Baseline white matter hyperintensities affect the course of cognitive function after small vessel disease-related stroke: a prospective observational study
Abstract
Background and purpose
Cognitive impairment is a common sequel of recent small subcortical infarction (RSSI) and might be negatively affected by preexisting cerebral small vessel disease (SVD). We investigated whether the course of cognitive function in patients with RSSI is influenced by the severity of white matter hyperintensities (WMH), an important imaging feature of SVD.
Methods
Patients with magnetic resonance imaging (MRI)-proven single RSSI were tested neuropsychologically concerning global cognition, processing speed, attention, and set-shifting. Deep and periventricular WMH severity was assessed using the Fazekas scale, and total WMH lesion volume was calculated from T1-weighted MRI images. We compared baseline function and course of cognition 15 months after the acute event in patients with absent, mild, and moderate-to-severe WMH.
Results
The study cohort comprised 82 RSSI patients (mean age: 61 ± 10 years, 23% female). At baseline, 40% had cognitive impairment (1.5 standard deviations below standardized mean), and deficits persisted in one-third of the sample after 15 months. After age correction, there were no significant differences in set-shifting between WMH groups at baseline. However, although patients without WMH (deep: p < 0.001, periventricular: p = 0.067) or only mild WMH (deep: p = 0.098, periventricular: p = 0.001) improved in set-shifting after 15 months, there was no improvement in patients with moderate-to-severe WMH (deep: p = 0.980, periventricular: p = 0.816). Baseline total WMH volume (p = 0.002) was the only significant predictor for attention 15 months poststroke.
Conclusions
This longitudinal study demonstrates that preexisting moderate-to-severe WMH negatively affect the restoration of cognitive function after RSSI, suggesting limited functional reserve in patients with preexisting SVD.
INTRODUCTION
Lacunar stroke is responsible for a quarter of all ischemic strokes. The neuroimaging correlate of a lacunar stroke is a recent small subcortical infarct (RSSI) in the supply area of a small perforating brain artery [1, 2]. Cognitive impairment is common in lacunar stroke patients and might be worsened by preexisting cerebral small vessel disease (SVD), suggested by magnetic resonance imaging (MRI) via the morphological features lacunes, white matter hyperintensities (WMH), microbleeds, or brain atrophy [3-6], especially concerning gray matter [7, 8]. SVD is a leading cause of vascular cognitive impairment and dementia, predominantly affecting the domains executive functions (e.g., set-shifting, the ability to shift attention between one task and another), attention, memory, processing speed, and verbal fluency [5, 6].
In general, cognitive outcomes vary between stroke patients, depending on factors such as brain atrophy, age, cognitive reserve (e.g., intelligence quotient, education), and depression [7, 9-11]. Recent studies in community-dwelling older people [12-17] highlighted a crucial contribution of WMH to cognitive impairment. The few longitudinal studies [7, 8, 18, 19] investigating the influence of WMH on poststroke cognitive impairment showed similar results, reporting an association between WMH progression and decline in general cognitive function. Due to distinct findings regarding the associations between cognition and deep WMH (dWMH) in comparison with cognition and periventricular WMH (pWMH), it has been suggested to classify WMH into deep (subcortical) and periventricular WMH scores [13, 17, 20]. However, it is still unclear whether total WMH volume provides additional information on vascular cognitive impairment compared to clinically applicable WMH scores [21].
Improved prediction for the likelihood and course of cognitive impairment in stroke patients would support planning of stroke care and inform cognitive rehabilitation, and thus could improve patients’ quality of life [22-24]. Therefore, in this longitudinal study, we aimed to assess whether the course of cognitive function of patients with SVD-related RSSI is influenced by WMH severity, while controlling for other SVD MRI features (e.g., lacunes, gray matter volume, size of RSSI, microbleeds, old cortical infarcts) and demographic covariates (e.g., age, education).
METHODS
Patients
Patients aged 18 to 75 years admitted to the Department of Neurology at the University Hospital Graz between 2012 and 2017 with an imaging-proven single RSSI were invited to participate in this prospective study. Exclusion criteria were an axial RSSI diameter >25 mm on diffusion-weighted MRI sequences, other acute brain infarcts, preexisting disability (modified Rankin Scale [mRS] score >1), and severe preexisting cognitive impairment (assessed by standardized questions regarding prestroke cognition prior to inclusion). For this analysis, only patients who attended clinical and neuropsychological assessment both at baseline (BL) and 15 months poststroke (FU) were included. A flowchart of the recruiting process is provided in the (Figure S1). This prospective study was approved by the ethics committee of the Medical University of Graz (permit number 24–260 ex 11/12). All participants gave written informed consent.
Clinical assessment
All patients had routine neurological examination (including medical history, assessment of stroke severity according to the National Institutes of Health Stroke Scale [NIHSS] score, mRS score), comprehensive etiological stroke workup, vascular risk factor assessment, and an extensive neuropsychological examination at baseline and 15 months poststroke. At baseline, all patients underwent brain MRI at 1.5T (Siemens MAGNETOM Espree; Siemens Healthcare, Erlangen, Germany); follow-up brain MRI at 15 months poststroke was performed on a 3T TimTrio or Prisma scanner (Siemens Healthcare). All MRI scans were reviewed by an expert (C.E.) according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) [2]. Exact location and size of the RSSI were assessed for every patient. Additionally, all scans were rated for deep and periventricular WMH severity according to the Fazekas scale, the presence of lacunes, microbleeds, and other structural brain abnormalities such as chronic infarcts. MRI reading was done blinded to clinical information. Further information on the brain MRI protocol is provided elsewhere [25].
Neuropsychological assessment
The neuropsychological test battery included the Montreal Cognitive Assessment (MoCA), the Symbol Digital Modalities Test (SDMT), and the Comprehensive Trail Making Test (CTMT; subtest 2 and 5). The MoCA is a screening tool for cognitive impairment that includes memory, attention, speech, and executive functions. Scores < 26 are considered to be indicative of global cognitive impairment. The SDMT measures processing speed, and the CTMT evaluates attention (subtest 2 [CTMT-2]) and set-shifting (subtest 5 [CTMT-5]). Set-shifting, which is part of the broader cognitive flexibility concept, is an executive function that involves the ability to shift attention between two different tasks. The CTMT-5 in part mimics the original Trail Making Test part B alphanumeric alternation sequence, but it adds the additional difficulty component of empty distractor circles. Depression was assessed with the Geriatric Depression Scale. All tests were evaluated using standardized published age- and education-related norms. Cognitive impairment was defined by 1.5 standard deviations below standardized mean [26, 27]. BL neuropsychological testing was performed on average 6 ± 3 days poststroke; FU assessment was performed at 15.9 ± 1.9 months poststroke.
Structural MRI analyses
WMH lesion masks were created on fluid-attenuated inversion recovery images by the lesion prediction algorithm [28] using the Statistical Parametric Mapping - Lesion segmentation toolbox subsequent to lesion identification by a single experienced rater (C.E.). WMH lesion volume was calculated for each patient from the generated lesion mask using FSLstats. After lesion filling with the FMRIB Software Library (FSL) lesion filling toolbox[29], normalized brain and gray matter volumes were assessed from T1-weighted images using Structural Image Evaluation, using Normalisation, of Atrophy for cross-sectional data, which is part of the FSL [30]. WMH lesion masks and lesion-filled T1-weighted images were visually checked for their accuracy.
Statistical analyses
Demographics and neuropsychological and clinical scores were analyzed with IBM SPSS Statistics 25 (IBM, Armonk, NY). The level of significance was set at 5%. Normal distribution was assessed with the Kolmogorov-Smirnov test and via skewness and kurtosis, and outliners were assessed via boxplots.
Group comparisons were conducted with unpaired t tests (for normally distributed continuous variables) and Mann-Whitney U tests (for nonnormally distributed variables). Longitudinal within- and between-group comparisons for deep and periventricular WMH groups were done with analysis of covariance (ANCOVA, correcting for age). Post hoc analyses were conducted with pairwise comparisons applying Bonferroni correction. Regarding the between-subject comparison of different WMH groups, RSSI patients were divided into three independent groups according to their WMH severity. Because of the small subsample with Fazekas score 3 regarding deep WMH (n = 9), we merged Fazekas 2 and 3 as moderate-to-severe WMH severity. Consequently, patients were divided into absent WMH (Fazekas grade 0), mild WMH (Fazekas grade 1), and moderate-to-severe WMH severity (Fazekas grades 2 or 3). Due to distinct clinical impairment between deep and periventricular WMH [13, 17, 20], all ANCOVAs were conducted separately for deep and periventricular WMH.
As different parameters (e.g., visual rating distinguishing deep and periventricular WMH vs. volumetric assessment) might be used to describe WMH burden, we compared both approaches in our analyses [31]. To assess which WMH operationalization (dWMH, pWMH, total WMH volume) provides most explanation of variance regarding the prediction of cognitive outcome, multiple linear regression (method FORWARD) was conducted. The prediction of cognitive outcome via demographic variables and MRI parameters was conducted with multiple linear regression (method ENTER). In a first step, we included the demographic covariates age and educational level as predictors for cognitive functioning (MoCA, SDMT, CTMT-2, CTMT-5) 15 months poststroke. In a second step, we included the MRI parameters total WMH volume, gray matter volume, size of the RSSI, presence of lacunes, old cortical infarcts, and microbleeds. Correlation analysis was performed with Spearman (ordinal data) and Pearson (metric data) correlations.
RESULTS
Patient characteristics
A total of 82 patients attended neuropsychological assessment at BL and FU. Demographics and clinical and neuroimaging characteristics of the study cohort are presented in Table 1. Mean age of RSSI patients was 61 ± 10 years at baseline and 23% were female. Overall stroke severity was mild (median NIHSS 2, interquartile range 1–4; median mRS at discharge 1, interquartile range 1–2) and improved after 15 months (median NIHSS 0, interquartile range 0–1, p < 0.001; median mRS 1, interquartile range 0–1, p < 0.001). Regarding dWMH, 28% of patients had no dWMH (Fazekas grade 0), 49% had mild dWMH (Fazekas grade 1), and 23% had moderate-to-severe dWMH (Fazekas grades 2 or 3). pWMH was slightly more prevalent; 21% of patients had no pWMH (Fazekas grade 0), 38% had mild pWMH (Fazekas grade 1), and 41% had moderate-to-severe pWMH (Fazekas grades 2 or 3). Mean total baseline WMH volume was 15.9 ± 17.5 cm3 and did not significantly change within 15 months poststroke (WMH volume FUP: 16.4 ± 17.8 cm3, p = 0.271). Correlations between demographics and MRI parameters are presented in Table 2. Age similarly correlated with dWMH (r = 0.44, p < 0.001), pWMH (r = 0.40, p < 0.001), and total WMH volume (r = 0.43, p < 0.001). Two patients (2.4%) had a recurrent stroke between BL and FU.
| Baseline, N = 82 | |
|---|---|
| Demographics | |
| Age in years, mean (SD) | 61 (10) |
| Sex, female, n (%) | 19 (23.2) |
| Education in years, mean (SD) | 12 (3) |
| Clinical characteristics, median (IQR) | |
| NIHSS | 2 (1–4) |
| mRS at discharge | 1 (1–2) |
| Vascular risk factors, n (%) | |
| Hyperlipidemia | 65 (79.3) |
| Hypertension | 61 (74.4) |
| Active smoking | 32 (39.0) |
| Diabetes mellitus | 12 (14.6) |
| Obesity | 10 (12.2) |
| Depression | 9 (11.0) |
| Location of RSSI, n (%) | |
| White matter | 35 (42.7) |
| Basal ganglia | 20 (24.4) |
| Thalamus | 17 (20.7) |
| Brainstem | 14 (17.1) |
| Deep WMH, n (%) | |
| Fazekas 0 | 23 (28.0) |
| Fazekas 1 | 40 (48.8) |
| Fazekas 2 | 10 (12.2) |
| Fazekas 3 | 9 (11.0) |
| Periventricular WMH, n (%) | |
| Fazekas 0 | 17 (20.7) |
| Fazekas 1 | 31 (37.8) |
| Fazekas 2 | 16 (19.5) |
| Fazekas 3 | 18 (22.0) |
| Structural MRI characteristics | |
| Total WMH volume, cm3, mean (SD) | 15.9 (17.5) |
| Normalized gray matter volume, cm3, mean (SD) | 719.0 (52.0) |
| Normalized brain volume, cm3, mean (SD) | 1479.9 (86.6) |
| Size of RSSI, mm, mean (SD) | 12.0 (4.5) |
| Microbleeds ≥1, n (%) | 11 (13.4) |
| Lacunes ≥1, n (%) | 27 (32.9) |
| Old cortical infarcts ≥1, n (%) | 5 (6.1) |
- Abbreviations: IQR, interquartile range; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; n, sample size; NIHSS, National Institutes of Health Stroke Scale; RSSI, recent small subcortical infarct; SD, standard deviation; WMH, white matter hyperintensities.
| Age, years | Education, years | Deep WMH | Periventricular WMH | Total WMH volume | Gray matter volume | Size of RSSI | No. of microbleeds | No. of lacunes | |
|---|---|---|---|---|---|---|---|---|---|
| Education, years |
r = −0.03 p = 0.783 |
||||||||
| Deep WMH |
r = 0.44 p < 0.001 |
r = 0.04 p = 0.742 |
|||||||
| Periventricular WMH |
r = 0.40 p < 0.001 |
r = 0.06 p = 0.612 |
r = 0.75 p < 0.001 |
||||||
| Total WMH volume |
r = 0.43 p < 0.001 |
r = −0.06 p = 0.624 |
r = 0.69 p < 0.001 |
r = 0.68 p < 0.001 |
|||||
| Gray matter volume |
r = −0.56 p < 0.001 |
r = 0.30 p = 0.007 |
r = −0.21 p = 0.066 |
r = −0.26 p = 0.018 |
r = −0.40 p < 0.001 |
||||
| Size of RSSI |
r = −0.16 p = 0.156 |
r = 0.06 p = 0.624 |
r = −0.03 p = 0.803 |
r = 0.02 p = 0.841 |
r = −0.10 p = 0.370 |
r = 0.04 p = 0.720 |
|||
| No. of microbleeds |
r = 0.01 p = 0.954 |
r = −0.02 p = 0.879 |
r = 0.25 p = 0.022 |
r = 0.34 p = 0.002 |
r = 0.26 p = 0.019 |
r = 0.07 p = 0.544 |
r = 0.20 p=.075 |
||
| No. of lacunes |
r = .04 p = 0.699 |
r = 0.00 p = 0.998 |
r = 0.31 p = 0.005 |
r = 0.37 p = 0.001 |
r = 0.31 p = 0.005 |
r = −0.14 p = 0.205 |
r = 0.02 p = 0.877 |
r = 0.08 p = 0.500 |
|
| No. of old cortical infarcts |
r = 0.15 p = 0.193 |
r = 0.18 p = 0.107 |
r = 0.03 p = 0.788 |
r = 0.23 p = 0.038 |
r = 0.37 p = 0.001 |
r = 0.01 p = 0.913 |
r = 0.13 p = 0.241 |
r = 0.09 p = 0.409 |
r = 0.20 p = 0.072 |
- Bold values are significant.
- Abbreviations: p, probability value; r, correlation coefficient; RSSI, recent small subcortical infarct; WMH, white matter hyperintensities.
Prevalence and course of cognitive impairment
Overall, 40% of RSSI patients showed cognitive impairment (1.5 standard deviations below standardized mean) in global cognition and set-shifting at baseline, with persistent deficits in global cognition in one-third of the entire sample after 15 months. A detailed figure on cognitive trajectories (i.e., how many remained stable vs. converted) is provided in the (Figure S2). Lower percentages of impairment at stroke onset were observed in processing speed (23%) and attention (4%, Table 3). Patients improved significantly in global cognition, processing speed, and set-shifting after 15 months (Table 3). Depressive symptoms were rare at BL and did not change over time (Table 3).
| Prevalence of cognitive impairment | ||
|---|---|---|
| Assessment | Baseline impaired, n (%) | 15 months follow-up impaired, n (%) |
| MoCA: global cognition (<26 raw score) | 33 (40.2) | 27 (32.9) |
| SDMT: processing speed | 19 (23.2) | 10 (12.2) |
| CTMT-2: attention | 3 (3.7) | 2 (2.5)* |
| CTMT-5: set-shifting | 30 (36.6) | 18 (22.2)* |
| Development of cognitive test performance | |||
|---|---|---|---|
| Assessment | Baseline mean (SD) | Follow-up mean (SD) | p |
| MoCA | 26 (3) | 27 (3) | 0.005 |
| SDMT | 34 (10) | 36 (11) | 0.002 |
| CTMT-2 in secondsa | 45 (17) | 46 (21) | 0.584 |
| CTMT-5 in secondsa | 116 (57) | 103 (52) | 0.002 |
| Median (IQR) | Median (IQR) | ||
| GDS: depressionb | 1 (3) | 1 (2) | 0.937 |
| NIHSS | 2 (3) | 0 (1) | <0.001 |
- Bold values are significant.
- Abbreviations: CTMT, Comprehensive Trail Making Test; GDS, Geriatric Depression Scale; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; n, sample size; NIHSS, National Institutes of Health Stroke Scale; p, probability value; SD, standard deviation; SDMT, Symbol Digital Modalities Test.
- a Missing in one patient.
- b Missing in three patients.
Influence of deep WMH on cognitive impairment
In the MoCA (global cognition), patients without dWMH (n = 23, BL = 26.8 ± 2.1, FU = 27.2 ± 2.6) and with mild dWMH (n = 40, BL = 25.9 ± 3.0, FU = 26.9 ± 2.6) performed better at both time points compared to patients with moderate to severe dWMH (n = 19, BL = 24.4 ± 2.5, FU = 25.3 ± 2.4, p = 0.009). All patients improved after 15 months, independent of WMH severity (p = 0.011). After correcting for age, there were no differences between dWMH groups in the MoCA (p = 0.109).
In the SDMT (processing speed), there were no differences between dWMH groups at BL and FU (p = 0.535), and all patients improved after 15 months, independent of dWMH severity (p = 0.010). When corrected for age, differences between dWMH groups became nonsignificant (p = 0.414).
In the CTMT-2 (attention), there were no differences between dWMH groups at BL and FU, neither in the noncorrected analysis of variance (ANOVA) (p = 0.210) nor in the age-corrected ANCOVA (p = 0.432).
In the CTMT-5 (set-shifting), there were no differences between dWMH groups at BL (p = 0.915). However, the course of cognitive function varied depending on dWMH severity (Figure 1). Although patients without dWMH improved (n = 23, p = 0.001), no such improvement was detected for patients with mild (n = 40, p = 0.086) and moderate-to-severe dWMH (n = 19, p = 0.801). When corrected for age, the same results were seen (absent dWMH: p < 0.001, mild dWMH: p = 0.098, moderate-to-severe dWMH: p = 0.980).
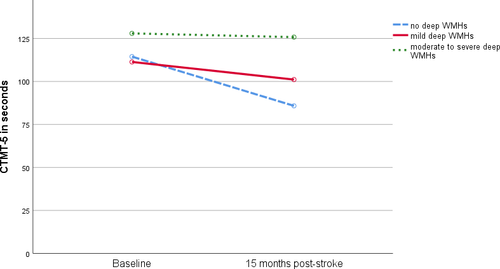
Influence of periventricular WMH on cognitive impairment
In the MoCA (global cognition) and SDMT (processing speed), there were no differences between pWMH groups at BL and FU, neither in the noncorrected ANOVA (MoCA: p = 0.627, SDMT: p = 0.212) nor in the age-corrected ANCOVA (MoCA: p = 0.753, SDMT: p = 0.518).
In the CTMT-2 (attention), there were no differences between pWMH groups at BL (p = 0.406). However, the course of cognitive function varied depending on pWMH severity (Figure 2). Although RSSI patients without pWMH (n = 17, p = 0.661) and mild pWMH (n = 31, p = 0.368) remained stable after 15 months, RSSI patients with moderate-to-severe pWMH (n = 34, BL = 44.6 ± 20.1, FU = 50.3 ± 26.2, p = 0.046) declined. After correcting for age, there was no longer an interaction between pWMH and development of CTMT-2 performance (p = 0.184).
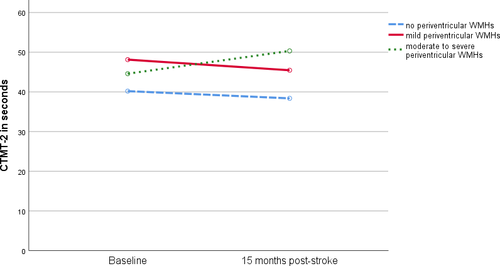
In the CTMT-5 (set-shifting), there were no differences between pWMH groups at BL (p = 0.321). However, the course of cognitive function varied depending on pWMH severity. Although RSSI patients with mild pWMH improved (n = 31, p = 0.001), there was no improvement in set-shifting in patients without pWMH (n = 17, p = 0.073) and moderate-to-severe pWMH (n = 34, p = 0.708). When corrected for age, the same relationships were observed (absent pWMH: p = 0.067, mild pWMH: p = 0.001, moderate-to-severe pWMH: p = 0.816).
MRI markers as predictors for poststroke cognitive impairment
The demographic covariates age and education predicted MoCA (global cognition), SDMT (processing speed), CTMT-2 (attention), and CTMT-5 (set-shifting) 15 months poststroke (MoCA: p = 0.015, R2 = 0.11; SDMT: p < 0.001, R2 = 0.38; CTMT-2: p < 0.001, R2 = 0.19; CTMT-5: p < 0.001, R2 = 0.33).
As an intermediate step, we added different operationalizations for WMH severity (dWMH, pWMH, total WMH volume) using the method FORWARD. Only WMH volume remained significant for the prediction of attention (p = 0.001); dWMH (p = 0.461) and pWMH (p = 0.878) were excluded from the model. Therefore, we used total WMH volume to describe WMH burden in our further analysis.
In addition to demographics, when we controlled for potential influences of gray matter volume, size of RSSI, lacunes, microbleeds, and old cortical infarcts, all models remained significant (MoCA: p = 0.046, R2 = 0.20; SDMT: p < 0.001, R2 = 0.47; CTMT-2: p < 0.001, R2 = 0.36; CTMT-5: p < 0.001, R2 = 0.35). However, only WMH volume (p = 0.002) significantly improved prediction of attention 15 months poststroke beyond demographics (p = 0.012), explaining incremental 17% of variance. Standardized beta coefficients for all predictors are presented in Table 4.
| MoCA | SDMT | CTMT-2a | CTMT-5a | |||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| 1. Step: demographic covariates | ||||||||
| Age, years | −0.18 | 0.096 | −0.48 | <0.001 | 0.39 | <0.001 | 0.44 | <0.001 |
| Education, years | 0.26 | 0.021 | 0.36 | <0.001 | −0.18 | 0.083 | −0.35 | <0.001 |
|
∆R2 = 0.09 p = 0.276 |
∆R2 = 0.09 p = 0.099 |
∆R2 = 0.17 p = 0.012 |
∆R2 = 0.02 p = 0.881 |
|||||
|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | |
| 2. Step: MRI parameters | ||||||||
| Age, years | −0.21 | 0.133 | −0.44 | <0.001 | 0.19 | 0.134 | 0.47 | <0.001 |
| Education, years | 0.28 | 0.017 | 0.34 | 0.001 | −0.14 | 0.197 | −0.37 | 0.001 |
| WMH volume | −0.05 | 0.733 | 0.12 | 0.303 | 0.40 | 0.002 | 0.04 | 0.766 |
| Gray matter volume | −0.02 | 0.874 | 0.19 | 0.118 | −0.13 | 0.349 | 0.05 | 0.695 |
| Size of RSSI | −0.18 | 0.116 | −0.18 | 0.055 | 0.01 | 0.954 | 0.09 | 0.361 |
| Lacunes ≥1 | 0.25 | 0.038 | 0.13 | 0.185 | −0.14 | 0.194 | −0.12 | 0.279 |
| Microbleeds ≥1 | −0.07 | 0.549 | −0.05 | 0.593 | −0.17 | 0.116 | 0.02 | 0.891 |
| Old cortical infarcts ≥1 | −0.04 | 0.764 | −0.13 | 0.209 | −0.02 | 0.855 | −0.03 | 0.810 |
- Bold values are significant.
- β, standardized regression coefficient; ∆R2, incremental variance of MRI parameters; CTMT, Comprehensive Trail Making Test (subtest 2: attention, subtest 5: set-shifting); MRI, magnetic resonance imaging; MoCA, Montreal Cognitive Assessment (global cognition); p, probability value; RSSI, recent small subcortical infarct; SDMT, Symbol Digital Modalities Test (processing speed); WMH, white matter hyperintensities.
- a Missing in one patient.
DISCUSSION
In this longitudinal study on patients with a single symptomatic RSSI, we aimed to assess whether the course of cognitive function is influenced by the severity of preexisting cerebral SVD-related white matter changes (i.e., deep, periventricular, and total WMH severity). Although overall median stroke severity was mild (NIHSS = 2) and improved after 15 months (NIHSS = 0), the prevalence of cognitive impairment was high in our cohort. Approximately 40% of RSSI patients showed cognitive impairment in global cognition and set-shifting at stroke onset, with persistent deficits in global cognition in one-third of the sample after 15 months. These results are comparable with previous studies that reported cognitive impairment in as much as 37% of patients after SVD-related stroke [3, 32].
In line with the few longitudinal studies investigating the influence of WMH on poststroke cognitive impairment [7, 8, 18], we found age-independent associations between higher WMH severity at baseline and worse recovery in set-shifting 15 months poststroke in patients with RSSI. Although patients without and with mild WMH improved in set-shifting after 15 months, there was no improvement in patients with moderate to severe WMH grades. Similar results were found in a large community-dwelling sample, suggesting a decline in cognitive performance as a function of WMH severity independent of age [16]. According to their results, cognitive decline becomes evident at progressed stages of white matter damage (starting from Fazekas grade 2), which corresponds to our definition of moderate-to-severe WMH. Within our specific lacunar stroke population with small vessel disease-related stroke, preexisting white matter damage appears to be associated with a diminished capacity for cognitive improvement after an RSSI.
Regarding the prediction of cognitive development 15 months poststroke, the burden of MRI assessable presumably microangiopathic white matter changes (total WMH volume) improved the prediction of attention beyond demographics (e.g., age, education). Considering all predictors including age and education, WMH volume at baseline was the sole significant factor that predicted attention at 15 months follow-up. This is a notable result because attention is highly dependent on demographic covariates such as age and education [33]. Similar results were found in general ischemic stroke patients and patients with transient ischemic attack [8, 18], concluding that WMH severity was the only predictor among age-corrected MRI markers (e.g., cortical thickness, lacunes, cerebral microbleeds, perivascular spaces) that predicted 12-months poststroke cognitive performance. Another study [7] investigating ischemic stroke patients identified WMH and stroke volume, but not grey matter volume as predictors for cognitive performance 12 months poststroke. In contrast, a study in patients with SVD found that cortical thickness mediates the association between WMH and cognitive performance [34].
A recent paper on knowledge gaps and opportunities regarding WMH addressed the question, whether it is clinically relevant to classify WMH into dWMH and pWMH [21]. A greater number of studies have found associations between pWMH and cognition compared to dWMH and cognition [13]. However, although neuropathological changes that underlie WMH differ between locations, it may be premature to discriminate deep from periventricular WMH in clinical practice [21]. In line with this conclusion, we found a high correlation (r = 0.75) between dWMH and pWMH in patients with RSSI and comparable results regarding cognitive development.
The observed pattern for improvement in set-shifting after 15 months for patients without dWMH, opposed to persistent deficits in patients with moderate-to-severe dWMH, was also observed in patients with mild pWMH. The nonsignificant result for improvement in patients without pWMH is likely due to unbalanced sample sizes (without pWMH: n = 17, mild pWMH: n = 31). The main clinical finding was that preexisting white matter damage (e.g., moderate-to-severe WMH) appears to be associated with a diminished capacity for cognitive recovery, independent of WMH classification.
Regarding the additional value of WMH volume over and above WMH grades, we unsurprisingly found strong correlations between WMH Fazekas grades and WMH volume (dWMH × WMH volume: r = 0.69, pWMH × WMH volume: r = 0.68). The comparability is further shown in the literature, where studies with different WMH operationalization came to similar findings. Studies that used WMH ratings found associations between higher WMH grades and higher impairments in attention [16, 18], memory [16, 18], executive function [18], processing speed [17], visual spatial [18], and verbal functioning [18]. In line with these observations, studies that used WMH volume found associations between higher WMH volume and higher impairments in memory [14], executive function [14], processing speed [14], and perceptional speed [12]. Greater WMH severity is associated with greater cognitive impairment, independent of the operationalization of WMH. Therefore, in clinical practice, the Fazekas rating would provide a simple and feasible assessment to support prediction of cognitive outcome and course of cognitive development in patients with RSSI. Clinical studies, however, might benefit from additional variance provided by total WMH volume to improve prediction of cognitive performance at group level, which was also confirmed by our study.
The main strength of our study is the homogenous study cohort of patients with symptomatic SVD-related RSSI. The few comparable studies analyzing the influence of WMH on poststroke cognitive impairment investigated rather heterogeneous stroke cohorts with different etiologies and infarct size/pattern [7, 8, 18, 19]. Furthermore, our longitudinal design allowed assessing the predictive value of MRI parameters for long-term cognitive impairment. All MRI scans were reviewed according to the current STRIVE criteria [2]. The multimethodological approach including thorough clinical neurological examination, extensive neuropsychological testing, and brain MRI further suggests that WMH severity leads to variation in cognitive improvement within lacunar stroke patients. Better prediction of the course of cognitive impairment is highly relevant in clinical practice for optimal patient follow-up care including planning of neurorehabilitation and social reintegration.
Besides these strengths, our study has also limitations. First, there has been some intended selection of patients, as we only included RSSI patients aged ≤75 years without preexisting disability (mRS ≤ 1) to minimize comorbidity and increase the chance of regular follow-up assessments. Therefore, our results from a comparatively young and less-impaired lacunar stroke cohort may not be generalizable to older and more-impaired stroke patients. However, if more severe brain damage in the acute stroke setting is already associated with a diminished capacity for cognitive improvement in such a cohort of stroke patients, it is likely to find even stronger effects in more strongly affected and older cohorts. Sex within our cohort was unevenly distributed (23% female), similar to previous studies that investigated the influence of WMH on poststroke cognitive impairment [7, 8, 18]. Second, the CTMT-5 measuring set-shifting is a relatively simple estimate of cognitive flexibility. Patients who perform well in the set-shifting task might still have trouble solving assignments that are more complex. However, an impaired performance in the CTMT-5 serves as marker for difficulties in executive functions. Third, baseline MRI was performed on a 1.5T scanner; however, baseline WMH volume did not differ from WMH volume 15 months poststroke (p = 0.271), which was measured on a 3T scanner. Fourth, given the complexity of potential contributors to SVD-related cognitive impairment, our sample size is moderate, reducing the statistical power [7]. Fifth, it is possible that in patients with severe SVD, extensive a priori structural damage might lead to cognitive impairment not altered by a single RSSI. Two matching cohorts of patients with severe WMH with and without RSSI would have to be studied longitudinally to clarify this point. Nevertheless, in this homogeneous cohort, important insights into the dynamics of cognitive improvement of SVD-related stroke could be revealed, stimulating further studies, which should be conducted in larger multicenter cohorts. As shown by the differential pattern of improvement in distinct cognitive domains, a more extensive neuropsychological assessment might have provided important additional cognitive trajectories on, for example, executive function. However, the duration of our assessment was a compromise between clinical practicability and scientific endeavor.
In conclusion, cognitive deficits are highly prevalent in patients with RSSI in the acute phase and at 15 months poststroke. Therefore, improved prediction for the course of cognitive impairment in patients with RSSI is important for planning acute stroke care and rehabilitation. Although in other diseases such as Alzheimer’s, the negative influence of WMH on cognition is better explored [35-37], and only a few longitudinal studies [7, 8, 18] have investigated the impact of WMH severity on cognition in stroke patients so far. Our results show that preexisting moderate-to-severe WMH are associated with a diminished capacity for cognitive improvement after RSSI. Furthermore, WMH volume at baseline improves prediction of attention beyond demographics and gray matter volume. Further longitudinal studies with lacunar stroke patients are needed to better understand the impact of WMH and other SVD related MRI markers on cognitive development within this high-risk population for cognitive impairment.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest that relate to the research covered in this article.
Open Research
Data availability statement
Data that support the findings of this study are available from the corresponding author upon reasonable request.




