Recent advances in the understanding of the aetiology and therapeutic strategies in burning mouth syndrome: Focus on the actions of cannabinoids
Abstract
Burning mouth syndrome (BMS) is a neuropathic pain disorder associated with a burning sensation on oral mucosal surfaces with frequently reported xerostomia, dysgeusia and tingling or paraesthetic sensations. However, patients present no clinically evident causative lesions. The poor classification of the disorder has resulted in a diagnostic challenge, particularly for the clinician/dentist evaluating these individuals. Major research developments have been made in the BMS field in recent years to address this concern, principally in terms of the pathophysiological mechanisms underlying the disorder, in addition to therapeutic advancements. For the purpose of this review, an update on the pathophysiological mechanisms will be discussed from a neuropathic, immunological, hormonal and psychological perspective. This review will also focus on the many therapeutic strategies that have been explored for BMS, including antidepressants/antipsychotics, non-steroidal anti-inflammatories, hormone replacement therapies, phytotherapeutic compounds and non-pharmacological interventions, overall highlighting the lack of controlled clinical studies to support the effectiveness of such therapeutic avenues. Particular focus is given to the cannabinoid system and the potential of cannabis-based therapeutics in managing BMS patients.
Abbreviations
-
- 2-AG
-
- 2-arachidonoyl-glycerol
-
- 5-HT
-
- serotonin
-
- AC
-
- adenylyl cyclase
-
- ACTH
-
- adrenocorticotropic hormone
-
- AEA
-
- anandamide
-
- BDNF
-
- brain-derived neurotrophic factor
-
- BMS
-
- burning mouth syndrome
-
- CB
-
- cannabinoid receptor
-
- CBC
-
- cannabichromene
-
- CBD
-
- cannabidiol
-
- CBG
-
- cannabigerol
-
- CBN
-
- cannabinol
-
- CBT
-
- cognitive behavioural therapy
-
- CGRP
-
- calcitonin gene-related peptide
-
- CNS
-
- central nervous system
-
- COX-2
-
- cyclooxygenase-2
-
- CRH
-
- corticotropin-releasing hormone
-
- DHEA
-
- dehydroepiandrosterone
-
- eCB
-
- endocannabinoid
-
- GABA
-
- gamma aminobutyric acid
-
- GMV
-
- grey matter volume
-
- GPCRs
-
- G-coupled protein receptors
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- IFN-γ
-
- interferon-gamma
-
- IL
-
- interleukin
-
- iNOS
-
- inducible nitric oxide synthase
-
- Klk13
-
- kallikrein 13
-
- LLLT
-
- low-level laser therapy
-
- MAPKs
-
- mitogen-activated protein kinases
-
- mPFC
-
- medial prefrontal cortex
-
- MRI
-
- magnetic resonance imaging
-
- MS
-
- multiple sclerosis
-
- MUC1
-
- mucin 1
-
- NA
-
- noradrenaline
-
- NADA
-
- N-arachidonoyl dopamine
-
- NAE
-
- N-acylethanolamine
-
- NF-κB
-
- nuclear factor kappa B
-
- NGF
-
- nerve growth factor
-
- NSAID
-
- non-steroidal anti-inflammatory drug
-
- PEA
-
- palmitoylethanolamide
-
- PI3K
-
- phosphoinositide 3-kinase
-
- PKB
-
- protein kinase B
-
- PNS
-
- peripheral nervous system
-
- PPARs
-
- peroxisome proliferator-activated receptors
-
- QOL
-
- quality of life
-
- QST
-
- quantitative sensory thresholds
-
- ROS
-
- reactive oxygen species
-
- rTMS
-
- repetitive transcranial magnetic stimulation
-
- SP
-
- substance P
-
- TG
-
- trigeminal nerve
-
- THC
-
- Δ9-tetrahydrocannabinol
-
- TLR
-
- Toll-like receptor
-
- TNF-α
-
- tumour necrosis factor alpha
-
- TRPV1
-
- transient receptor potential vanilloid 1
1 INTRODUCTION
Primary, or idiopathic burning mouth syndrome (BMS), is defined by the International Headache Society as an “intraoral burning or dysaesthetic sensation, recurring daily for more than two hours per day over more than three months, without clinically evident causative lesions”. In addition to the oral burning or stinging sensation of the tongue, lips or other oral mucosal surfaces, BMS patients frequently report dry mouth (xerostomia), taste disturbance (dysgeusia) and tingling or paraesthetic sensations (IHS, 2018). Although some reports indicate a decrease in unstimulated salivary flow in BMS (Lee, Hong, Na, & Eun, 2015), recent data suggest that the alteration in both unstimulated and stimulated salivary flow is associated with the medication used by the patient(s), and the presence of other systemic diseases, rather than the syndrome per se (Acharya, Hagglin, et al., 2018). Moreover, Lee et al. (2015) did not identify significant differences in salivary gland function when using salivary scintigraphy. According to ICD-10 codes, BMS is also known as glossodynia, orodynia, stomatodynia, and sore or burning tongue and is currently described by the International Headache Society as a “painful cranial neuropathy”, highlighting the neuropathic mechanisms underlying the disorder. BMS was previously viewed as a “psychogenic pain” due to its close association with psychological factors including anxiety, depression and carcinophobia (Browning, Hislop, Scully, & Shirlaw, 1987). This shift in classification has been increasingly supported by data from more recent histological, neurophysiological, brain imaging and quantitative sensory testing that provide evidence of neuropathic alterations in BMS patients, as reviewed extensively elsewhere (Jaaskelainen & Woda, 2017). A secondary BMS may also occur due to local (candidiasis, lichen planus, hyposalivation) or systemic (medication-induced, anaemia, deficiencies of vitamin B12 or folic acid, Sjögren's syndrome, diabetes) causes. In the case of secondary BMS, once the underlying factors are treated, the symptoms cease (IHS, 2018). Primary BMS is the focus of this review.
1.1 Clinical features of BMS
The clinical features of BMS vary from patient to patient, including variations in pain intensity and duration. The onset of symptoms can be gradual or sudden, with no identifiable trigger. However, some cases have been linked to dental procedures, trauma, new medication use, illness or stressful life events (Bender, 2018). In most cases, the pain progresses from mild to moderate throughout the day (Lopez-Jornet, Molino Pagan, Andujar Mateos, Rodriguez Agudo, & Pons-Fuster, 2015b) and may be continuous or intermittent, most commonly affecting the anterior two-thirds of the tongue; however, the pain may impact other mucosal surfaces. Furthermore, the symptoms tend to be bilateral and symmetric (Bender, 2018; Wada et al., 2017). Based on these differences, some authors have classified different subtypes of BMS (Moghadam-Kia & Fazel, 2017); nonetheless, no clear classification has been validated to date. More recent reports to divide BMS into three major categories depending on the neuropathic origin of the disorder include the following: (a) peripheral small fibre neuropathy, (b) trigeminal neuropathy and (c) hypofunction of dopaminergic neurons in the basal ganglia (Jaaskelainen & Woda, 2017).
Several factors can modify the painful symptoms reported in BMS, including the consumption of food/drink, speech (Bender, 2018) and sleep quality (Lopez-Jornet, Lucero-Berdugo, et al., 2015). Even though some patients report pain relief while eating, most patients avoid hot, spicy, acidic food/drinks or alcoholic beverages, because they intensify the pain sensation (Bender, 2018). Poor sleep quality is frequently reported in BMS and correlates with syndrome severity (Adamo et al., 2018). Furthermore, stress and fatigue have a major impact on pain intensity, and the disruption of the circadian rhythm can affect inflammation (Kizaki et al., 2015), pain thresholds and pain sensitivity (Haack, Simpson, Sethna, Kaur, & Mullington, 2019).
1.2 Diagnosis of BMS
The diagnosis of primary BMS is a clinical challenge and can only be established by exclusion of other disorders that may cause oral cavity pain (Bender, 2018; Moghadam-Kia & Fazel, 2017), in particular Sjögren's syndrome (Aljanobi, Sabharwal, Krishnakumar, & Kramer, 2017). A clinical evaluation is made when the pain complaints are consistent with the oral burning pain sensation, and there is no physical pathology associated with the complaint. To establish a diagnosis, the clinician must gather a comprehensive medical and dental history, detailing the characteristics of the pain (onset, duration, anatomical location, exacerbating/ameliorating factors), current medication use, coexisting manifestations of xerostomia, denture use and history of psychiatric disease (anxiety disorder, depression, personality disorder). A history of previous upper respiratory tract infections, middle ear disease or surgery associated with damage to the chorda tympani nerve (branch of cranial nerve VII) should also be recorded (Bender, 2018). This is particularly relevant because taste input mediated by the chorda tympani nerve is known to inhibit the trigeminal somatic input at the central nervous system (CNS). Therefore, BMS may result from the loss of the normal chorda tympani inhibitory control over somatic afferents, thus intensifying trigeminal sensations including pain (Bartoshuk et al., 2005). A physical evaluation should also investigate signs of parafunctional habits, erythema, irritation, mucosal ulceration or other abnormalities. Furthermore, laboratory studies should collate blood cell counts, iron, zinc and vitamin levels, fasting blood glucose levels/glycosylated haemoglobin and thyroid function (Bender, 2018; Moghadam-Kia & Fazel, 2017).
Due to difficulties in diagnosing BMS, patients commonly attend several clinical consultations prior to a definitive diagnosis, resulting in delays in treatment initiation and commonly exacerbating anxiety. Hence, further characterization of BMS is needed, in addition to clear diagnostic tools. This is particularly relevant taking into consideration the older age demographic of BMS patients, usually with multimorbidity and polypharmacy that increases the risk of adverse drug effects commonly associated with the symptoms of BMS (hyposalivation, dysgeusia) (Ni Riordain, O'Dwyer, & McCreary, 2019). With that aim, several attempts have been made to identify specific biomarkers for the disorder. Indeed, some promising candidates include interleukin (IL)-18 (a proinflammatory cytokine associated with interferon-gamma (IFN-γ) synthesis (Banu et al., 2015)), kallikrein (Klk) 13 (a serine protease involved in regulating inflammation (Ehrenfeld, Bhoola, Matus, & Fig ueroa, 2018)) and α-amylase (a stress-related enzyme (Imura, Shimada, Yamazaki, & Sugimoto, 2016)). Such candidates are upregulated in saliva from BMS patients compared with control subjects and may be relevant to the pathophysiology of the disease (Imura et al., 2016; Ji et al., 2017). Furthermore, recent technological advances in clinical neurophysiology suggest that electrogustometry readings (for taste disturbances) and quantitative sensory thresholds (QST) (for detection thresholds) may act as sensitive diagnostic tools, or tools that can be used to identify BMS subtypes (Jaaskelainen & Woda, 2017).
1.3 Epidemiology and aetiology of BMS
Depending on the study, BMS prevalence ranges from 0.7% to 15% in the general population (Tait, Ferguson, & Herndon, 2017), and such variability is due to the lack of objective diagnostic criteria and clear distinction between idiopathic and secondary BMS subtypes. In fact, a more recent study estimated a lower prevalence of 0.1% when using more stringent criteria for diagnosis (Kohorst, Bruce, Torgerson, Schenck, & Davis, 2015). Although BMS may affect younger women and men, it is most prevalent at older ages, especially in postmenopausal women (Kohorst et al., 2015). Age-adjusted incidence is higher in women than men (18.8 versus 3.7/100,000 person-year), with the highest values between 50 and 89 years of age (maximum of 70.3/100,000 person-year between 70 and 79 years of age; Kohorst, Bruce, Torgerson, Schenck, & Davis, 2014). The ratio of male: female BMS is approximately 1:4, and hence, the main predictive factors for BMS onset are age and sex (Rabiei, Leili, & Alizadeh, 2018). Furthermore, sociodemographic studies commonly link BMS onset with a stressful life event, particularly unemployment (Adamo et al., 2015). Unfortunately, the prognosis for such patients is poor, with a significant impact on quality of life (QOL) (Kim & Kho, 2018).
Despite much research investigating BMS, its aetiology still remains unclear. It is believed to be a multifactorial condition that involves alterations in the expression profile of hormones and local neuroactive steroids related to menopause and anxiety states. This, combined with external or internal factors (environmental, medical procedures, pharmacological or systemic disease-related agents), may be deleterious to the function of the nervous system, especially in genetically susceptible individuals (Chimenos-Kustner et al., 2017; Jaaskelainen & Woda, 2017). Indeed, some authors have categorized BMS risk factors as local and systemic (Table 1; Bender, 2018); local factors can induce oral burning sensation via direct irritation, ischaemia or compression of the oral tissue, while systemic factors are linked to axonal damage (Jaaskelainen & Woda, 2017). Overall, both local and systemic factors may impair neural function (Kang, Kim, Chang, & Kho, 2017; Robinson, 2000), and once this occurs, even if the causative factors are removed/treated (secondary BMS excluded), the damage is not reverted (Jaaskelainen & Woda, 2017). The same applies to hormone replacement therapy, which may be effective at reverting neuropathic changes at early stages, but ineffective at later stages when irreversible fibre damage has taken place (Jaaskelainen & Woda, 2017). Genetic susceptibility should also be further investigated in BMS because polymorphisms in IL-1β have been linked to BMS pathogenesis (Guimaraes et al., 2006). Furthermore, some studies point to a heightened taste perception and increased number of fungiform papillae on the tongue of patients, linking the taste receptor TAS2R38 gene to BMS susceptibility (Kolkka-Palomaa et al., 2015). Reports of xerostomia and skin disease or symptoms (rosacea, eczema and dry skin) have also been strongly linked to BMS (Acharya, Carlen, Wenneberg, Jontell, & Hagglin, 2018a). At the CNS, dopamine D2 receptor polymorphism C957T has also been associated with BMS aetiology, because such polymorphisms result in reduced synaptic dopamine concentrations and a subsequent reduction in pain inhibition (Jaaskelainen et al., 2014).
| Local Factors | Systemic Factors |
|---|---|
|
|
2 PATHOPHYSIOLOGY OF BMS
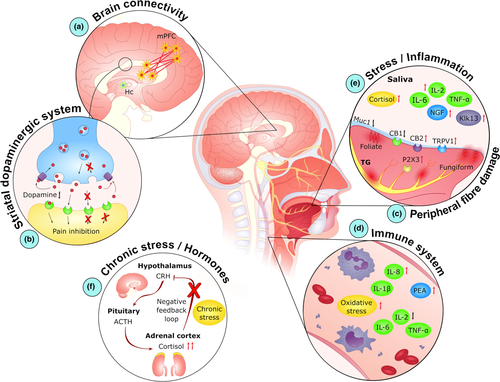
There have been major developments in BMS research in recent years, which has resulted in classification shifts, particularly from psychogenic to neuropathic (2018; Jaaskelainen, 2018). In fact, although anxiety and depression are reported in BMS patients, such conditions commonly arise only after BMS onset (Sikora et al., 2018). Moreover, much evidence links BMS with lesions and/or dysfunction in the CNS and peripheral nervous system (PNS) (Valenca, Oliveira, & Martins, 2015). In addition, both the immune and endocrine systems are closely associated with the onset and progression of BMS (Koike et al., 2014). Indeed, allergies (Acharya, Carlen, et al., 2018; Marino, Capaccio, Pignataro, & Spadari, 2009), and genotypes associated with inflammatory diseases (Guimaraes et al., 2006; Kim, Kim, Chang, Kim, & Kho, 2017), confer a higher risk for BMS. Overall, the pathophysiology of BMS is complex, with multiple mechanisms associated with BMS pathogenesis (Bender, 2018). For the purpose of this review, these pathophysiological mechanisms will be discussed under four subject areas: neuropathic, immunological, hormonal and psychological (summarized in Figure 1). It is important to note that although these mechanisms have been correlated with BMS, much of this data is from cross-sectional study analysis. Therefore, it is not clear whether these mechanisms are present prior to BMS development, or whether they manifest as a result of BMS. To overcome this limitation, further studies using longitudinal approaches are needed.
2.1 Neuropathic
Much data demonstrate relevant structural and functional changes in both the CNS and PNS of patients with BMS (Valenca et al., 2015). Indeed, significant alterations in the structure/function of both the hippocampus and the medial prefrontal cortex (mPFC) have been reported in BMS patients, compared with healthy individuals (Khan, Keaser, Meiller, & Seminowicz, 2014; Wada et al., 2017). Specifically, Khan et al. (2014) report an increase in hippocampal grey matter volume (GMV) in BMS patients, alongside a decrease in the mPFC (Khan et al., 2014). The latest published data indicate a decrease in GMV in the thalamus and middle temporal gyrus of BMS patients when compared to control subjects, in addition to a decrease in cerebral blood flow in the middle temporal gyrus and insula (Lee, Jahng, Ryu, & Byun, 2019; Liu et al., 2015). Such thalamic atrophy may play a key role in BMS pathogenesis because this area mediates nociceptive signalling to the cortex, and thalamic lesions are associated with chronic pain disorders (Giesecke et al., 2004). In support of this, recent data from both Lee et al. (2019) and Sinding et al. (2016) correlate pain intensity with a decrease in GMV in BMS patients. Moreover, a decrease in cerebral blood flow may also correlate with the depressive symptomatology in BMS (Liu et al., 2015).
Functional magnetic resonance imaging (MRI) studies indicate several differences between BMS patients and healthy subjects. Indeed, BMS patients exhibit higher functional connectivity between the mPFC and areas associated with pain processing (anterior insula cortex and anterior cingulate cortex), while in the hippocampus, a decreased connectivity with areas associated with working memory and attention (dorsolateral prefrontal cortex) is reported (Khan et al., 2014) (Figure 1a). Strikingly, those differences increase from morning to afternoon and correlate with a pain/burning state, which may explain the exacerbation of BMS symptoms during the day (Khan et al., 2014). Such structural and functional alterations may be linked to anxiety/depression that patients endure related to their ongoing pain (Khan et al., 2014).
CNS involvement in BMS is also evident in neurophysiological studies that link this disorder to dysfunction in the striatal dopamine system (Hagelberg et al., 2003) (Figure 1b). The depletion of dopamine in the putamen results in deficient pain inhibition in the trigeminal brainstem complex in BMS patients (Hagelberg et al., 2003; Wood, 2008). In support of this, BMS patients exhibit low serum levels of neurokinin A, a neuropeptide associated with pain and inflammation (Boras et al., 2010). Furthermore, reduced dopaminergic tone may also be associated with the personality traits and/or psychiatric disorders commonly reported in BMS (Taiminen et al., 2011).
Neural function can be assessed by testing the blink reflex via stimulation of trigeminal nerve (cranial nerve V) branches, specifically the supraorbital, mental and lingual nerves. Interestingly, with this approach, BMS has been correlated with trigeminal dysfunction in approximately 20% of the patients (Jaaskelainen & Woda, 2017). Furthermore, data from Puhakka et al. (2016) indicate that BMS is linked to large fibre neuropathy in the form of elevated vibratory detection thresholds and enhanced mental nerve blink reflexes. The electrical thresholds that elicit the blink reflex are higher in BMS patients than in control subjects, and these finding suggest trigeminal tactile Aβ fibre hypofunction (Jaaskelainen & Woda, 2017; Puhakka et al., 2016). BMS is also related to alterations in taste and sensory systems, as patients frequently report bitter, metallic or foul tastes (Bender, 2018; Jaaskelainen & Woda, 2017). Indeed, electrogustometry studies indicate that BMS patients have lower taste sensitivity in fungiform and foliate taste buds (Braud, Descroix, Ungeheuer, Rougeot, & Boucher, 2017). Furthermore, Bartoshuk et al. (2005) hypothesize that BMS may result from a convergence of pain and taste sensations and that this may be due to the loss of inhibition of the trigeminal nerve due to chorda tympani nerve damage (Bartoshuk et al., 2005). Due to the close physiological interaction between the trigeminal and facial nerves, damage to either nerve may influence the function of the other (Bartoshuk et al., 2005; Schobel et al., 2012).
BMS has also been linked to peripheral small fibre damage (Figure 1c) as shown by psychophysical QST or neurophysiological recordings of thermal and pain-evoked potentials, in addition to nociceptive reflexes (Jaaskelainen & Woda, 2017). Although the first thermal QST found lower tolerance to painful heat stimulus only at the tip of the tongue in BMS (Grushka, Sessle, & Howley, 1987), recent studies indicate broader dysfunction of the thermal detection thresholds at the tongue mucosa (Hartmann et al., 2017; Puhakka et al., 2016) that correlate with symptom duration (Watanabe et al., 2018). In most cases, hypoaesthesia or anaesthesia to innocuous and painful thermal stimuli is recorded (Forssell, Jaaskelainen, Tenovuo, & Hinkka, 2002; Puhakka et al., 2016). However, reports elsewhere indicate cold hyperalgesia in BMS patients (Hartmann et al., 2017; Yilmaz, Egbuniwe, & Renton, 2016), and despite contradictory findings, the lower reactivity to thermal stimuli may be correlated with a reduced volumetric activation throughout the brain (Albuquerque et al., 2006). In terms of the type of fibres affected, even though some patients exhibit C fibre hypofunction within the lingual nerve, it is more common to detect small myelinated Aδ fibre damage/hypofunction in BMS (Puhakka et al., 2016). This suggests that decreased signalling of Aδ fibres results in a deficient inhibition of the unmyelinated C fibres, and their continuous signalling may be the cause of the burning pain sensation perceived in BMS (Jaaskelainen & Woda, 2017; Moura, Ferreira, DosSantos, & Janini, 2018; Puhakka et al., 2016). These findings are supported by neuropathological investigations using oral mucosal biopsies from BMS patients, which report a reduced density of epithelial small fibres and subpapillary tongue nerve fibres, while preserving the subepithelial large fibres (Puhakka et al., 2016). The damage may result from consumption of excessively hot food or beverages or from common dental procedures (Bender, 2018). Dysregulation in mucosal blood flow has also been reported in BMS patients, and the increased vasoreactivity in BMS might either result from, or affect, the neurovascular microcirculatory unit (Heckmann et al., 2001).
It should be noted that the activity of several receptors located on the tongue is dysregulated in BMS. Indeed, the classical G protein-coupled cannabinoid receptor-1 (CB1) is downregulated in the tongue epithelia in BMS patients, while cannabinoid receptor-2 (CB2) and the transient receptor potential vanilloid 1 (TRPV1) receptor are upregulated (Borsani et al., 2014; Yilmaz et al., 2007). Both receptor types are relevant in the endogenous cannabinoid (endocannabinoid; eCB) system, since CB1/2 are “classical” and TRPV1 “non-classical” receptors for eCBs (Di Marzo, Stella, & Zimmer, 2015) (discussed in section 4). Likewise, both nerve growth factor (NGF) (Yilmaz et al., 2007) and artemin (Shinoda et al., 2015) are overexpressed in BMS patients, which is important as both of these neurotrophic factors are regulators of TRPV1 expression. The upregulation of TRPV1 is particularly relevant because this receptor is commonly activated by noxious heat and capsaicin (chilli pepper extract), and its expression correlates with ongoing pain symptoms (Yilmaz et al., 2007). Similarly, enhanced immunoreactivity of the sensory purinergic receptor P2X3 has been reported in BMS, which is primarily expressed on small neurons of sensory ganglia (Beneng et al., 2010). A higher density of fungiform papillae usually leads to a higher taste perception that may lower tolerance to bitter foods and irritants (Bartoshuk et al., 2005). However, there is still controversy regarding dysregulation of its expression since the distribution of fungiform papillae appear asymmetrical on both sides of the tongue in BMS patients, thus indicating an asymmetrical innervation (Naud, Benca, Drangsholt, LeResche, & Coldwell, 2018). The taste thresholds within both the fungiform and foliate papillae are also impaired in BMS patients (Braud et al., 2017).
2.2 Immunological
Although few studies have been published focusing on the role of immunological factors in BMS, there is growing evidence that a dysregulation of inflammatory mechanisms is a key factor in disease onset and development (Figure 1d, e). The dysregulation of several inflammatory mediators in both plasma and saliva from BMS patients, when compared to control subjects (Barry, O'Halloran, McKenna, McCreary, & Downer, 2018a; Chen, Xia, Lin, Zhou, & Li, 2007; Pekiner, Demirel, Gumru, & Ozbayrak, 2008; Pezelj-Ribaric et al., 2013; Simcić et al., 2006), strengthens the hypothesis of a neuroinflammatory mechanism.
Proinflammatory cytokines and chemokines contribute to nociceptive signalling, and their expression profiles are altered in neuropathic pain disorders and under conditions of stress (Lechner & von Baehr, 2015; Lewis, Grace, Hutchinson, Maier, & Watkins, 2017). Indeed, cytokine imbalance may increase the risk of depressive symptomatology by modulating central neurotransmitter systems (Liu, Ho, & Mak, 2012). In agreement with this, recent data from a pilot study in our laboratory demonstrate that the expression of the proinflammatory chemokine IL-8 is enhanced in plasma isolated from BMS patients, when compared to control subjects (Barry, O'Halloran, McKenna, McCreary, & Downer, 2018). The same study also indicates a correlation between the ratio of plasma IL-8:IL-10 and depressive symptomatology in BMS patients (Barry, O'Halloran, McKenna, McCreary, & Downer, 2018). Interestingly, genetic polymorphisms related to the overexpression of the cytokine IL-1β are correlated with BMS development (Guimaraes et al., 2006) and psychological asthenia (Kim et al., 2017), and similarly, the expression of the IL-18 cytokine (a member of the IL-1 family) is also upregulated in BMS saliva (Ji et al., 2017). Although saliva is a promising non-invasive methodology to assess BMS, the data from saliva assessment in BMS are more variable, with some studies not identifying differences in salivary cytokines (Boras, Brailo, Lukac, Kordic, & Blazic-Potocki, 2006; Suh, Kim, & Kho, 2009). However, a body of research indicates that the expression of the inflammatory cytokines IL-6 (Chen et al., 2007), IL-2 and tumour necrosis factor alpha (TNF-α) (Pekiner et al., 2008) is reduced in plasma, while significantly upregulated in saliva (Pezelj-Ribaric et al., 2013; Simcić et al., 2006), in BMS cohorts, when compared to healthy subjects. The poor classification of the disease, and differences in the methodologies adopted in each study, may explain the variability in published data related to cytokine signatures in both BMS plasma and saliva.
In the search for BMS biomarkers in saliva, several inflammatory-related molecules have also been proposed, with evidence that their expression profiles are altered in BMS; namely Klk (Ji et al., 2017) and α-amylase (Imura et al., 2016; Nosratzehi, Salimi, & Parvaee, 2017). In addition, the expression of membrane-bound mucin 1 (MUC1) in oral epithelial cells (Kang et al., 2017), α-enolase (Ji et al., 2017) and cystatin (Cabras et al., 2019) are increased in saliva from BMS patients. This is particularly relevant because both mucus gel production (Kang et al., 2017) and α-enolase (Ji et al., 2017) participate in the first-line immune defence against pathogens, and a higher neutral cystatin reflects a mechanism of defence against ongoing inflammatory processes (Cabras et al., 2019). Reports also indicate that the expression of neuropeptides in saliva is also dysregulated in BMS. Among them, NGF is upregulated, and substance P (SP) (Borelli et al., 2010) and calcitonin gene-related peptide (CGRP) are downregulated (Zidverc-Trajkovic et al., 2009). NGF can trigger mast cell degranulation at nerve lesion sites that, in turn, may exacerbate its expression in BMS (Borelli et al., 2010). Both SP and CGRP are associated with neurogenic pain and are secreted simultaneously. Their reduced expression is indeed a distinct feature from other painful conditions (Borelli et al., 2010; Zidverc-Trajkovic et al., 2009). Opiorphin, an inhibitor of enkephalin-inactivating peptidases (Wisner et al., 2006), is also upregulated in saliva from BMS patients, which may represent an adaptive response to chronic pain (Salaric, Sabalic, & Alajbeg, 2017). In addition, Boucher et al. (2017) found significant differences in the expression of opiorphin in blood samples from BMS patients, which may reflect a systemic dysregulation related to environmental stress conditions and psychological distress.
Another poorly investigated pathophysiological mechanism in BMS is the imbalance in the eCB system (discussed in section 4), which consists of endogenous ligands for the cannabinoid receptors (Storozhuk & Zholos, 2018). Recent data from our laboratory suggest that the expression profile of the non-cannabinoid N-acylethanolamine (NAE) molecule palmitoylethanolamide (PEA) is increased in plasma isolated from newly diagnosed BMS patients compared with healthy subjects, and that plasma PEA levels correlate with pain and depressive symptomatology (Barry, O'Halloran, McKenna, McCreary, Harhen, et al., 2018). In fact, much evidence indicates that the eCB system can regulate nociceptive signalling and the immune response to inflammation, by acting both in the CNS and in the PNS. In addition, the peripheral eCB system is also closely associated with stress and/or depressive disorders (Di Marzo et al., 2015; Hill et al., 2013).
The contribution from oxidative stress must also be considered, given that stress-related hormonal alterations in postmenopausal BMS patients may affect MUC1 expression and burning pain perception (Kang et al., 2017). Stress may, in fact, play a relevant role on BMS pathophysiology, because the stress-related enzyme α-amylase is also upregulated in saliva from BMS patients (Imura et al., 2016; Nosratzehi et al., 2017). Reports suggesting altered levels of reactive oxygen species (ROS) in saliva (Tvarijonaviciute, Aznar-Cayuela, Rubio, Ceron, & Lopez-Jornet, 2017), in addition to alterations in total oxidant capacity and biological antioxidant potential as iron-reducing activity in blood (Tatullo et al., 2012) of BMS patients, support the contribution of oxidative stress to BMS pathogenesis.
2.3 Hormonal
During menopause, several changes occur in gonadal, adrenal and neuroactive steroid levels (Woda, Dao, & Gremeau-Richard, 2009), and the higher prevalence of BMS in peri- and postmenopausal women (Rabiei et al., 2018) supports the role of female sex hormones in BMS pathogenesis. Indeed, hypoestrogenism at menopause is associated with xerostomia and taste alterations (Friedlander, 2002) as seen in BMS, and the salivary levels of 17β-estradiol in postmenoupausal BMS patients correlate with the symptoms of the disorder (Kang et al., 2017). The neuroendocrine stress response is controlled via the hypothalamic–pituitary–adrenal (HPA) axis, the activation of which promotes an increase in the levels of circulating corticosteroids to impact several systems (Pecoraro et al., 2006; Figure 1f). Importantly, chronic stress can disrupt adrenal steroid production by impairing the supply of precursors for neuroactive steroids both in the skin, mucosa and nervous system (by glial cells in the CNS and by Schwann cell in the PNS). The lower gonadal steroid production inherent to menopause, combined with chronic stress dysregulation, can contribute to irreversible neurodegenerative alteration in the PNS (small nerve fibres in oral mucosa) and (or) CNS (Woda et al., 2009). As the production of protective neurosteroids is decreased, those regions may be more vulnerable to the action of corticoids (Woda et al., 2009), which are increased in saliva from BMS patients (Kim, Kim, Chang, Ko, & Kho, 2012). As these hormones can interact locally with benzodiazepine receptors, it may also explain the localization of pain in BMS (Dias Fernandes et al., 2009; Pajot, Ressot, Ngom, & Woda, 2003). Furthermore, the over-production of cortisol may be a consequence of prolonged anxiety or stress (dysregulation of the HPA axis) (Koike et al., 2014; Nosratzehi et al., 2017), and both the excessive production and depletion of cortisol may be deleterious to neural tissues (Koike et al., 2014).
The adrenal steroid dehydroepiandrosterone (DHEA) is an androgen and oestrogen precursor associated with the production of male and female sex hormones. Interestingly, levels of this hormone are reduced in saliva from BMS patients, suggesting a possible correlation of DHEA deficiency with the development of disease (Dias Fernandes et al., 2009). Although there is a clear correlation of menopause with BMS onset, it is likely that other factors must be present in combination with the hormonal imbalance for the disease onset, because hormone replacement therapy alone achieves conflicting outcomes (Zakrzewska, Forssell, & Glenny, 2005).
2.4 Psychological
Many BMS patients have suffered a stressful life event, either recently or in early life (Lamey, Freeman, Eddie, Pankhurst, & Rees, 2005). While psychological disorders are not considered as causative factors in BMS, several studies have associated BMS with psychologic factors (somatization and psychoticism) (Yoo et al., 2018), anxiety and depression (Davies et al., 2016). Furthermore, BMS patients commonly present with characteristics of type C personality disorders, including fear and neurosis, in addition to low levels of novelty seeking (Taiminen et al., 2011; Tokura et al., 2015). Individuals with BMS also commonly report a fear of having cancer and commonly overreact to trivial stress stimulants (pain catastrophizing) (Rogulj, Richter, Brailo, Krstevski, & Boras, 2014). Indeed, Kim, Kim, and Kho (2018) report that psychological factors increase the number and severity of BMS symptoms, especially in terms of taste disturbances (Kim et al., 2018). Psychologic distress can also influence BMS pain perception (Yoshino et al., 2017). It is interesting to note that many personality disorders, commonly observed in BMS, are associated with low dopaminergic tone in the CNS (Taiminen et al., 2011). Indeed, chronic anxiety and stress promote alterations in adrenal steroid physiology (Woda et al., 2009). In terms of HPA dysfunction, BMS is associated with hypercortisolism (Amenabar et al., 2008; Koike et al., 2014), and reports suggest an inverse correlation between openness personality traits and stress-related salivary biomarkers, including cortisol, in BMS patients (de Souza et al., 2015).
The correlation between BMS and psychological factors is under much investigation, with several contradictory reports published. Honda et al. (2019) recently suggested that pain on the tongue in elderly female patients with BMS is more related to psychological factors than disturbances in mechanical sensitivity, because no QST alterations were determined (Honda et al., 2019). Also, BMS patients that receive objective information and reassurance about their condition tend to be less negative, report lower pain and have a better QOL (Brailo et al., 2016). This is supported by the responsiveness to antidepressants and cognitive behavioural therapy (CBT) observed in this patient cohort (discussed in section 3). However, the former is not independent of the analgesic activity (Tu et al., 2019), and the latter is usually correlated with better coping mechanisms rather than an aetiological cure (Zakrzewska & Buchanan, 2016). Moreover, BMS can occur without psychological problems, and furthermore, the symptoms in BMS do not meet the criteria for a diagnosis of a formal psychiatric disorder (Kim et al., 2018). In fact, the psychological distress in BMS may arise only after the pain symptoms, hence being more related to the delayed diagnosis. Overall, it is very difficult to ascertain the contribution of psychological problems in BMS, leaving the question of a causal or aggravating factor in the pathophysiology of this syndrome (Kim & Kho, 2018).
3 BMS THERAPIES
Although there are few data describing the natural course of the disease, the duration of symptoms in BMS can prolong for several years. When untreated, a small percentage of patients (approximately 3%) may achieve complete remission within five years after the onset of the clinical manifestations (Sardella et al., 2006). The treatment of idiopathic BMS is challenging because there are limited evidence-based management strategies, and most therapeutic approaches yield limited success (Tu et al., 2019). Indeed, data indicate that approximately 30% of the patients benefit from neuropathic pain medication (Sardella et al., 2006). Clinically, there is a reliance on drug therapies that target the clinical manifestations of the disease in combination with CBT (McMillan et al., 2016).
The chronic pain, delayed diagnosis and lack of effective treatment greatly impair the QOL of BMS patients (Braud & Boucher, 2016). Moreover, patients that have previously experienced unsuccessful therapies tend to feel more negative emotions and report fewer positive results from new treatments (Varoni, Lodi, & Sardella, 2015). Therefore, there is a need for the clinician to inform and reassure the patient to avoid negative thinking and behavioural patterns (Brailo et al., 2016). A full understanding of the pathophysiological mechanisms of the disease, together with a better diagnostic classification, will facilitate better disease management. As an example, current evidence indicates that a CNS-related BMS is more responsive to treatments that target the dopaminergic system (Carcamo Fonfria et al., 2017), whereas a PNS-related BMS is more responsive to topical administration of the benzodiazepine clonazepam, in addition to nerve blockers (Gremeau-Richard, Dubray, Aublet-Cuvelier, Ughetto, & Woda, 2010).
3.1 Pharmacological therapy
Several studies have investigated pharmacological approaches to manage BMS, but the number of well-designed trials to support pharmacological targets is limited (Liu, Kim, Yoo, Han, & Inman, 2018; McMillan et al., 2016). Several authors consider tricyclic antidepressants the first-line therapeutic choice in BMS due to their wide use in other chronic neuropathic pain conditions (Moore, Derry, Aldington, Cole, & Wiffen, 2015). α-Lipoic acid (an antioxidant), clonazepam (benzodiazepine) or gabapentin (antiepileptic/anticonvulsant) are considered alternatives only when other medications are contraindicated or poorly tolerated (Tu et al., 2019). There is also increasing evidence that serotonin (5-HT) and noradrenaline (NA) reuptake inhibitors may be considered as an alternative treatment, particularly when patients are refractory to other medications. These pharmacological agents can act as analgesics by interacting with 5-HT, NA, gamma aminobutyric acid (GABA) and enkephalin descending pain signalling (Kim, Lee, & Shim, 2014; Mitsikostas, Ljubisavljevic, & Deligianni, 2017). Furthermore, capsaicin is also considered for the management of BMS due to its ability to reduce pain by decreasing the functionality of TRPV1 nociceptive signalling (Jorgensen & Pedersen, 2017). Elsewhere, alternative therapeutic strategies have been explored for BMS, including hormone replacement therapies (Tarkkila, Linna, Tiitinen, Lindqvist, & Meurman, 2001), other antidepressants/antipsychotics (Takenoshita, Motomura, & Toyofuku, 2017), anaesthetics (Treldal et al., 2016), lafutidine (histamine H2-receptor antagonist) (Toida et al., 2009), benzydamine hydrochloride (non-steroidal anti-inflammatory drug; NSAID) (Sardella et al., 1999) and phytotherapeutic compounds (Valenzuela, Pons-Fuster, & Lopez-Jornet, 2016). Unfortunately, there are limited controlled clinical studies to support the effectiveness of such compounds. Indeed, many studies concerning BMS therapeutics are not standardized, lacking clinically validated pain assessment tools specific to BMS, and consensus on how to effectively rate the pain associated with BMS. As a consequence, inconsistencies arise when comparing studies (McMillan et al., 2016).
The most common pharmacological strategies currently employed in BMS and their mechanism of action, alongside adverse side effects, are listed in Table 2. From these pharmacological strategies, a recent systematic review (de Souza, Marmora, Rados, & Visioli, 2018) highlights clonazepam and α-lipoic acid as the only pharmacological compounds with the proclivity to significantly reduce BMS clinical manifestations compared with placebo in clinical trials. More recently, a promising clinical study targeted the eCB-like compound PEA. In this study, BMS patients received a sublingual dose of ultramicronized PEA (600 mg/twice daily; one sachet every 12 hr) for 60 consecutive days. During this period, PEA exhibited significant pain-relieving efficacy when compared to the placebo group. Moreover, 4 months after ceasing PEA administration, this effect still persisted although less pronounced (Ottaviani et al., 2018). Overall, data assessing the cannabinoid system as a bone fide therapeutic strategy in BMS are still lacking.
| Pharmacological Therapies | Mechanism of Action | Adverse effects |
|---|---|---|
| Systemic and Topical therapies | ||
| Antidepressants | ||
|
Tricyclic antidepressants
|
|
|
|
Serotonin and/or Norepinephrine reuptake inhibitors
|
|
|
|
Benzodiazepines
*Also topical (short effect duration) |
|
|
|
Anticonvulsants
**Alone or in combination with α-lipoic acid |
|
|
|
Vitamin-like antioxidants
|
|
|
| Topical | ||
|
Local anaesthetics/ Non-steroidal anti-inflammatories
|
|
|
3.2 Non-pharmacological therapies
Non-pharmacological therapeutic strategies in BMS are diverse and include low-level laser therapy (LLLT) (Al-Maweri et al., 2017), repetitive transcranial magnetic stimulation (rTMS) (Umezaki et al., 2016), acupuncture (Jurisic Kvesic et al., 2015), psychotherapy (Miziara, Filho, Oliveira, & Rodrigues dos Santos, 2009) and tongue protectors (a transparent polyethylene cover) (López-Jornet, Camacho-Alonso, & Andujar-Mateos, 2011). Although there are limited data published to date, LLLT poses some promise for reducing pain and symptoms in BMS, while being a non-invasive technique with no serious side effects. Indeed, LLLT demonstrates analgesic, anti-inflammatory and biostimulatory propensity due to an enhancement of 5-HT and β-endorphin synthesis and release, while reducing bradykinin secretion and blocking the depolarization of C fibres (Al-Maweri et al., 2017). Also, evidence indicates that rTMS, when delivered over the dorsolateral prefrontal cortex, can reduce pain in BMS patients; however, some patients report headaches as a side effect (Umezaki et al., 2016). Some evidence, albeit limited, suggests that acupuncture can improve BMS symptoms (Jurisic Kvesic et al., 2015). Overall, CBT has shown beneficial effects in at least one clinical trial (Zakrzewska, Forssell, & Glenny, 2003), and its combination with pharmacotherapy can also be beneficial (Femiano, Gombos, & Scully, 2004). CBT focuses on educating BMS patients to parafunctional habits that are detrimental to their condition, such as clenching, bruxism and tongue habits (Matsuoka, Chiba, Sakano, Toyofuku, & Abiko, 2017). In addition, tongue protectors can be beneficial by controlling parafunctional habits for certain periods during the day (López-Jornet et al., 2011). Overall, it is recommended that BMS patients should select oral care products that avoid formulations containing alcohol, flavouring agents and other known irritants and should also improve their diet and sleep patterns (Tu et al., 2019).
4 POTENTIAL USE OF CANNABINOIDS FOR BMS
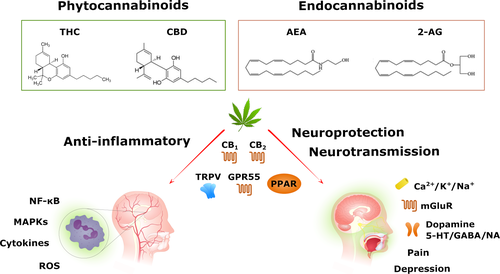
Cannabinoids include phytocannabinoids synthesized by the annual dioecious plant Cannabis sativa L. (C. sativa), endogenous cannabinoid ligands (the eCBs) and synthetic cannabinoid compounds (Lu & Mackie, 2016). The most common phytocannabinoids include Δ9-tetrahydrocannabinol (THC), a psychoactive phytocannabinoid, in addition to cannabinol (CBN) (an oxidized metabolite of THC), cannabidiol (CBD), cannabichromene (CBC) and cannabigerol (CBG) (Booth & Bohlmann, 2019; Chandra, Lata, Elsohly, Walker, & Potter, 2017). The eCBs represent a group of lipid messengers, synthesized on demand, that can interact with cannabinoid receptors in the eCB system. Examples include N-arachidonoylethanolamine (anandamide; AEA), 2-arachidonoyl-glycerol (2-AG), O-arachidonoyl ethanolamine (virodhamine), N-arachidonoyl dopamine (NADA) and 2-arachidonoyl-glycerol ether (noladin ether) (Storozhuk & Zholos, 2018). Lastly, synthetic cannabinoids are derivates of the phytocannabinoids developed to exert receptor-specific effects within the eCB system.
4.1 Cannabinoid targets
Cannabinoid molecules can exert their action by interacting with the eCB system both in the CNS and in the PNS. This system consists of two “classical” G-coupled protein receptors (GPCRs), CB1 and CB2, and other “non-classical” receptors, including the TRPV channel, orphan GPCRs (GPR119/GPR55) and nuclear peroxisome proliferator-activated receptors (PPARs) (Di Marzo et al., 2015; Figure 2). The “classical” CB1 receptors are distributed predominantly throughout the nervous system and are detected on a diverse array of cells/tissues in the body, including the immune, reproductive and digestive systems (Croxford, 2003). In contrast, CB2 receptors are found primarily in the periphery, particularly located in cells/tissues of the immune system. However, under certain pathological conditions (i.e. nerve injury), CB2 is expressed in some populations of neurons (Van Sickle et al., 2005). Thus, CB1 interaction is usually related to analgesic effects, while CB2 signalling is commonly related to the immunomodulatory properties of these molecules (Pellati et al., 2018). Depending on the structure, cannabinoid molecules have different affinities to these receptors and can interact as agonists, antagonists and (or) inverse agonists. Resulting from these complex interactions, cannabinoid molecules can modulate multiple intracellular signalling pathways involving adenylyl cyclase (AC), mitogen-activated protein kinases (MAPKs), phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB) and voltage-dependent ion channels (K+, Ca2+, Na+) (Demuth & Molleman, 2006). In terms of the “non-classical” receptors, TRPV1 is widely expressed in mammalian cells, mediating a variety of physiological processes including temperature sensation, pain and inflammation (Yoo et al., 2019). Several studies have shown that the anti-inflammatory effects of cannabinoids are also mediated by PPAR activation (Paterniti et al., 2013), which inhibit proinflammatory signalling pathways such as nuclear factor kappa B (NF-κB) and promote the expression of anti-inflammatory mediators (Cheng et al., 2019). Moreover, activation of PPARα might also contribute to pain control by triggering TRPV1 signalling and its desensitization (Ambrosino, Soldovieri, De Maria, Russo, & Taglialatela, 2014). Cannabinoids can also activate GPR55 receptor, which can control inflammation and pain (at the periphery), together with processes involved in memory, anxiety and glutamate release in the hippocampus (at the CNS) (Marichal-Cancino, Fajardo-Valdez, Ruiz-Contreras, Mendez-Diaz, & Prospero-Garcia, 2017).
4.2 Cannabinoid therapeutic properties
Cannabinoid-induced responses are complex due to the pharmacological differences in cannabinoid ligands and the involvement of multiple signalling mechanisms governing cannabinoid-mediated effects. Evidence shows that the eCB system has an important role in nervous system homeostasis and neuroprotection (Xu & Chen, 2015), and the use of cannabinoids affords a combination of neuroprotective, anti-inflammatory, antioxidative and antiapoptotic properties (Castillo, Tolon, Fernandez-Ruiz, Romero, & Martinez-Orgado, 2010; Iuvone et al., 2004; Figure 2). Considering the previously described pathophysiological mechanisms of BMS (section 2), such effects of cannabinoids might prove beneficial in the treatment of this syndrome and warrants full investigation.
4.2.1 Neuroprotection
Much evidence indicates that cannabinoids have neuroprotective properties in models of neurodegeneration. The eCB system is believed to play an important role in synaptic plasticity by regulating both excitatory and inhibitory synapses in response to certain events. These neurochemical changes contribute to processes such as learning, memory and behavioural adaptation (Xu & Chen, 2015). In particular, eCBs released in postsynaptic neurons suppress the release of neurotransmitters presynaptically. Therefore, cannabinoids may be beneficial in the treatment of BMS by preventing glutamate-induced excitotoxicity (Kano, 2014). In brain ischaemic injury, cannabinoids can prevent neuronal damage and promote cell survival, by inhibiting mitochondrial dysfunction (Ma et al., 2018). In neurodegenerative models, cannabinoids can also prevent oxidative stress-related neurotoxicity by modulating endoplasmic reticulum stress signalling (Vrechi, Crunfli, Costa, & Torrao, 2018), reducing ROS accumulation and lipid peroxidation (Iuvone et al., 2004). Neuroprotection is also achieved by reducing neurodegeneration caused by neuroinflammatory processes (detailed in section 4.2.3; Esposito et al., 2007). Furthermore, some evidence, albeit limited, indicates that cannabinoids stimulate NGF (Velasco, Ruiz, Sanchez, & Diaz-Laviada, 2001) and brain-derived neurotrophic factor (BDNF) (D'Souza, Pittman, Perry, & Simen, 2009) production. Considering the neuropathic nature of BMS, the neuroprotection afforded by cannabinoids represents a promising therapeutic strategy for BMS patients. Moreover, the role for neurotrophins in cannabinoid-mediated effects (and vice versa) may be critically important given that BMS patients exhibit important changes in GMV (Lee et al., 2019).
4.2.2 Neurotransmission
As aforementioned, cannabinoids can also modulate neurotransmission, with associated effects on emotion, mood, anxiety and depression (Xu & Chen, 2015). Indeed, an understanding of role of the eCB system in depression and anxiety disorders has increased over the last number of years, and there is some indication of the therapeutic potential of cannabinoid-based drugs in disorders such as anxiety and posttraumatic stress disorders (Chadwick, Rohleder, Koethe, & Leweke, 2019). Adding to the inhibition of excitatory glutamatergic system (Colizzi, McGuire, Pertwee, & Bhattacharyya, 2016), several studies indicate that activation of CB1 receptors can affect a large range of neurotransmitter systems, including dopamine, 5-HT, GABA and NA signalling (Fantegrossi, Wilson, & Berquist, 2018; Mendiguren, Aostri, & Pineda, 2018). The eCB system is also a key player in the initiation/termination of HPA axis responses to stressful conditions, and much evidence supports the role of the eCB system as a regulator of the stress response (Morena, Patel, Bains, & Hill, 2016). Given the diverse roles of such systems in functional connectivity of the nervous system, pain processing, stress, cognition, mood and depression/anxiety, the impact of cannabinoids on these systems is critical when considering BMS from a therapeutic standpoint. Importantly, our laboratory has shown that the expression of the eCB-like compound, PEA, is increased in plasma from BMS patients, and that this correlates with depressive symptomatology (Barry, O'Halloran, McKenna, McCreary, Harhen, et al., 2018). PEA has potential antidepressant effects (De Gregorio et al., 2019), and the potential therapeutic role of PEA in BMS and neuropathic orofacial pain warrants full investigation.
Analgesia is one of the principal therapeutic targets of the cannabinoid system, and multiple studies have demonstrated the efficacy of cannabinoids in the treatment of neuropathic pain (McDonough, McKenna, McCreary, & Downer, 2014). Indeed, some evidence indicates that the cannabis-based therapeutic Sativex® (discussed in section 4.3) can be useful in the management of trigeminal neuropathic pain (Gajofatto, 2016).
4.2.3 Anti-inflammatory
Cannabinoids demonstrate anti-inflammatory propensity in various disorders including multiple sclerosis (MS) (Annunziata, Cioni, Mugnaini, & Corelli, 2017), traumatic brain injury (Braun et al., 2018), spinal cord injury (Su et al., 2017) and Parkinson's disease (Viveros-Paredes et al., 2017). In these studies, the anti-inflammatory action of cannabinoids is predominantly mediated by activation of CB2. Furthermore, in vitro data in CNS cells indicate that cannabinoids exert anti-inflammatory propensity against IFN-γ (Ehrhart et al., 2005)-, amyloid-β (Esposito et al., 2006)-, IL-1β (Sheng et al., 2005)- and Toll-like receptor (TLR) (Downer et al., 2011)-induced inflammation. Different cannabinoids can modulate proinflammatory cytokine and chemokine secretion by targeting several inflammatory mechanisms, including NF-κB activation (Downer et al., 2011), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), and TNF-α (de Lago, Moreno-Martet, Cabranes, Ramos, & Fernandez-Ruiz, 2012). There is growing evidence that several inflammatory mediators are dysregulated in both plasma and saliva isolated from BMS patients, when compared to control subjects (Barry, O'Halloran, McKenna, McCreary, & Downer, 2018; Chen et al., 2007; Pekiner et al., 2008; Pezelj-Ribaric et al., 2013; Simcić et al., 2006), while polymorphisms in IL-1β are associated with BMS pathogenesis (Guimaraes et al., 2006). Therefore, modulation of these, and other proinflammatory molecules, is an important approach to consider in the management of BMS.
4.3 Cannabinoids in the clinic
Several cannabinoid-based therapies, including Sativex®, Epidiolex®, Marinol® and Cesamet®, have been approved in several countries for use in the clinic for a range of disorders (details on indication, mechanism of action and side effects described in Table 3).
| Name | Composition | Indication | Administration Route | Postulated mechanism of action | Common Side Effects |
|---|---|---|---|---|---|
| Sativex® |
THC:CBD 1:1 |
|
|
|
|
| Epidiolex® | CBD (highly purified) |
(Antiepileptic for treatment of seizures) |
|
|
|
| Marinol® (Dronabinol) | THC |
|
|
|
|
| Cesamet® (Nabilone) | Synthetic analogue of THC |
|
|
|
|
Sativex® is a combination of THC and CBD (2.7 mg THC and 2.5 mg CBD/0.1 ml; 1:1 ratio) approved for the treatment of spasticity and pain in adult patients with MS (Feliu, 2015). The co-administration of both cannabinoids benefits from the mitigation of THC adverse effects (Vaney et al., 2004). Sativex® is administered as an oromucosal spray, with the advantage of fast onset of action and high bioavailability (Scott, White, White, Wilbraham, & Guy, 2013). Patients self-titrate the dosage according to their need and tolerance of the drug. Interestingly, a THC/CBD spray has been shown to produce an improvement in peripheral neuropathic pain (Serpell et al., 2014). Also, a case study in an individual with MS receiving Sativex® for spasticity reported complete resolution of trigeminal neuralgia episodes and background facial discomfort, which were consistently present before treatment (Gajofatto, 2016).
Epidiolex®, a purified solution of CBD (100 mg/ml), is effective in the treatment of epileptic seizures and has been approved for patients (2 years of age and older) with Lennox–Gastaut and Dravet syndromes (Ali, Scheffer, & Sadleir, 2019). In double-blind placebo-controlled trials, CBD demonstrates efficacy in reducing convulsive seizure frequency (Devinsky et al., 2017). As pure CBD is not associated with psychoactive properties, Epidiolex® is a particularly attractive therapeutic option.
Marinol® is a pharmaceutical formulation of synthetic THC (2.5 mg, 5 mg or 10 mg soft gelatin capsules) indicated for anorexia associated with loss of appetite in AIDS patients and for the treatment of nausea and vomiting in patients undergoing chemotherapy (Badowski & Yanful, 2018). Phase III studies assessing neuropathic pain symptoms in MS demonstrate a clinically relevant decrease in pain during a 16-week Marinol® treatment period, when compared to placebo (Schimrigk et al., 2017).
Lastly, Cesamet®, a synthetic analogue of THC (1mg capsule), is approved in a number of countries for the treatment of chemotherapy-induced nausea and vomiting (Zurier & Burstein, 2016). Additionally, a number of trials have evaluated the efficacy of Cesamet® in the treatment of pain disorders, including neuropathic pain, chronic non-cancer pain and fibromyalgia (Tsang & Giudice, 2016). Reports indicate that Cesamet® has a higher bioavailability when compared to Marinol® (Turcott et al., 2018).
5 CONCLUSION FOR FUTURE PERSPECTIVES
BMS remains a true challenge for both patients and healthcare providers. Due to its multifactorial aetiology and involvement of multiple physiological processes, its definitive diagnosis is difficult, and no cure for the disorder has been identified. There is an urgent need for well-designed translational research programmes to study the mechanisms underlying this syndrome, in addition to developing novel therapeutics in this area. Indeed, many studies concerning BMS therapeutics are not standardized, lacking clinically validated pain assessment tools specific to BMS, and consensus on how to effectively rate the pain associated with BMS. Therefore, inconsistencies arise when comparing studies. This review identifies cannabinoid-based therapeutics for consideration in the management/treatment of BMS. Given the known neuroprotective, anti-inflammatory, antioxidative and antiapoptotic properties of cannabinoids, a full investigation of the cannabinoid system as a bone fide therapeutic strategy in BMS is warranted.
ACKNOWLEDGEMENTS
This work is supported by Enterprise Ireland (IP/2018/0740 to EJD).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTIONS
SRP, JTV, SD and EJD drafted the manuscript. BI, JPK and CM reviewed the original draft and provided additional input. All authors critically revised the manuscript and approved its final version.




