Alzheimer's disease
Abstract
Alzheimer's disease, the commonest cause of dementia, is a growing global health concern with huge implications for individuals and society. In this review, current understanding of the epidemiology, genetics, pathology and pathogenesis of Alzheimer's disease is outlined, before its clinical presentation and current treatment strategies are discussed. Finally, the review discusses how our enhanced understanding of Alzheimer pathogenesis, including the recognition of a protracted preclinical phase, is informing new therapeutic strategies with the aim of moving from treatment to prevention.
Introduction
Alzheimer's disease (AD) is recognized by the World Health Organization as a global public health priority. Despite large gains in our understanding of AD pathogenesis and how the disease is conceptualized since Alois Alzheimer reported the first case in 1907 1 there are still no disease-modifying treatments. Here an overview of current thinking in AD, with respect to epidemiology, genetics, pathology and pathogenesis, is provided before its clinical presentation, current treatment options and future therapeutic strategies are considered.
Epidemiology
Dementia – acquired progressive cognitive impairment sufficient to impact on activities of daily living – is a major cause of dependence, disability and mortality. Current estimates suggest that 44 million people live with dementia worldwide at present. This is predicted to more than triple by 2050 as the population ages, when the annual cost of dementia in the USA alone may exceed US$600 billion 2. In England and Wales, dementia is the leading cause of death overall, accounting for 11.6% of all deaths registered in 2015 3. Recent studies suggest that the incidence of dementia, particularly in men, may be declining in western countries; it is unclear which causes of dementia are declining, and this may be underpinned by better management of vascular risk 4, 5. In coming years, the largest increase in dementia prevalence is expected in low and middle income countries, which show patterns of increasing cardiovascular disease, hypertension and diabetes 2. AD is the single biggest cause of dementia, accounting for 50%–75%, and is primarily a condition of later life, roughly doubling in prevalence every 5 years after age 65 2.
Aetiology
Whilst the vast majority of AD occurs on an apparently sporadic basis, mutations in three genes – amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) – cause a rare (<0.5%) familial form of AD (fAD). Symptoms develop earlier than in sporadic AD, typically between 30 and 50 years of age 6.
‘Typical’ late onset AD is likely to be driven by a complex interplay between genetic and environmental factors. It is now thought that ~70% of AD risk is attributable to genetic factors. The APOE gene, which has three variants, ε2, ε3 and ε4, is the single biggest risk for sporadic AD: compared to non-ε4 carriers, ε4 heterozygotes have an odds ratio (OR) for AD of ~3, rising to ~12 in homozygotes 7. Genome-wide association studies using many thousands of samples have identified more than 20 genetic risk factors, implicating inflammatory, cholesterol metabolism and endosomal-vesicle recycling pathways 8. In particular, microglial activation in response to amyloid deposition is now recognized to play a key role in AD pathogenesis. These relatively common risk genes each confer only a very small increased risk, but when combined in a polygenic risk score can almost double case prediction from chance 9. Focused genetic approaches and studies using next generation sequencing have also revealed a number of other low frequency genes conferring relatively high risk for AD, which in turn are providing insights into pathogenesis (Fig. 1).
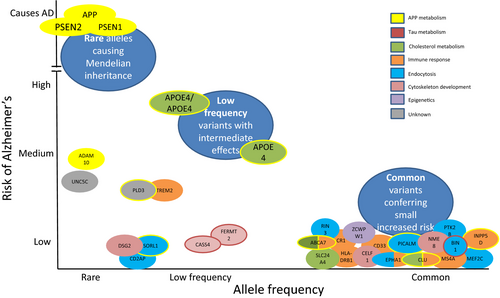
Epidemiological evidence suggests education and physical exercise may protect against AD, whereas mid-life hypertension and diabetes adversely influence risk 10. Obesity has long been considered a risk for dementia and AD, but this has recently been questioned 11. The mechanisms by which vascular risk factors might influence AD remain unclear, not least as few epidemiological studies have pathological confirmation of diagnosis. Vascular risk factors may increase the risk of clinical AD through a ‘double-hit’ with superimposed cerebrovascular damage, or vascular damage might influence the development of AD pathology directly.
Pathology
The cardinal features of Alzheimer pathology are amyloid plaques and neurofibrillary tangles (NFTs) (Fig. 2). In addition, neuropil threads, dystrophic neurites, associated astrogliosis and microglial activation are seen, and cerebral amyloid angiopathy frequently coexists 12. The downstream consequences of these pathological processes include neurodegeneration with synaptic and neuronal loss leading to macroscopic atrophy. Mixed pathology frequently occurs particularly in older individuals and includes vascular disease and Lewy bodies 13. Indeed, even in fAD cases Lewy body pathology often coexists, the mechanism for which remains uncertain 14. TDP-43 pathology is increasingly recognized as an important co-pathology 15.
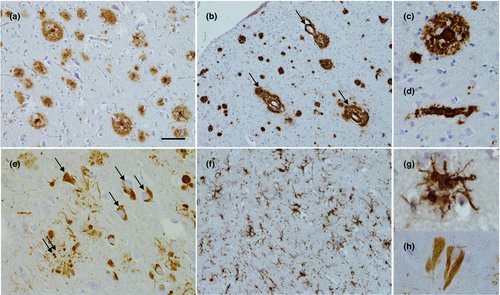
Amyloid plaques are extracellular accumulations principally composed of abnormally folded Aβ with 40 or 42 amino acids (Aβ40 and Aβ42), two by-products of APP metabolism. Aβ42 is more abundant than Aβ40 within plaques due to its higher rate of fibrillization and insolubility. Amyloid deposition does not always follow a stereotypical pattern of progression but broadly speaking develops in the isocortex, and only latterly affects subcortical structures. Unlike NFTs, amyloid plaques involve the entorhinal cortex and hippocampal formations to a lesser extent 12. Different staging systems for Aβ include those from Braak and Braak 16, the Thal criteria 17 and the Consortium to Establish a Registry for Alzheimer Disease (CERAD) 18.
Neurofibrillary tangles are primarily composed of paired helical filaments consisting of hyperphosphorylated tau. Tau pathology typically begins in the allocortex of the medial temporal lobe (entorhinal cortex and hippocampus) before spreading to the associative isocortex. Primary sensory, motor and visual areas tend to be relatively spared. Neuronal and synapse loss typically parallel tangle formation, and as such the clinical features and severity of AD are better correlated with NFT pathology 12, whilst β-amyloid pathology reaches a plateau early in the symptomatic phase of the disease 19.
Several criteria have been proposed for the pathological diagnosis of AD. Early attempts using either amyloid plaques or NFTs were limited by low specificity or sensitivity 20. Previous pathological criteria for AD from the National Institute of Aging and the Reagan Institute combined the CERAD neuritic plaque score with the Braak and Braak NFT staging, deriving three diagnostic categories: high, intermediate and low likelihood. An AD diagnosis could only be made if criteria for high or intermediate likelihood of AD were met in conjunction with a dementia diagnosis 21. A limitation of this system is that it did not address individuals dying with a high burden of AD pathology but without clinical symptoms. Updated National Institute on Aging and the Alzheimer's Association (NIA-AA) neuropathological guidelines attempt to address this, acknowledging the potential for disconnect between the clinical picture and neuropathological changes 22.
Pathogenesis
The amyloid hypothesis, the prevalent theory of AD pathogenesis, suggests that accumulation of pathological forms of Aβ produced by sequential cleavage of the APP by the β- and γ-secretase enzymes in the brain is the primary pathological process, driven through an imbalance between Aβ production and Aβ clearance. The formation of NFTs and subsequent neuronal dysfunction and neurodegeneration, perhaps mediated via inflammation, are thought to be downstream processes 23 (Fig. 3). Strong support for a central role for Aβ comes from genetics: all fAD mutations are involved in either Aβ generation or processing and result in relative overproduction of toxic forms of β-amyloid. Conversely, an APP missense mutation (A673T) results in a lifelong decrease in APP cleavage by β-secretase conferring a reduced clinical risk of AD 24. In sporadic disease, ApoE is involved in amyloid clearance, as are many other risk genes (Fig. 1).
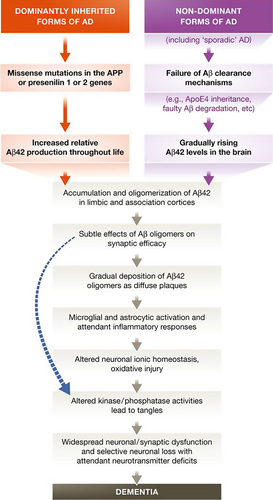
Whilst fibrillar amyloid within dense-core plaques was originally thought to be critical to the development of AD, it is now thought that soluble Aβ oligomers may be the most pathological forms: oligomers purified from AD brains and applied to neurons in vitro inhibit long-term potentiation, cause synaptic dysfunction, damage dendritic spines and cause neuronal death 25, 26. Human oligomers also induce hyperphosphorylation of tau at AD-relevant epitopes and cause neuritic dystrophy in cultured neurons 27. Plaques may therefore act as a ‘reservoir’ from which amyloid oligomers diffuse, or may even act as a protective mechanism sequestering toxic Aβ series until they reach a physiological saturation point 28.
Whilst accumulation of Aβ is necessary for a diagnosis of AD, the fact that a significant proportion of elderly individuals die with evidence for significant β-amyloid deposition without symptoms shows that it is not sufficient for AD dementia. The Aβ soluble oligomer:plaque ratio may be lower in patients with asymptomatic amyloidosis than in patients with AD dementia, supporting the concept that plaques may act as a protective reservoir 29. Tau is clearly a vital part of the process that leads to AD, as evidenced by the requirement for both Aβ and tau pathology for a diagnosis for AD and the close association between neurodegeneration and tau load. However, whilst mutations in the tau gene lead to accumulation of tau and a variety of neurodegenerative dementias within the frontotemporal dementia spectrum 30, unlike mutations in β-amyloid genes, tau mutations alone do not cause AD.
The advent of cerebrospinal fluid (CSF) and positron-emission tomography (PET) biomarkers of Aβ and tau pathology has led to numerous studies exploring the progression and interaction between these pathologies in vivo. Such studies in healthy elderly individuals and patients with both sporadic AD 31 and fAD 32 provide further evidence that amyloid pathology develops many years before clinical symptoms and precedes changes in CSF tau and tau PET which in turn are proposed to predate magnetic resonance imaging (MRI) changes and finally clinical symptoms 31. These models – which as discussed below have led to new criteria for AD – continue to evolve as more data become available; and whilst there are considerable data to suggest that β-amyloid is upstream of tau pathology in AD, some healthy elderly individuals show evidence for tau pathology without β-amyloid which may be part of the normal ageing process or reflect a non-AD neurodegenerative pathway 33.
There is much interest in the mechanisms by which AD proteins target certain brain regions but not others and how they spread through the brain. Abnormally folded Aβ and tau have been shown to induce conformational change in structurally normal peptides, much as occurs in prion disease. These may be transferred trans-synaptically from one neuron to another 34. The site of the original pathological event might then determine which cortical networks are affected and, through differential network breakdown, explain the phenotypic diversity seen in AD.
Whilst amyloid and tau pathology are clearly critical in the pathogenesis of AD, how the two are mechanistically linked is unclear. A number of lines of evidence suggest that the innate immune system plays a critical role in AD pathogenesis and may provide this link. Activated microglia co-localize with amyloid plaques at postmortem 35. A number of AD-risk genes, including CR1, CD33 and TREM2, are involved in immune system pathways 36, 37. Clinical studies using PET ligands which bind to activated microglia provide further in vivo evidence for a role of neuroinflammation in AD 38, 39. The question of whether (or when) neuroinflammation is protective, detrimental or perhaps both may well depend on disease stage and genotypes, and remains to be fully elucidated.
Clinical features
The commonest presentation of AD is of an elderly individual with insidious, progressive problems centred on episodic memory. At this stage, the patient may fulfil criteria for amnestic mild cognitive impairment (MCI). Topographical difficulties subsequently commonly emerge, alongside difficulties with multi-tasking and loss of confidence. As the condition progresses, cognitive difficulties become more profound and widespread so as to interfere with activities of daily living; at this stage a patient can be diagnosed with AD dementia. Increasing dependence is the rule, and later in the disease behavioural change, impaired mobility, hallucinations and seizures may emerge. Death is on average 8.5 years from presentation 40.
A number of atypical (non-memory) clinical syndromes are also recognized, particularly in younger-onset cases. These include posterior cortical atrophy (PCA), logopenic aphasia (LPA) and the frontal variant of AD. In PCA, whilst amyloid is widely distributed, the burden of tau pathology and atrophy is at least initially focused on the parieto-occipital lobes, and patients typically present with prominent visuospatial and visuoperceptual problems and dyspraxia with relatively preserved memory 41. In LPA, patients present with prominent word finding pauses, anomia and impairments in working memory 42. Frontal AD, which is rare, can closely resemble behavioural variant frontotemporal dementia 43. fAD tends to have a typical amnestic presentation, albeit at a much younger age. Some PSEN1 mutations are associated with additional features including prominent myoclonus, seizures and spastic paraparesis 44.
Diagnostic criteria
With the recognition that the pathological changes occur years prior to symptoms, and the advent of biomarkers of β-amyloid and tau pathology and MRI measures of atrophy, diagnostic criteria have evolved to allow for the diagnosis to be made both earlier and with increased molecular specificity. The most recent diagnostic criteria from both the National Institute of Aging (NIA) and the International Working Group (IWG-2) now incorporate one or more preclinical AD phases, where biomarker evidence of AD pathology exists in the absence of symptoms 45-47. Whilst a definitive diagnosis of AD still requires pathological confirmation, the NIA-AA criteria allow dementia, or mild cognitive impairment, to be attributed to underlying Alzheimer pathology with high, intermediate or low likelihood by incorporating biomarker information. Both sets of criteria also recognize atypical, non-amnestic presentations 46, 48 (Table 1).
| NIA-AA criteria 45, 48, 59 | IWG-2 criteria 46 | Proposed preclinical AD criteria from Dubois et al. 47 | |
|---|---|---|---|
| Asymptomatic individuals | |||
| Evidence of Aβ pathology only | Stage 1 preclinical AD | Asymptomatic at risk for AD | Asymptomatic at risk for AD (asymptomatic A+) |
| Evidence of tau pathology only | – | – | Asymptomatic at risk for AD (asymptomatic T+) |
| Evidence of both Aβ and tau pathology |
Stage 2 preclinical AD Stage 3 preclinical AD (when additional subtle cognitive changes exist which do not meet criteria for mild cognitive impairment) |
Asymptomatic at risk for AD | Preclinical AD |
| Symptomatic individuals | |||
| Symptoms that do not meet criteria for dementia |
Mild cognitive impairment due to AD
|
Prodromal AD (typical or atypical) Requires consistent history plus biomarker evidence (CSF Aβ and tau or amyloid PET) |
|
| Symptoms that meet criteria for dementia (both typical and atypical phenotypes) |
Dementia due to AD
|
Typical or atypical AD Requires consistent history plus biomarker evidence (CSF Aβ and tau or amyloid PET) |
|
- The IWG-2 criteria do not specifically differentiate between mild cognitive impairment and dementia, focusing on diagnosing the underlying disease process rather than the clinical syndrome. In NIA-AA criteria, neuronal injury biomarkers include elevated CSF tau, medial temporal lobe atrophy on structural MRI or hypometabolism on FDG PET. These latter two biomarkers can be used instead of tau pathology for defining stage 2/3 preclinical AD.
Important differential diagnoses
In patients presenting to clinic with memory complaints, the differential diagnosis is broad. Causes other than AD include individuals anxious about perceived memory loss in the absence of objective evidence for impairment, the so-called ‘worried well’; individuals with affective disorders; and the effects of drugs and alcohol. Other mimics of AD include other neurodegenerative disorders including Lewy body dementia and frontotemporal dementia; vascular cognitive impairment; infectious, inflammatory and metabolic conditions; and a range of miscellaneous causes including transient epileptiform amnesia and obstructive sleep apnoea.
Diagnostic approach
The mainstay of the diagnosis of AD remains the clinical assessment, and in particular the clinical interview with the patient and an informant, and a cognitive and focused physical examination. Neuropsychology allows for the quantification of both the pattern and severity of cognitive deficits against age-related norms.
Blood tests are performed routinely to exclude conditions which may cause, or more commonly contribute, to cognitive symptoms and typically include full blood count, renal function, thyroid function, vitamin B12 and folate. Depending on the clinical scenario, it may be appropriate to exclude a range of inflammatory, metabolic and infective causes with specific serological tests (e.g. anti-nuclear, anti-neuronal, Lgi1 antibodies, syphilis and HIV serology).
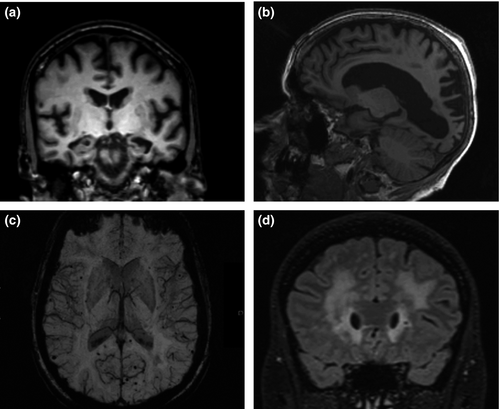
Structural imaging, using computed tomography or ideally MRI, is recommended for all patients investigated for cognitive impairment to exclude structural abnormalities and provide positive diagnostic information 49. The presence of focal symmetrical medial temporal atrophy has predictive value for AD 50. In PCA AD, there is typically parieto-occipital atrophy with relative sparing of the hippocampi, at least early in the disease 41. MRI allows for the other neurodegenerative diseases to be excluded and for the presence and extent of cerebrovascular disease (e.g. white matter hyperintensities and lacunar infarcts) which can mimic, or very commonly co-occur with, AD to be evaluated. Cerebral microbleeds may be evaluated using iron-sensitive sequences: deep microbleeds are more likely to be due to hypertension, whilst lobar microbleeds are more likely to be caused by cerebral amyloid angiopathy 51 and, in the correct clinical context, to have positive predictive value for AD. Figure 4 shows representative MR images. 18F-fluorodeoxyglucose (FDG) PET hypometabolism in the parieto-temporal association areas, posterior cingulate and precuneus is supportive of an AD diagnosis 52.
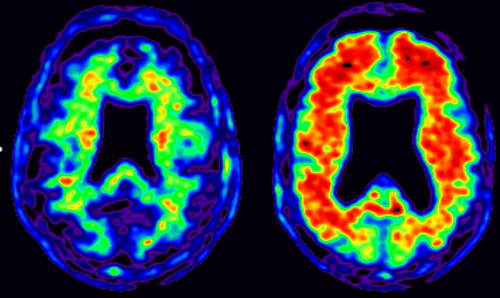
Amyloid PET imaging (Fig. 5) is now available clinically, with three agents approved by the European Medicines Agency and the US Food and Drug Administration. Florbetapir, flutemetamol and florbetaben all bind fibrillary β-amyloid and closely correlate with β-amyloid burden at postmortem 53-55. To date, amyloid PET is not routinely reimbursed in most countries; a number of ongoing studies are currently evaluating its clinical utility and cost-effectiveness 56. Tau PET imaging, using tracers such as AV1451, is a recent development which is currently only used for research purposes 57.
Cerebrospinal fluid examination can be used to exclude rare, reversible causes of cognitive impairment but also to aid in a positive, molecular diagnosis of AD. The typical CSF pattern in AD is low Aβ42 and elevated levels of both tau and phospho-tau; this pattern also has value in predicting which individuals with MCI will develop AD 58 and as a result is included in diagnostic criteria 59. There are to date no AD-specific blood based biomarkers in routine clinical use 60.
Genetic testing, with appropriate consents, can be used to identify autosomal dominant causes of AD where these are suspected. The increasing availability of genetic panels using next generation sequencing allows for large numbers of genes to be tested concurrently at reasonable cost. Routine testing of genetic risk factors (e.g. ApoE status) is not currently recommended.
Treatment/management
Disease-modifying treatments, i.e. those proven to alter the underlying disease pathology or disease course, are not yet available. Optimal management needs to be tailored to the individual patient and their specific circumstances, and to adapt as the disease progresses. Both the patient and carers should be involved in decision-making, with all reasonable steps taken to allow for patient involvement even as cognition declines; a multidisciplinary approach including medical professionals, nurses, social services and charities/support services is vital. Important issues to consider include driving, noting that a diagnosis of AD does not necessarily preclude driving if symptoms are mild and executive and parietal functions are relatively preserved; support at home; finances; and future planning especially whilst the individual has capacity to make decisions. Referral to palliative care to discuss end-of-life planning can be particularly valuable, ideally in advance of end-stage dementia.
Acetyl-cholinesterase inhibitors (AChEIs) (donepezil, galantamine and rivastigmine) are the mainstay of symptomatic treatment, increasing acetylcholine availability by inhibiting its breakdown in the synapse. Peripheral cholinergic side effects such as leg cramps and gastrointestinal upset are common but usually well tolerated, especially when the drugs are introduced at low dose and titrated slowly. AChEIs should be avoided or used with caution in individuals with heart conduction defects due to the risk of brady-arrhythmias. AChEIs have proven beneficial effects in mild to severe AD, with most evidence at the mild to moderate stage 61. There are fewer data on measures of behavioural disturbance and activities of daily living, but some evidence of benefit. In all domains, however, the benefit observed in clinical trials is modest at best. There is no evidence that one drug in the class is more efficacious than another 61; differences in the frequency of dosing, dose variation, timing of escalation, and delivery (oral and transdermal) provide options that can be tailored to individual patients. The DOMINO-AD study demonstrated that withdrawal of donepezil in moderate to severe AD increased the risk of nursing home placement in the following 12 months of treatment, but not in the following 3 years. The authors suggested that withdrawal of treatment may have potential risks, even when the benefits of continuing are not clear 62.
Memantine is an alternative symptomatic treatment, licensed for moderate to severe AD. Memantine, a low affinity N-methyl-d-aspartate receptor antagonist, aims to reduce l-glutamate excitatory neurotoxicity without interfering with its physiological actions. Side effects include constipation and headache. Memantine has been shown to have a small but clinically appreciable benefit on cognition and functional decline in patients with moderate to severe AD, with some evidence that it reduces the likelihood of patients developing agitation 63. There is now some evidence for combination therapy using an AChEI and memantine. A recent meta-analysis found weak evidence for improved cognition with dual therapy, but there was evidence of improved behavioural symptoms in moderate to severe AD 64.
Concurrent psychiatric disturbances are common and often difficult to treat. Depression and anxiety are frequently seen in AD and have significant impact on quality of life, caregiver burden and risk of institutionalization 65. There is only limited evidence of benefit for antidepressant treatment for depression 66. There is some evidence for benefit of psychological treatments in reducing depression and, to a lesser extent, anxiety in patients with dementia 65. Tricyclic antidepressants can worsen confusion and should be avoided.
Agitation, aggression and psychosis may develop in later-stage dementia. Atypical antipsychotics are usually favoured over typical agents but, regardless of drug, benefits are moderate 67, and no treatments are licensed for behavioural symptoms in dementia. Serious adverse events include chest infection, stroke and death. Consequently, antipsychotics should be avoided if possible and limited to those with neuropsychiatric symptoms, particularly psychosis, that are severe, debilitating or pose safety risks 68; ongoing use needs regular review. Where required, the best evidence is for low dose risperidone 69. Non-pharmacological approaches are preferred and include communication skills training, music therapy and person-centred care training which have some evidence of benefit 70.
Future prospects
Although our understanding of AD has increased dramatically over recent years, it remains far from complete. Next generation genetic studies have implicated a number of pathways important to the pathogenesis of AD: these are currently being explored in cellular and animal models and are already leading to the identification of novel drug targets. A more nuanced model of the preclinical phase of AD, no longer viewing β-amyloid, tau and inflammation as steps along a sequential pathway but as part of a cellular phase of AD pathogenesis 71, will also lead to a more sophisticated approach to treatment and prevention.
The failure of a number of major phase 3 clinical trials using monoclonal antibodies targeting cerebral β-amyloid has prompted scepticism about the amyloid hypothesis, but perhaps more worryingly about prospects for disease modification in AD more generally. It is important to note, however, that many of these studies have been complicated by concern over target engagement and patient selection 72: not only did a proportion of individuals recruited for some of these trials not have evidence for AD pathology 28, but most studies have targeted patients with later-stage AD, by which time β-amyloid may no longer be the most appropriate target. There are a number of ongoing clinical trials which will report over the next few years – with encouraging preliminary findings from a trial of aducanumab, targeting AD at an earlier stage and showing reduction in amyloid burden and delay in disease progression at 1 year in prodromal and mild AD patients 73; a number of other studies in MCI and mild AD patients are ongoing 74. There is a concerted effort to use strategies to either clear amyloid using immunotherapy or prevent the formation of pathological forms (either with β-site APP cleaving enzyme BACE or γ-secretase inhibitors/modulators) in the preclinical phase. In fAD cohorts, the DIAN-TU and API-ADAD studies are identifying at-risk individuals with genetic screening 75, 76. The Generation study is recruiting ApoE4 individuals; and the A4 study is recruiting healthy elderly individuals with asymptomatic amyloidosis 77. Alternative targets including tau pathology are attracting interest, with a number of clinical trials ongoing. Targeting neuroinflammation also has potential, although there have been no positive trials to date 35.
If disease-modifying therapies do provide a signal for efficacy in patients with established disease, it will be vital to ensure both that they are affordable and that they can be rolled out quickly and equitably to all who will benefit, which will be a major challenge for existing healthcare systems. For disease prevention, it will be necessary to identify accurately which individuals are at risk. The development of new disease-specific biomarkers using PET, CSF and, in due course, blood has already provided important insights into the pathways leading to the development of AD. Application of these technologies to ever larger cohorts, particularly when combined with genetic data, will improve our ability to detect individuals at risk of developing AD. Longer term follow-up will allow for the development of risk models and biomarkers that can predict not only if an individual is at risk of AD but when – information vital both for clinical trials and eventually for personalized medicine. Combining this information with epidemiological approaches will provide a rational evidence base for the extent to which AD can and cannot be prevented by interventions in early or mid-life 78.
Ultimately, a time can be foreseen when polygenic risk score and other health measures proven to be risk factors can be combined to create an individualized risk score (much like the Framingham cardiovascular risk calculator). At an appropriate age, high risk individuals can then be referred for more invasive tests of AD pathology, e.g. amyloid imaging, and other perhaps blood based biomarkers to predict proximity to disease, with bespoke treatments with a range of different agents being targeted to that individual's stage of disease. Whilst this model of personalized disease prevention may be some way off, advances on numerous fronts make this vision, if not yet a reality, at least in sight.
Acknowledgements
JMS acknowledges the support of the NIHR Queen Square Dementia Biomedical Research Unit, the NIHR UCL/H Biomedical Research Centre, Wolfson Foundation, EPSRC (EP/J020990/1), MRC (MR/L023784/1), ARUK (ARUK-Network 2012-6-ICE; ARUK-PG2017-1946; ARUK-PG2017-1946), Brain Research Trust (UCC14191) and European Union's Horizon 2020 research and innovation programme (grant 666992). JH receives grant support from an Anonymous Foundation, and is a co-grantee with Cytox from Innovate UK, UK Department of Business.
Disclosure of conflicts of interest
Dr Lane has no disclosures. Professor John Hardy has no disclosures. Dr Schott has received research funding from Avid Radiopharmaceuticals (a wholly owned subsidiary of Eli Lilly), has consulted for Roche Pharmaceuticals and Eli Lilly, and serves on a Data Safety Monitoring Committee for Axon Neuroscience SE.




