Huntington's disease: a clinical review
Abstract
Huntington's disease (HD) is a fully penetrant neurodegenerative disease caused by a dominantly inherited CAG trinucleotide repeat expansion in the huntingtin gene on chromosome 4. In Western populations HD has a prevalence of 10.6–13.7 individuals per 100 000. It is characterized by cognitive, motor and psychiatric disturbance. At the cellular level mutant huntingtin results in neuronal dysfunction and death through a number of mechanisms, including disruption of proteostasis, transcription and mitochondrial function and direct toxicity of the mutant protein. Early macroscopic changes are seen in the striatum with involvement of the cortex as the disease progresses. There are currently no disease modifying treatments; therefore supportive and symptomatic management is the mainstay of treatment. In recent years there have been significant advances in understanding both the cellular pathology and the macroscopic structural brain changes that occur as the disease progresses. In the last decade there has been a large growth in potential therapeutic targets and clinical trials. Perhaps the most promising of these are the emerging therapies aimed at lowering levels of mutant huntingtin. Antisense oligonucleotide therapy is one such approach with clinical trials currently under way. This may bring us one step closer to treating and potentially preventing this devastating condition.
Introduction
In 1872 George Huntington wrote an account of hereditary chorea, which is now known as Huntington's disease (HD). He described its hereditary nature, associated psychiatric and cognitive symptoms and the manifestation of the disease in adult life between 30 and 40 years of age. He outlined the progressive nature of the disease stating, ‘Once it begins it clings to the bitter end’ 1. However, the monogenic nature and full penetrance of HD makes it perhaps one of the most treatable neurodegenerative diseases. This has become particularly apparent in the last decade with the advent of new therapeutic approaches that can directly target the HD gene and prevent production of the toxic mutant huntingtin protein 2.
Aetiology
Huntington's disease is caused by an autosomal dominantly inherited CAG trinucleotide repeat expansion in the huntingtin (HTT) gene on chromosome 4. This results in the production of a mutant huntingtin (mHTT) protein with an abnormally long polyglutamine repeat 3. Those with greater than 39 CAG repeats are certain to develop the disease, whilst reduced penetrance is seen between 36 and 39 repeats. Anticipation can be seen when the gene is passed down the paternal line, such that a father with a CAG repeat length in the intermediate range may have a child with an expanded pathogenic repeat length. This is because sperm from males shows greater repeat variability and larger repeat sizes than somatic tissues 4.
Epidemiology
Huntington's disease has a prevalence of 10.6–13.7 individuals per 100 000 in Western populations. Japan, Taiwan and Hong Kong have a much lower incidence of HD with a prevalence of 1–7 per million; in South Africa lower rates are seen in black populations compared to white and mixed populations. The difference in disease prevalence across ethnic groups relates to genetic differences in the HTT gene. Populations with a high prevalence have longer average CAG repeats. For example those of European ancestry have an average of 18.4–18.7, whilst those of Asian ancestry have an average of 16.9–17.4 5.
Pathogenesis
Molecular pathogenesis
Mutant huntingtin results in neuronal dysfunction and death through a number of mechanisms. These include direct effects from the exon 1 mHTT fragment, the propensity of mHTT to form abnormal aggregates and its effects on cellular proteostasis, axonal transport, transcription, translation, mitochondrial and synaptic function 6, 7 (Fig. 1). Medium spiny neurons (MSNs) of the striatum are selectively vulnerable to the effects of mHTT. Striatal pathology follows a biphasic course with initial loss of MSNs of the indirect pathway leading to a hyperkinetic phenotype followed by loss of MSNs of the direct pathway resulting in a hypokinetic phenotype 8. The cause for the selective vulnerability of indirect pathway MSNs is unclear; however, dopamine D2 receptors may be a factor as they are expressed by indirect but not direct MSNs and have been implicated in HD pathogenesis 9; other hypotheses include the loss of brain derived neurotrophic factor, glutamate excitotoxicity from cortico-striatal projections and toxic effects of repeat associated non-ATG translation proteins 6, 10.
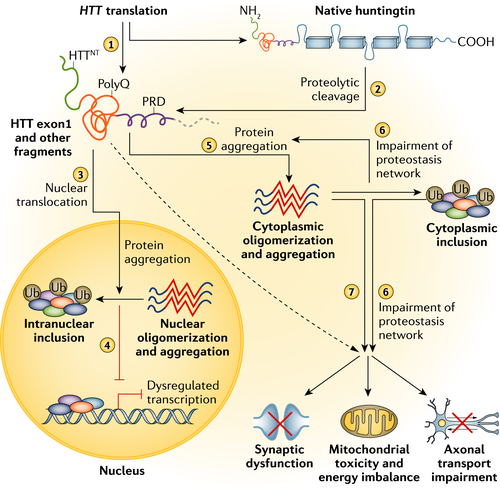
Macroscopic pathology
Post-mortem studies reveal diffuse atrophy of the caudate and putamen with degeneration occurring along a caudo-rostral, dorso-ventral and medio-lateral gradient. The globus pallidus and nucleus accumbens are also affected but to a lesser extent 11. A classification system for HD pathology has been developed which consists of five grades: grade 0, clinical evidence for HD but no gross or microscopic abnormalities that could be related to HD; grade 1, no macroscopic abnormalities in the caudate or putamen but moderate fibrillary astrocytosis at the microscopic level; grade 2, macroscopic changes in the caudate and putamen but no macroscopic changes in the globus pallidus; grade 3, lateral segment of the globus pallidus showing fibrillary astrocytosis with the medial segment of the globus pallidus unchanged; grade 4, shrunken caudate yellow-brown in colour, widened anterior horn of lateral ventricle and smaller nucleus accumbens 12.
At grades 3 and 4 changes are also seen in other brain regions including the thalamus, sub-thalamic nucleus, white matter and cerebellum. With respect to the cerebral cortex atrophy is variable even in stages 3 and 4 12. More recently advances in magnetic resonance imaging (MRI) have confirmed these early pathological findings in vivo, particularly loss of caudate and putamen grey matter volume and loss of both striatal and cortical white matter 13.
Clinical features
Diagnosis
Diagnosis of HD is based on a confirmed family history or positive genetic test and the onset of motor disturbance as defined by the Unified HD Rating Scale (UHDRS) total motor score (TMS) diagnostic confidence score. This score ranges from 0 (no motor abnormalities suggestive of HD) to 4 (≥99% to be due to HD), with a score of 4 defining motor onset or ‘manifest’ HD. However, subtle motor, cognitive and psychiatric deficits can be identified up to 10–15 before the onset of manifest disease and this is referred to as the pre-manifest stage of the disease 14.
CAG length and clinical phenotype
The full penetrance of HD in mutation carriers with >39 CAG repeats makes it an ideal model for studying the preclinical phase of neurodegeneration, as it is possible to predict who will develop the disease many years before symptom onset. Reduced penetrance is seen between 36 and 39 repeats 15, whilst 27–35 is considered the intermediate range and below 27 is normal. CAG repeat length accounts for approximately 56% of the variability in age of onset 16 and is also correlated with progression of motor and cognitive deficits 17.
Genetic modifiers
Genetic factors independent of CAG repeat length have also been shown to modify HD. The largest genome-wide association study (GWAS) in HD identified a number of genes involved in DNA repair that can alter the age of motor onset. Two genes on chromosome 15, FAN1 (Fanconi anemia FANC1/FANCD2-associated endonuclease) and MTMR10 (myotubularin related protein 10) were shown to be the most significant. On chromosome 8 significant associations were also seen with RRM2B (a subunit of DNA damage p53-inducible ribonucleotide reductase M2 B) and URB5 (an HECT domain E3 ubiquitin-protein ligase). In addition, genetic pathway analysis implicated gene pathways involved in DNA repair, mitochondrial fission and oxidoreductase activity 18. Similarly a recent GWAS has revealed association between HD progression and a genetic variant in MSH3, a DNA repair gene, associated with CAG somatic instability 19.
Natural history studies
In recent years a number of multi-centre natural history studies have been pivotal in our understanding of disease onset and progression and in the search for clinical and imaging biomarkers. The largest study to date is REGISTRY, which is a European study spanning 16 countries with over 17 000 participants collecting motor, cognitive, behavioural and biosample data 20. The Cooperative HD Observation Research Trial (COHORT) 21 and the Prospective Huntington At Risk Observational Study (PHAROS) 22 are both prospective longitudinal studies tracking changes in motor, cognitive and behavioural variables.
In addition to extensive clinical data PREDICT and TRACK-HD collected imaging data across multiple time points. PREDICT included over 1000 participants followed up over 10 years and focused on identifying measures that predict conversion to manifest HD 23. TRACK-HD was a study focused on the evaluation of biomarkers for clinical trials and included 123 controls, 120 pre-manifest and 123 with manifest disease, followed up over 3 years 24. This has now extended to Track-On HD, which aims to identify functional markers of pre-manifest HD and study mechanisms of brain compensation over three time points 1 year apart 25.
Motor disturbance
In keeping with the biphasic course of striatal pathology with initial loss of MSNs of the indirect pathway followed by loss of MSNs of the direct pathway 8, movement disturbance in HD can be split into a hyperkinetic phase with prominent chorea in the early stages of the disease, which then tends to plateau 26. The hypokinetic phase is characterized by bradykinesia, dystonia, balance and gait disturbance. The hypokinetic movement disorder shows association with disease duration and CAG length whilst chorea does not 27.
Assessment of motor disturbance is based on the UHDRS TMS, which assesses eye movements, speech, alternating hand movements, dystonia, chorea and gait. Whilst the UHDRS TMS is sensitive to change over time 28 it is also subject to inter-rater variability 29. More quantitative assessments such as the (quantitative) Q-motor battery, which includes tongue force variability, grip force, speeded and self-paced tapping 30, have shown sensitivity to longitudinal change 28.
Cognitive disturbance
Cognitive disturbance can be seen many years before symptom onset and follows a sub-cortical pattern characterized by impaired emotion recognition, processing speed, visuospatial and executive function 31. In early manifest disease longitudinal changes can be demonstrated over 12 and 24 months by performance on the symbol digit modalities test which assesses psychomotor speed, Stroop word reading which assesses executive function, indirect circle tracing which is used to assess visuospatial performance and the emotion recognition test 13, 32. This extends to pre-manifest HD at 36 months, with Stroop word reading demonstrating the highest sensitivity for those furthest from disease onset 28.
Neuropsychiatric disturbance
A wide variety of neuropsychiatric symptoms occur in HD, including apathy, anxiety, irritability, depression, obsessive compulsive behaviour and psychosis. Whilst high rates are seen in manifest disease 33, psychiatric disturbance is also common many years before symptom onset in the pre-manifest stage 24. The most recent study from the REGISTRY cohort, which includes both pre-manifest and manifest participants, shows that apathy is the most common occurring in 28%, whilst depression, irritability and obsessive compulsive behaviour occur in around 13%. Psychosis is relatively rare, occurring in 1% 34.
Whilst apathy, irritability and depression are all related to functional decline, apathy is the only neuropsychiatric symptom that has been shown consistently to progress with disease 28. This may be due to the lack of effective treatments for apathy in comparison to the use of anti-depressants and anti-psychotics for depression, anxiety and irritability.
Quality of life
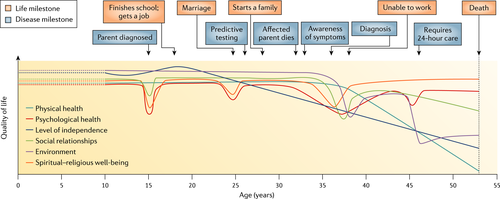
Huntington's disease has a profound effect on quality of life, which begins with the diagnosis of a parent. In one study over 50% of at risk adults reported adverse childhood events related to a diagnosis of HD in the family 35. Reduced total functional capacity is seen after the onset of symptoms with the loss of employment and the need for job modification in the early stages. As the disease progresses to the end stage there is a need for 24 h care (Fig. 2). Motor and cognitive decline are predictive for long-term placement in care 7.
Differential diagnoses
In the absence of an mHTT mutation the triad of chorea, cognitive and neuropsychiatric disturbance is known as an HD phenocopy. Whilst diagnosis can only be achieved in around 3% 36 of these cases there are a number of genetic conditions that may present as HD phenocopies. The most common of these in European populations are C9orf72 37 and spinocerebellar ataxia (SCA) 17 36. Additional features such as ataxia or peripheral neuropathy may suggest other diagnoses such as SCA 1−3 or Friedrich's ataxia. In the case of seizures dentatorubral-pallidoluysian atrophy should be considered. Iron accumulation disorders such as neuroferritinopathy and neurodegeneration with brain iron accumulation may reveal abnormal MRI imaging. In the case of neuroacanthocytosis abnormal acanthocytes can be seen on peripheral blood films. Huntington's disease like syndrome 2 (HDL2) is the most common cause of HD phenocopies in African populations 37, 38.
Isolated chorea can be caused by acquired conditions including striatal pathology, chorea of pregnancy, systemic lupus erythematosus/anti-phospholipid syndrome, thyrotoxicosis, post-infectious syndromes, polycythaemia rubra vera and drugs 38.
Investigations
Genetic testing
Genetic testing for the mHTT mutation can be either diagnostic or predictive. In the case of a diagnostic test this may be performed when a patient presents with typical motor features of HD. Prior to testing it is important to inform the patient about HD and its hereditary nature as a positive test has implications both for the patient and his/her family. Delivering the news of a positive genetic test should be done face-to-face with the patient and his/her family. The option of referral to a specialist HD management clinic should also be available 39.
Predictive testing is done prior to symptom onset in adults who are at risk of inheriting the HTT gene mutation. International guidelines were established shortly after the identification of the HTT gene in 1993 3 and have been updated in 2013 40. The protocol for predictive testing consists of pre-test counselling where the candidate is provided with information in order to make an informed decision regarding the risks and benefits of testing. After a period of time this is followed by a neurological examination to ensure the candidate is not symptomatic and then psychological screening to identify those at high risk of suicide in the event of a positive result. Post-test follow-up is also carried out to monitor the effects of the test result and assess if the candidate requires any further support 41. Predictive testing is commonly performed for reproductive reasons. Reproductive options for at risk individuals include prenatal diagnosis and termination of pregnancy in the event that the foetus carries the expanded CAG or pre-implantation genetic diagnosis performed during in vitro fertilization where only embyros without the CAG expansion are transferred. These options are also available to those unaware of their gene status by use of an exclusion test, which tests for the mutant HTT allele of the affected grandparent 42.
Management
The optimal management of HD involves a multidisciplinary approach involving physicians, nurses, physiotherapists, speech and language therapists, dieticians and other healthcare professionals. The aim is to optimize quality of life and pre-empt the changing needs of the patient as the disease progresses. This usually involves a combination of pharmacological and non-pharmacological interventions. In many instances the evidence base for pharmacological treatments is sparse and decisions are made based on expert opinion and clinical experience 43.
Motor symptoms
Chorea is one of the most prominent symptoms in HD and occurs early in the disease. The only drug specifically licensed to treat chorea is tetrabenazine 44. This is a synaptic vesicular amine transport inhibitor, which provides a sustained anti-choreic effect at doses in the range 50–75 mg per day. Side effects include sleep problems, depression, anxiety and restlessness 45.
Deutetrabenazine is a modified version of tetrabenazine that contains deuterium molecules. This results in a prolonged half-life and less metabolism variability. The FIRST-HD study revealed that compared to placebo deutetrabenzine significantly reduces chorea 46 and, whilst no head-to-head studies have been performed comparing tetrabenazine and deutetrabenazine, there is the suggestion that deutetrabenazine may result in fewer side effects such as depression and somnolence 47.
Sulpiride, a neuroleptic, has shown efficacy in treating chorea in a randomized controlled trial (RCT). In clinical practice other neuroleptics including olanzapine, respiridone and quetiapine are also commonly used, with sedation and weight gain being the most common side effects 45. Other motor symptoms such as abnormal gait, poor balance and frequent falls are commonly treated with physiotherapy.
Psychiatric symptoms
There is limited evidence with regard to the treatment of psychiatric symptoms in HD; therefore treatment decisions are based on clinical consensus and expert opinion. Depression, anxiety, obsessive compulsive disorder and irritability may be treated with non-pharmacological interventions such as cognitive behavioural therapy or psychodynamic therapy; however, these approaches may be limited in the context of cognitive impairment. Pharmacological interventions include selective serotonin uptake inhibitors (citalopram, fluoxetine, paroxetine and sertraline) and mirtazepine and venlafaxine, which have serotonergic and noradrenergic effects. Neuroleptics can be useful in treating aggression and psychosis. A number of medications including methylphenidate, atomoxetine, modafinil, amantadine, bromocriptine and bupropion have been used to treat apathy; however, no RCTs have been performed 48.
Cognitive symptoms
Two RCTs have assessed the use of anti-cholinesterase inhibitors for cognition in HD; however, participant numbers were small and results were conflicting 49. Another RCT found no effect of citalopram on cognitive function 50. Coping strategies to deal with cognitive deficits can be beneficial, e.g. requesting that employers change the type of work or work setting, arrange work in a quiet environment or change to work that requires less multi-tasking 51.
Biomarkers
Clinical
The cognitive measures Stroop word reading, symbol digit modalities and circle tracing (direct and indirect) are sensitive to longitudinal change in HD over 24 months; however, relatively little change is seen in pre-manifest HD over this time course 32. Quantitative measures of chorea, grip force and speeded tapping show changes in HD over 24 months, with speeded tapping also showing longitudinal change in pre-manifest HD 52. Longitudinal change in psychiatric measures is more variable and is only seen in apathy 28.
Biofluid biomarkers
A number of potential blood biomarkers have emerged. The most promising of these is neurofilament light (NFL) protein. Baseline plasma levels of NFL protein show correlation with progression in brain atrophy and motor and cognitive measures and over time. In pre-manifest HD baseline plasma NFL is associated with disease onset over a 3-year period. Plasma NFL is highly correlated with cerebrospinal fluid (CSF) NFL, suggesting that peripheral blood sampling may be sufficient to detect NFL accurately, therefore avoiding the need for more invasive CSF collection 53. Transcription studies using RNA derived from blood reveal abnormal gene expression in HD compared to controls; however, results have been conflicting 54, 55. Increases in immune proteins in plasma, using a proteomics approach, also show correlation with disease stage 56. More recently it has been possible to detect mHTT from blood-derived monocytes, with levels correlating with disease burden and caudate atrophy 57. Longitudinal studies are yet to reveal whether these approaches can detect change over time.
Much research has focused on searching for CSF biomarkers 58. Perhaps the most promising of these is the detection of mHTT in CSF. mHTT in the CSF correlates with disease burden and is also associated with cognitive and motor performance 59. Other CSF markers are also linked to disease stage, such as tau, NFL and measures of inflammation 60, 61. HDClarity is a large multi-site initiative that has recently been set up in order to collect large numbers of CSF samples to facilitate further CSF biomarker investigation (http://hdclarity.net).
Imaging
Structural MRI has been the most extensively studied imaging modality in HD to date. TRACK-HD evaluated changes in both grey and white matter volume at three time points 1 year apart. This study revealed grey matter volume loss in the striatum and loss of white matter volume around the striatum, within the corpus callosum and in the posterior white matter tracts in the pre-manifest stage extending to widespread loss of white matter volume, and to a lesser extent grey matter, in the manifest stage 13, 28, 52. The limited decline in cognitive and motor function in the pre-manifest stage coupled with this grey and white matter volume has led to the suggestion that compensatory mechanisms such as neuroplasticity and network reconfiguration enable those in the pre-manifest stage to maintain normal function 52.
Diffusion weighted MRI can be used to measure the diffusion of water in vivo and is therefore capable of providing information about the microstructure of white matter fibre tracts in the brain 62. In keeping with regional changes in white matter volume, microstructural white matter changes are also seen around the striatum, within the corpus callosum and in the posterior white matter tracts 63. More recently studies using diffusion tractography, which can delineate white matter connections, have shown selective vulnerability of cortico-striatal white matter connections in pre-manifest HD, extending to widespread loss of white matter connections in the manifest stage 64.
Other imaging modalities such as functional MRI have shown abnormalities both in pre-manifest and manifest disease 65. Positron emission tomography using a phosphodiesterase 10A tracer has been able to detect change in pre-manifest HD up to 25 years before symptom onset even before grey and white matter changes occur 66.
Clinical trials
Over the past two decades 99 clinical trials have been performed in HD investigating 41 different compounds. However, the success rate is low with only 3.5% of trials progressing to the next stage 67. Currently there are 23 active clinical trials in HD registered with ClinicalTrials.gov (Table 1). In this section a number of studies that have completed recently will be reviewed.
| Clinical trial |
|---|
| Open-label extension study of pridopidine (ACR16) in the symptomatic treatment of Huntington disease |
| Safety, tolerability, pharmacokinetics, and pharmacodynamics of IONIS-HTTRx in patients with early manifest Huntington's disease |
| Deep brain stimulation (DBS) of the globus pallidus (GP) in Huntington's disease (HD) |
| Resveratrol and Huntington disease |
| Exploring computerized cognitive training for people with Huntington's disease |
| Exercise effects in Huntington's disease |
| Beta in Huntington's disease (HD) |
| Feasibility of a video-oculography in patients with Huntington's disease VOG-HD study |
| A pilot evaluation of mindfulness-based cognitive therapy for people with Huntington's disease |
| A comparative phase 2 study assessing the efficacy of triheptanoin, an anaplerotic therapy in Huntington's disease |
| Follow-up measurement of brain PDE10A enzyme levels in Huntington's disease gene expansion carriers |
| A clinical study in subjects with Huntington's disease to assess the efficacy and safety of three oral doses of laquinimod |
| A study to evaluate sigma-1 and dopamine-2 receptor occupancy by pridopidine in the human brain of healthy volunteers and in patients with Huntington's disease |
| Tolerability, safety and activity of SRX246 in irritable subjects with Huntington's disease |
| Effect of tetrabenazine on Stroop interference in HD |
| Social cognition in Huntington's disease: cognitive study and functional and morphological imaging |
| Assessment of the psychological, cognitive and social resources of applicants for Huntington's disease and presymptomatic genetic testing |
| Alternatives for reducing chorea in HD |
| Brain stimulation in movement disorders |
| Validation and standardization of a battery evaluation of the socio-emotional functions in various neurological pathologies (GREFEX II) |
| Electronic-health application to measure outcomes remotely clinical trial |
| Laughter therapy effects on mood, stress and self-efficacy in people with neurological diseases |
| ExAblate transcranial MRgFUS for the management of treatment-refractory movement disorders |
Pridopidine, a dopamine modulator, has been studied in three large phase 3 trials: MermaiHD 68, HART 69 and Pride-HD 70. Unfortunately none of these studies reached their primary end-points. PBT2 is a metal protein-attenuating compound, which acts to reduce metal induced aggregation of mHTT. In a phase 2 trial the Reach2HD study showed that this drug was safe and well tolerated and plans are currently under way for a phase 3 trial 71.
Cysteamine, used in the treatment of cystinosis, increases brain-derived neurotrophic factor, a growth factor depleted in the brains of HD patients 6. The effect of cysteamine on motor progression in HD has been evaluated in a 3-year phase 2/3 trial. This revealed that it was safe and well tolerated; however, effects on motor progression did not reach statistical significance 72. SIRT1 is a member of the sirtuin family and causes reduction of mHTT protein levels 73. This molecule has been investigated in a phase 2 study, but no effect on the UHDRS TMS was seen 74.
Phosphodiesterase 10A (PDE10A) is found in the striatum and is reduced in HD patients many years before the onset of manifest disease 66. Inhibition of this enzyme using PDE10A inhibitors has been shown to restore basal ganglia circuitry in HD animal models 75. This compound was recently tested in the Pfizer Amaryllis phase 2 trial. Unfortunately this failed to show significant improvement in motor, cognitive or behavioural measures 76.
DNA and RNA targeting therapies for HD
Perhaps the most promising approaches with regard to disease modification are the emerging therapies aimed at lowering levels of mHTT by targeting either the DNA or RNA of the mHTT gene (Fig. 3) 2. RNA targeting can be achieved by using antisense oligonucleotides (ASOs), RNA interference (RNAi) or small molecule splicing inhibitors. ASOs are currently being trialled in a first human phase 1b/2a study 77. They are delivered intra-thecally and catalyse the degradation of HTT mRNA by RNAse H, thereby reducing the production of the mHTT protein (Fig. 3). In animal models this results in up to 80% sustained reduction in HTT mRNA levels 78.
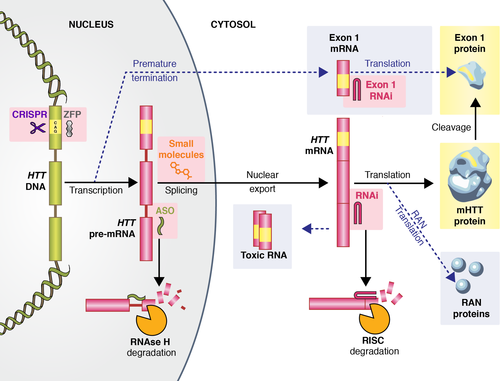
In RNAi based approaches RNA molecules bind to mRNA in the cytoplasm, prompting its removal by argonaute 2, the RNAse enzyme within the RNA-induced silencing complex 79, Fig. 3. Therapeutic strategies using this approach are currently in the preclinical phase. RNAi delivery is more invasive than ASOs requiring intracranial injection into the striatum. However, a single treatment may provide permanent HTT lowering 2. Small molecule splicing modifiers have shown promise in animal models of small muscular atrophy 80 and screening is currently under way to identify small molecule splicing modulators of mHTT 81.
Targeting the DNA of mHTT can be achieved using two approaches, zinc finger proteins and the CRISPR/Cas9 (clustered inter-spaced short palindromic repeats) system. Zinc fingers are proteins forming a structural motif that bind to DNA. Synthetic zinc finger transcription factors targeting CAG have been used to reduce levels of mHTT protein in animal models. However, as they create non-native proteins they have the potential to cause immune reactions − thus further work is needed 82.
CRISPR/Cas9 is used by bacterial immune systems in order to cleave foreign DNA. In recent years the system has been harnessed as a tool for genome editing with a multitude of applications to human disease. This technology has been used in fibroblasts of an HD patient to excise the promoter regions, transcription start site and the CAG mutation expansion of the mHTT gene. This resulted in permanent and mutant allele-specific inactivation of the mHTT gene 83. Recently the method was successfully tested in an HD rodent model 84. This affirms the feasibility of this approach but much preclinical work is needed to bring these rapidly evolving technologies to the clinic, especially given recent concerns about unexpected off-target mutations with CRISPR/Cas9 gene editing 85.
Conclusions
Huntington's disease is a progressive and devastating disease. Over the last decade there has been a rapid growth in our understanding of the natural history of HD and pathogenesis at both the cellular and macroscopic level. To date few treatments are available and a number of clinical trials have failed. However, the development of therapeutic strategies capable of targeting mHTT directly heralds a new era for HD research. Now more than ever there is a real potential to modify and prevent HD.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.




