Nucleus accumbens shell and core dopamine responsiveness to sucrose in rats: role of response contingency and discriminative/conditioned cues
Abstract
This study investigated by microdialysis the role of response contingency and food-associated cues in the responsiveness of dopamine transmission in the nucleus accumbens shell and core to sucrose feeding. In naive rats, single-trial non-contingent presentation and feeding of sucrose pellets increased dialysate shell dopamine and induced full habituation of dopamine responsiveness to sucrose feeding 24 and 48 h later. In rats trained to respond for sucrose pellets on a fixed ratio 1 (FR1) schedule, dialysate dopamine increased in the shell but not in the core during active responding as well as under extinction in the presence of sucrose cues. In rats yoked to the operant rats, the presentation of sucrose cues also increased dialysate dopamine selectively in the shell. In contrast, non-contingent sucrose presentation and feeding in FR1-trained and in yoked rats increased dialysate dopamine to a similar extent in the shell and core. It is concluded that, whereas non-contingent sucrose feeding activated dopamine transmission in the shell and core, response-contingent feeding activated, without habituation, dopamine transmission selectively in the shell as a result of the action of sucrose conditioned cues. These observations are consistent with a critical role of conditioned cues acquired during training and differential activation of shell vs. core dopamine for response-contingent sucrose feeding.
Introduction
Two main subdivisions have been distinguished in the nucleus accumbens (NAc) (a ventro-medial shell and a dorso-lateral core), to which have been attributed different functional properties and roles in behaviour (Alheid & Heimer, 1988; Di Chiara, 2002; Saddoris et al., 2013).
Microdialysis and voltammetry studies have shown that shell dopamine (DA) is preferentially activated by drugs of abuse after non-contingent (passive) and contingent exposure (Pontieri et al., 1995, 1996; Tanda et al., 1997; Lecca et al., 2006a,b, 2007a,b; Aragona et al., 2008). Non-contingent palatable food stimulates in vivo DA transmission in the shell and core. This response undergoes single-trial habituation by 2 h specifically in the shell (Bassareo & Di Chiara, 1997, 1999a,b; Bassareo et al., 2003; Gambarana et al., 2003; Rada et al., 2005; Danielli et al., 2010). Habituation lasts for at least 24 h and recovers within 5 days (Bassareo & Di Chiara, 1997). However, core DA is eventually potentiated by non-contingent single-trial exposure to palatable food (Bassareo & Di Chiara, 1997). In contrast to food, drugs of abuse do not induce habituation of shell DA after repeated non-contingent as well as contingent exposure (Bassareo et al., 2003; Lecca et al., 2006a,b, 2007a,b).
Response contingency drastically affects the responsiveness of DA transmission in the shell and core under repeated drug exposure. Thus, whereas response-contingent heroin and cocaine preferentially activate shell DA, non-contingent exposure blunts DA responsiveness in the shell and sensitizes it in the core (Cadoni & Di Chiara, 1999; Lecca et al., 2007a,b). In the case of food, various microdialysis studies are available that compared shell vs. core DA transmission during operant and free food consumption but none has directly tested the contingent vs. non-contingent issue by a yoked paradigm (Sokolowski et al., 1998; Cheng & Feenstra, 2006; Ostlund et al., 2011; Segovia et al., 2011).
However, many discrepancies exist between microdialysis and voltammetry studies even within the same laboratory (see 4; Sokolowski et al., 1998; Roitman et al., 2008; Brown et al., 2011; Segovia et al., 2011; Wheeler et al., 2011; Cacciapaglia et al., 2012).
In order to investigate the role of response-contingency and sucrose-related cues in the responsiveness of DA transmission in NAc shell and core, rats were fully trained on fixed ratio 1 (FR1) to respond for sucrose pellets and dialysate DA was then monitored in the NAc shell and core under three different conditions: response-contingent (active) presentation of sucrose, extinction in the presence of cues associated with sucrose, and non-contingent presentation of sucrose. A group that was implanted in the shell and core and yoked to rats responding for sucrose was trained in parallel and then tested as for the operant rats.
Materials and methods
Animals
Male Sprague–Dawley rats (Harlan Italy, Udine, Italy) weighing 250–275 g were housed in groups of six per cage with standard food (MIL topi e ratti, GLP diets; Stefano Morini, S. Polo D'Enza, RE, Italy) and water ad libitum for at least 1 week in the central animal room, under constant temperature (23 °C), humidity (60%) and a 12-h light/dark cycle (lights on from 08:00 to 20:00 h).
All animal experiments were conducted in accordance with the statement that was revised and approved by the Society for Neuroscience in January 1995, with the guidelines for the care and use of experimental animals of the European Economic Commission (EEC Council 86/609; DL 27.01.1992, no. 116), and in accordance with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Surgery
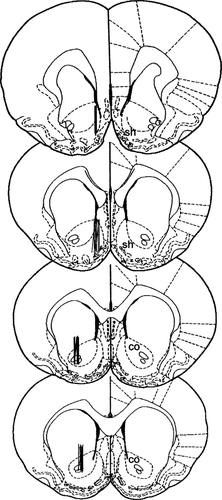
Rats were anaesthetized by 400 mg/kg, i.p. of chloral hydrate (Carlo Erba, Milano, Italy). A guide cannula (Plastics One, Roanoke, VA, USA) was stereotaxically implanted at the following coordinates: NAc shell (A: 2.0; L: 1 from bregma; V: −3.6 from dura) and NAc core (A: 1.6; L: 1.9 from bregma; V: −3.4 from dura) according to the atlas of Paxinos & Watson (1998) (Fig. 1). Guide cannulas were plugged with a dummy cannula.
After surgery, rats were housed in individual cages (45 × 21 × 24 cm) under the same conditions as above. Rats were left to recover for 10 days. During the first 5 days they were administered with gentamicin sulphate (40 mg/kg, s.c.). Rats were manipulated once a day for 5 min during the whole training period.
After recovery, rats were fed 15 g of standard food (MIL topi e ratti, GLP diets; Stefano Morini) in order to keep their weight at around 90% of their ad-libitum weight. Water was available ad libitum for the whole duration of the experiments.
Microdialysis
Probe preparation
Microdialysis probes were prepared according to the method of Lecca et al. (2006a,b) using AN69 membrane (Hospal Dasco, Italy). The dialyzing portion of the probe was 1.5 mm long. A new probe was utilized for each experimental session.
Microdialysis experiments
At the beginning of each microdialysis session, the microdialysis probes were connected to an infusion pump and perfused with a Ringer's solution (147 mm NaCl, 4 mm KCl, 2.2 mm CaCl2) (see Lecca et al. (2006b) on the use of 2.2 mm Ca2+ in the Ringer's solution) at a constant rate of 1 μL/min; the dummy cannula was removed and the microdialysis probe was inserted through the guide cannula. Dialysate samples (5 μL) were taken every 5 min and injected without purification into a high-performance liquid chromatograph equipped with a reverse phase column (LC-18 DB, 15 cm, 5 μm particle size; Supelco) and a coulometric detector (Coulochem II; ESA, Bedford, MA, USA) to quantify DA. The first electrode of the detector was set at +125 mV (oxidation) and the second at −175 mV (reduction). The composition of the mobile phase was as follows: 50 mm NaH2PO4, 0.1 mm Na2-EDTA, 0.5 mm n-octyl sodium sulphate, and 15% (v/v) methanol, pH 5.5 (obtained by adding Na2HPO4). With these conditions the sensitivity of the assay for DA was 5 fmol/sample.
At the end of each microdialysis session the probe was removed and the guide cannula was plugged again with a sterilized dummy cannula.
Sucrose
Sucrose pellets of 45 mg each were utilized as food (Test Diet, Richmond, IN, USA).
Passive sucrose pellet feeding
In order to investigate whether repeated feeding of sucrose induced habituation of NAc shell DA responsiveness, rats naive to sucrose and implanted with a microdialysis probe inserted through the guide cannula were placed in the Skinner boxes and dialysate samples were taken every 5 min. After about 1 h, when the DA basal levels in the dialysates were stable, sucrose pellets were delivered in the food dispenser for 10 min at a constant speed of 4 pellets/min (total of 40 pellets). The test was repeated on each of the next 2 days. Rats were then anaesthetized with chloral hydrate (400 mg/kg) and processed for histology.
Operant and yoked training
At 10 days after guide cannula implant, rats (n = 30) were randomly assigned to operant and yoked groups. Each yoked rat was paired to one operant rat. Rats were trained every day for 2 weeks, apart from weekends. Sessions lasted 1 h and took place between 09:00 and 14:00 h in acoustically isolated and ventilated operant cages (Coulbourn Instruments, Allentown, NJ, USA). Two nose-poke holes were placed on one wall at 2 cm from the cage floor. The active nose-poke was illuminated by a green/yellow light and the inactive nose-poke by a red light. The food dispenser was placed between the nose-poke holes. On the same wall was located a loudspeaker emitting a tone of 4500 Hz.
Operant rats were trained to respond to sucrose under an FR1 schedule. Yoked rats received sucrose contingent upon responding by the operant rat to which they were paired. The number of nose-pokes made and rewards earned were recorded by Graphic State 2 software (Coulbourn Instruments).
For operant rats each 1-h session was composed of a cyclic alternation of three phases.
- Phase 1: lasting for 15 s during which the house light and nose-poke lights were turned on and a tone was activated to signal reward availability. Failure to respond correctly for more than 15 s resulted in the switch off of visual and auditory cues and start of phase 3 without going into phase 2.
- Phase 2: a sucrose pellet was dropped into the food dispenser and, after 5 s, phase 3 was initiated.
- Phase 3: all cues were turned off and reward was not available for 7 s. Asymptotic responding for at least three consecutive sessions was taken as the criterion for full training. Yoked rats underwent the same three phases except that both sucrose and the relative cues were contingent upon responding by the operant rats to which they were yoked.
Microdialysis after operant and yoked training
After the completion of training, operant and yoked rats were tested on three microdialysis sessions performed each day on three consecutive days. Operant and yoked sessions were started as soon as the DA basal dialysate levels had become stable (i.e. after approximately 1 h).
- First day: operant rats were tested under FR1 responding for sucrose. Yoked rats were presented with sucrose pellets contingent upon responding of the paired operant rat in the presence of all cues.
- Second day: operant rats were tested under extinction, i.e. in the presence of all of the stimuli preceding and following each response, but without sucrose pellet delivery. Yoked rats were exposed to the same cues activated under responding by the operant rats.
- Third day: both operant and yoked rats were presented non-contingently with sucrose pellets through the food dispenser at the same mean rate of operant responding but in the absence of discriminative cues signalling sucrose availability. Only cues associated with pellet delivery were present.
Day 1 of testing was an operant FR1 session and, as such, sucrose was presented in a response-contingent manner, as in all of the preceding training sessions. Because of this we did not find it necessary to introduce a further group tested under non-contingent sucrose pellet presentation on day 1.
Reverse order group
In order to control for the influence of session order on the response pattern of dialysate DA, an additional group of nine rats was trained on FR1 responding for sucrose and then tested on a reverse order, i.e. on day 2 under non-contingent sucrose pellet presentation and on day 3 under extinction (see Supporting Information).
Histology
At the end of all experimental procedures, rats were anaesthetized with chloral hydrate (400 mg/kg, i.p.) and transcardially perfused with 50 mL of saline, followed by 100 mL of 4% paraformaldehyde solution. The brains were then removed and post-fixed for 2 days. The brains were cut in alternate 40-μm-thick and 100-μm-thick serial coronal slices on a Vibratome (Technical Products International, St Louis, MO, USA). To establish the location of the dialysis probes, 100-μm-thick sections were examined under a stereomicroscope, whereas the 40-μm-thick sections were processed for tyrosine hydroxylase immunohistochemistry, as previously described (Lecca et al., 2006a).The location of the probes was reconstructed and referred to the atlas of Paxinos & Watson (1998) (Fig. 1).
Statistics
Statistical analysis was carried out by Statistica for Windows. Basal dialysate DA was calculated as the mean of the last three consecutive samples, differing by no more than 10%, collected during the 60-min period preceding experimental sessions. Basal dialysate DA values (expressed as fmol/5 μL dialysate) were compared between groups by one-way anova. Changes in dialysate DA were expressed as a percentage of the respective baseline values and were analysed by two-way anova for repeated measures. To assess the normality assumption we considered separate normal Q–Q plots of our response variable for each combination of the groups (levels) of the two independent variables, as well as Q–Q plots of the residuals. None of the plots indicated a significant violation of the normality assumption. Results from treatments showing significant overall changes were subjected to post-hoc Tukey's test with P < 0.05 as statistically significant.
Results
The basal values of dialysate DA (means ± SEM) were 26 ± 2 fmol/sample (n = 50) in the shell and 25 ± 2 fmol/sample (n = 38) in the core, and they were not different between the two areas. These values were consistent among different groups. Thus, one-way anova of basal dialysate DA obtained in the different groups did not show significant differences between microdialysis sessions of the different groups in both the shell (nine sessions: first, second and third session of habituation group; first, second and third session of FR1-trained group; first, second and third session of yoked group; F8,41 = 0.43; P = 0.89) and core (six sessions: first, second and third session of FR1-trained group; first, second and third session of yoked group; F5,32 = 0.01; P = 1). The absence of a statistical difference in absolute basal levels between groups justified the subsequent analysis of changes in dialysate DA as a percentage of baseline.
Habituation of nucleus accumbens shell dopamine responsiveness to sucrose feeding
The habituation of DA responsiveness to feeding of palatable foods has been shown by us for a salty food (Fonzies®) as well as for a sweet food (Kinder®) (Bassareo & Di Chiara, 1997, 1999b). In order to establish whether habituation also applies to sucrose, dialysate DA was monitored every day for 3 days during feeding of experimenter-administered sucrose pellets in rats that had never been previously exposed to sucrose. Figure 2 shows the time-course of dialysate DA in the NAc shell on three successive daily sucrose-feeding trials. The basal value of NAc shell DA (mean ± SEM) was 27 ± 3 fmol/sample (n = 16). This value did not change over days in the same subject (two-way anova, F2,13 = 0.028; P = 0.97).
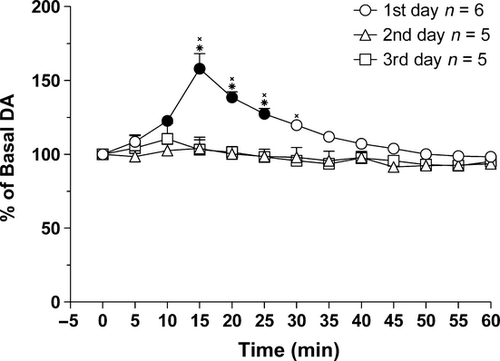
As shown in Fig. 2, NAc shell DA started to increase during sucrose consumption (between 5 and 10 min), peaking at 5 min after the end of pellet delivery. Rats continued to explore the food magazine for a few minutes after the last pellet delivery.
Two-way anova showed an effect of day (F2,13 = 17.28; P < 0.01) and time (F12,156 = 12.64; P < 0.01), and a day × time interaction (F24,156 = 5.44; P ≤ 0.01). Tukey's test showed that sucrose feeding increased dialysate shell DA on the first day but not on the following days.
Acquisition of FR1 responding for sucrose
Figure 3 shows the number of cumulative active and inactive nose-pokes during training of FR1 responding for sucrose. Nose-poking increased selectively on the active hole, reaching asymptote on the seventh session. No difference was observed in the temporal profile of acquisition or in asymptotic responding in the group implanted in the shell as compared with the group implanted in the core. Three-way anova showed a main effect of nose-poke (F1,32 = 130.54; P < 0.01) and session (F9,288 = 24.59; P < 0.01), and a nose-poke × session interaction (F9,288 = 18.24; P < 0.01).
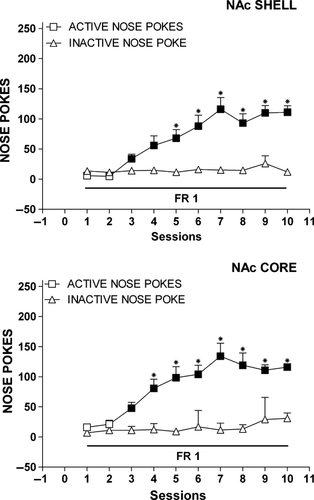
Results superimposable to those above were obtained in the reverse order group (Fig. S1).
Monitoring dialysate dopamine after training
Monitoring dialysate dopamine in FR1-trained (master) rats
The basal values of DA (means ± SEM) were 25 ± 3 fmol/sample (n = 20) in the NAc shell and 26 ± 4 fmol/sample (n = 26) in the core. As shown by two-way anova, basal dialysate DA did not change within subjects over the 3 days of testing, in both the shell (F2,17 = 0.58; P = 0.56) and core (F2,17 = 0.0001; P = 1).
Responding for sucrose
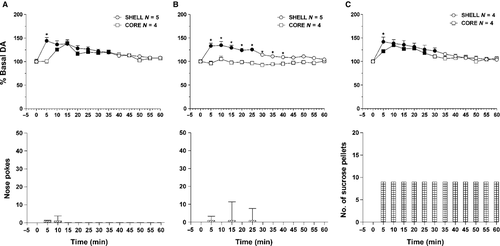
Figure 4A shows the time-course of dialysate DA in the NAc shell and core and the number of active nose-pokes during FR1 responding for sucrose. Responding for sucrose increased dialysate DA only in the shell. Core DA did not change for the whole session. Shell DA peaked on the first sample and returned to the basal level on the sixth sample. Responding remained high for one more sample beyond the increase of DA in the shell. Two-way anova showed an effect of area (F1,18 = 15.83; P < 0.01) and time (F6,108 = 7.77; P < 0.01), and an area × time interaction (F6,108 = 4.79; P ≤ 0.01). Post-hoc test showed an increase of dialysate DA in the NAc shell but not in the core.
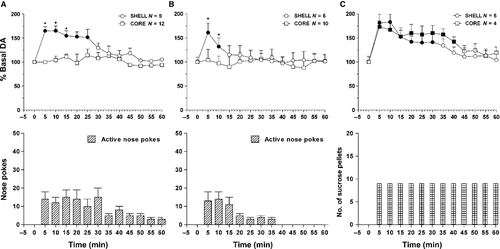
Figure S2A shows that similar results were obtained under responding for sucrose in the reverse order group.
Responding under extinction
Figure 4B shows the time-course of dialysate DA in the NAc shell and core, and that of active nose-pokes under extinction in the presence of cues signalling sucrose availability and associated with sucrose delivery.
Responding under extinction was short-lasting and was associated with a selective increase of DA in the shell. NAc core DA did not change for the whole session. Shell DA peaked on the first sample and remained high for one more sample. As in the case of responding for sucrose, the high rate of responding exceeded by one sample the increase of dialysate DA in the shell. Two-way anova showed an effect of area (F1,14 = 8.46; P = 0.011) and time (F6,84 = 11.29; P < 0.01), and an area × time interaction (F6,84 = 9.75; P < 0.01). Post-hoc test showed that DA increased in the NAc shell but not in the core.
Figure S2C shows that when the extinction session was performed on the third day, i.e. on the day after that of non-contingent sucrose presentation (reverse order group), results similar to those above (Fig. 4B) were obtained.
Non-contingent sucrose presentation
Figure 4C shows the time-course of DA in the NAc shell and core following non-contingent sucrose presentation. In contrast to responding for sucrose and to extinction, non-contingent sucrose presentation was associated with an increase of dialysate DA in both the NAc shell and core. The increase was maximal in both areas from the first sample and the DA time-course was almost superimposable in the two areas. In the NAc core the increase over the basal level was maintained for two more samples (seventh and eighth) compared with shell DA. Bars show the number of pellets presented every 5 min. Two-way anova showed an effect of time (F8,48 = 10.61; P < 0.01). Post-hoc test showed an increase of DA in both the shell and core.
Figure S2B shows that when the non-contingent sucrose session was performed on the day before that of the extinction session (reverse order group), similar results to those above (Fig. 4C) were obtained.
Monitoring dialysate dopamine in yoked rats
The basal values of DA (means ± SEM) in 5-min samples were 24 ± 2 fmol/sample (n = 14) in the NAc shell and 25 ± 2 fmol/sample (n = 12) in the core. As shown by two-way anova, basal DA did not change within subjects over the 3 days of testing, in both the shell (F2,8 = 0.76; P = 0.50) and core (F2,9 = 0.0001; P = 1).
Yoked sucrose pellet presentation
Figure 5A shows the time-course of dialysate DA in the NAc shell and core, and the number of nose-pokes of yoked rats during presentation of sucrose pellets contingent upon responding of rats to which they were paired. Yoked presentation of sucrose pellets increased DA in the shell from the first sample and with a delay of one sample in the core but the maximal increase and duration (up to the seventh sample) was similar in the two areas. Two-way anova showed a main effect of time (F12,72 = 29.61; P < 0.01) and an area × time interaction (F12,72 = 9.56; P ≤ 0.01). Post-hoc test showed that dialysate DA increased in the shell and core, with a higher response in the shell on the first sample.
Yoked extinction
Figure 5B shows the time-course of dialysate DA in the NAc shell and core, and of nose-pokes in rats yoked to operant rats under extinction. Yoked extinction resulted in a selective increase of shell DA lasting up to the fifth sample. Two-way anova showed an effect of area (F1,6 = 35.90; P < 0.01) and time (F12,72 = 13.76; P < 0.01), and an area × time interaction (F12,72 = 11.22; P < 0.01). Post-hoc test showed an increase of DA in the NAc shell but not in the core.
Yoked non-contingent sucrose pellet presentation
Figure 5C shows the time-course of DA in the shell and core during sucrose presentation non-contingent with responding of the operant paired rat but at the same mean rate of the operant sessions. Bars show the number of pellets presented every 5 min. Two-way anova showed an effect of time (F12,72 = 44.31; P < 0.01) and an area × time interaction (F12,72 = 2.76; P < 0.01). Post-hoc test showed that, although dialysate DA increased to a similar level in the shell and core, the increase took place earlier in the shell and lasted longer than in the core.
Discussion
The main goal of this study was to investigate the role of sucrose-related cues and response contingency in the responsiveness of NAc shell and core DA transmission. In fully-trained rats, FR1 responding for sucrose as well as extinction in the presence of sucrose-associated cues increased dialysate DA in the shell but not in the core. In contrast, response non-contingent sucrose feeding increased DA in the shell and core with a similar time-course. These observations were independent of the order of the extinction vs. non-contingent sucrose session as they occurred when extinction either preceded or followed the non-contingent sucrose session (see 2.7).
Rats that were yoked for the whole training to the operant rats did not perform any active nose-pokes but showed an increase of dialysate DA in both the NAc shell and core in response to sucrose and only in the shell in response to sucrose-related cues alone.
Responding for sucrose
Our observation that FR1 responding for sucrose was associated with a rapid increase of DA in the shell and no change in the core is at variance with Sokolowski et al. (1998) who reported that, during responding for food, dialysate DA increased to a similar extent in the shell and core, with a higher DA response in the shell only during the post-instrumental phase. In the present study, changes in shell DA increased over the basal level from the first sample and only during high nose-poking. Experimental differences (food instead of sucrose as reward and bar-pressing instead of nose-poking) might account for these differences. However, a recent study from the same laboratory by Segovia et al. (2011), in rats trained on FR1 to respond for food pellets (Bioserv), failed to observe changes in dialysate DA in both the shell and core. However, in rats bar-pressing for food on a random-ratio of 10 paradigm, Ostlund et al. (2011) observed an increase of DA in the shell and core with no differences between the two areas.
Cheng & Feenstra (2006) reported that, in rats learning to respond for sucrose/cellulose pellets during two sessions (2 h apart), dialysate DA increased to a similar extent in the shell and core, but, on the first session, rats that learned to criterion showed a higher shell DA response compared with rats that did not learn. These results are difficult to compare with ours due to the short duration of the training period.
With regard to voltammetry, there are basic differences from microdialysis that make it difficult to compare the results. Thus, voltammetry estimates changes in DA signals in a very short time interval (usually 100 ms) over or below background noise rather than basal DA levels, whereas microdialysis estimates absolute basal and stimulated levels of DA over a 100× longer interval (5–20 min; Robinson et al., 2008). As a result of this, voltammetry would not detect slow changes, taking place over tens of minutes, such as changes in basal NAc DA in rats responding for cocaine, whereas microdialysis may not detect changes resulting from discrete and isolated cues signalling the start of a behavioural sequence.
In spite of these basic differences, Cacciapaglia et al. (2012) reported that presentation of visual/auditory cues signalling sucrose availability elicits a larger phasic increase of DA in the shell than in the core. This response is followed by a slower response, selectively in the shell, that coincides with sucrose reward. If one considers the differences in time-scale between microdialysis (5 min) and voltammetric measurements (100 ms), the observations of Cacciapaglia et al. (2012) are not in contrast to ours. Thus, changes in NAc core DA might be too small in absolute terms to modify basal dialysate DA, whereas this may not be the case for NAc shell DA, which is released both in response to conditioned stimuli (CS) preceding sucrose delivery as well to sucrose itself.
It should be pointed out, however, that, as in the case of microdialysis, large discrepancies are found between voltammetry studies. Thus, in contrast to Brown et al. (2011), Cacciapaglia et al. (2012) observed phasic activation of DA in the core but no change in the shell after presentation of discriminative cues signalling food availability. In turn, whereas Brown et al. (2011) report an increase of phasic DA in the core but not in the shell in response to food, Roitman et al. (2008) and Wheeler et al. (2011) found just the opposite.
From this analysis it appears that large discrepancies do exist not only between our study and those of other laboratories but even between studies from the same laboratory. Nonetheless, the differences between the shell and core in the responsiveness of DA transmission observed in the present study are consistent with those reported in the voltammetric study of Cacciapaglia et al. (2012).
Extinction in master and yoked rats
During extinction sessions, all cues, either preceding or following responding, were still present, except that the pellet was prevented from falling into the dispenser. Under these conditions, rat responses were still emitted on the active nose-poke and DA increased selectively in the shell, just as in rats earning sucrose contingent upon their response. The same pattern of selective shell DA activation was observed in rats trained while yoked to the operant rats and therefore exposed to the same cues except that, in this case, any conditioning of sucrose-related cues would have been acquired by pavlovian rather than instrumental learning.
As far as concerns the literature, to our knowledge, no microdialysis studies are available comparing shell and core DA responsiveness to food-related cues presented under extinction by rats trained to respond for food. As for voltammetry, the study of Cacciapaglia et al. (2012) has been discussed previously.
Non-contingent sucrose feeding (including yoked feeding)
A main purpose of the present study was to compare the effect of response-contingent and response non-contingent sucrose feeding on in vivo DA transmission in the NAc shell and core. In naive rats repeatedly fed with sucrose pellets presented non-contingently, dialysate DA increased on the first trial and this response habituated on a second and third trial (24 and 48 h apart). These observations extend to sucrose the observations of previous studies from our laboratory and those of others made with various palatable foods (Bassareo & Di Chiara, 1997, 1999a,b; Gambarana et al., 2003; Rada et al., 2005; Danielli et al., 2010).
In rats previously trained to respond for sucrose on FR1, response non-contingent sucrose feeding elicited a robust and sustained increase in dialysate DA in the shell and core. Thus, in spite of the fact that these rats had been eating sucrose daily during training, no habituation of NAc shell DA responsiveness to sucrose was observed. However, this lack of habituation of shell DA transmission might only be apparent as, as observed during extinction sessions, NAc shell DA can be activated in trained rats, either yoked or operant, by conditioned/discriminative stimuli. These stimuli are likely to replace unconditioned stimuli as determinants of NAc shell DA activation.
As to the DA response in the NAc core, the fact that it is consistently and robustly observed under non-contingent sucrose feeding but is absent under extinction and in yoked rats exposed to sucrose-related cues suggests that it is either unconditioned in nature or is due to conditioned stimuli arising from stimuli intrinsic to sucrose (e.g. taste) and therefore evoked only by sucrose feeding. This conclusion makes even more striking the fact that, under responding for sucrose, when rats eat sucrose as a result of their instrumental behaviour, activation of DA transmission is observed in the shell but not in the core. We suggest therefore that the responsiveness of NAc core DA transmission to sucrose is actively suppressed during instrumental responding.
These observations are consistent with the hypothesis that DA responsiveness in the NAc shell and core is driven by different stimuli and is differentially affected by response contingency. This in turn suggests that DA transmission in these two areas plays different roles in instrumental behaviour. The robust and selective activation of DA transmission in the shell during responding for sucrose and under extinction is consistent with a role of conditioned stimuli and with the property of encoding a prediction of the intrinsic rewarding value of the action outcome (sucrose). Because of this, the activation of shell DA transmission might be intrinsically rewarding, whereas DA release in other terminal areas (NAc core, dorsal striatum and prefrontal cortex) might play more cognitive functions, such as signalling reward for learning purposes (Schultz et al., 1997). This hypothesis is consistent with the rewarding and reinforcing properties of amphetamine-like and cocaine-like psychostimulants, which preferentially increase DA in the NAc shell (Di Chiara & Bassareo, 2007; Lecca et al., 2007a) and depend for their reinforcing properties on DA receptors in this area (Pisanu et al., 2014). The suppression of DA activation in the core during responding for sucrose might be related to the need to prevent species-specific, automatic actions that would interfere with goal-directed action under conditions, such as those of FR1 responding, that do not require a decision among different reward options (Saddoris et al., 2013).
Conclusions
The present study shows that, under operant conditions, responding for sucrose stimulates DA transmission in the shell but not in the core. As non-contingent sucrose presentation and feeding also activate DA in the core, it is hypothesized that operant responding for sucrose suppresses the ability of sucrose to stimulate DA transmission in the core. This inhibition might serve to prevent impulsive and inappropriate responses, thus increasing the efficiency of goal-directed action.
No habituation of shell responsiveness was obtained under operant sucrose feeding. This observation, coupled with the fact that responding under extinction was also associated with the stimulation of shell but not core DA, suggests that activation of DA transmission during responding for sucrose is dependent upon conditioned rather than unconditioned sucrose stimuli. This might also apply to the stimulation of DA transmission in the shell by non-contingent sucrose presentation and feeding. Thus, following habituation, sucrose unconditioned stimulus (US) are replaced by sucrose CS as determinants of shell DA activation. These observations are consistent with a critical role of conditioned cues acquired during training and of differential activation of shell vs. core DA for response-contingent sucrose feeding.
Acknowledgements
This work was supported by grants from the Fondazione Banco di Sardegna (2011.1047, prot. no. U1140.2013/AI.1059.MGB) and RAS LR 7, 2007 (CRP-59764-F71J12000990002). F.C. gratefully acknowledges the financial support of her PhD scholarship (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007–2013, Axis IV Human Resources, Objective 1.3, Line of Activity 1.3.1.).
Abbreviations
-
- CS
-
- conditioned stimuli
-
- DA
-
- dopamine
-
- FR
-
- fixed ratio
-
- NAc
-
- nucleus accumbens
-
- US
-
- unconditioned stimuli




