by improved functional outcome, almost two-thirds of the effect was independent of the beneficial effect of alteplase on the mRS. More severe strokes or larger stroke lesion volume, previous medication with an antidepressant, and history of depression were predictors of PSD and might help screen for patients at risk of developing PSD.
ACKNOWLEDGEMENTS
The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
Open Access funding enabled and organized by Projekt DEAL.
WOA Institution: Universitatsklinikum Hamburg-Eppendorf
Blended DEAL: Projekt DEAL
DISCLOSURES
AK reports grants from the European Union 7th Framework Programme during the conduct of the study. BC reports grants from the European Union 7th Framework Programme during the conduct of the study and personal fees from Bayer Vital and Abbott, all outside the submitted work. CZS is supported by a research grant from Novo Nordisk, outside the submitted work. VT has received personal fees as consultant or lecturer from Boehringer-Ingelheim, outside the submitted work. MEn reports grant support from Bayer, the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), the German Center for Neurodegenerative Diseases (DZNE), the German Center for Cardiovascular Research (DZHK), the European Union, Corona Foundation and Fondation Leducq; and fees paid to the Charité from Boehringer Ingelheim, Bristol-Myers Squibb, BMS/Pfizer, Daiichi Sankyo, Amgen, GlaxoSmithKlineGSK, Sanofi, Covidien, Ever, Novartis, all outside the submitted work. JBF reports consulting, lecture and advisory board fees from BioClinica, Cerevast, Artemida, Brainomix, Merck and Lundbeck as well as a grant from the German Federal Ministry of Education and Research (01EO0801 and 01EO01301), outside the submitted work. JBF is holding European Patent No. 17179320.01-1906. RL is a senior clinical investigator of FWO Flanders. KWM has participated in advisory boards for Boehringer Ingelheim. Boehringer Ingelheim supplies medication for the ATTEST-2 clinical trial, funded by the Stroke Association and British Heart Foundation, for which he is Chief Investigator. SP reports personal fees from Lundbeck, outside the submitted work. CG reports personal fees from Boehringer Ingelheim, outside the submitted work. GT reports personal fees as consultant or lecturer from Acandis, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb/Pfizer, Daiichi Sankyo, Portola, Stryker, and research grants from Bayer, Federal Ministry for Economic Affairs and Energy (BMWi), Corona Foundation, German Research Foundation (DFG), Else Kröner-Fresenius Foundation, European Union (Horizon 2020), German Innovation Fund, all outside the submitted work. All remaining authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Alina Königsberg: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); visualization (equal); writing original draft (equal); writing review and editing (equal). Susanne Sehner: Methodology (equal); software (equal). Sönke Arlt: Conceptualization (equal); supervision (equal). Bastian Cheng: Data curation (equal); software (equal). Claus Simonsen: Data curation (equal). Florent Boutitie: Data curation (equal); formal analysis (equal); methodology (equal). Joaquin Serena: Data curation (equal). Vincent Thijs: Data curation (equal). Martin Ebinger: Data curation (equal). Mathias Endres: Data curation (equal). Jochen B. Fiebach: Data curation (equal). Robin Lemmens: Data curation (equal). Keith W. Muir: Data curation (equal). Norbert Nighoghossian: Data curation (equal). Salvador Pedraza: Data curation (equal). Christian Gerloff: Data curation (equal); project administration (equal); supervision (equal). Götz Thomalla: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); writing review and editing (equal).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




