Exploration of the dynamics between brain regions associated with the default-mode network and frontostriatal pathway with regards to task familiarity
Abstract
Specific brain regions have consistently been reported to be activated during resting state period, and they were described as being part of a particular network called the default-mode network (DMN). It has been shown that the DMN would deactivate during goal-directed tasks, but the actual relationship between them is still a matter of debate. In a previous study, we reported a specific pattern of activation of the frontostriatal regions during a set-shifting task in which these regions were increasing their activity as set-shifts were performed continuously and decreasing when the same rule was executed repeatedly. The present study aimed at assessing the relationship between the frontostriatal regions and the DMN. We hypothesized that the DMN would be anticorrelated with the frontostriatal regions so the DMN would be more deactivated as set-shifts are executed for a long period, but would start increasing when the same rule is being executed for a long period. Here, 15 participants underwent functional magnetic resonance imaging while performing a card-sorting task. We observed increased activity in the frontostriatal regions as more set-shifts are being performed while the DMN gets more deactivated. Interestingly, as decreased activity was observed in the frontostriatal regions during the execution of the same rule for a long period, the DMN showed increasing activity. We argue that there is an anticorrelation between the frontostriatal regions and the DMN, but also that the DMN could show positive activation during performance of a familiar goal-directed task.
Introduction
The default-mode network (DMN) is known to be activated during rest period (Gusnard & Raichle, 2001; Greicius et al., 2003), and includes various regions, such as the medial prefrontal cortex (mPFC), the posterior cingulate cortex, temporal cortex and posterior parietal cortex (Gusnard & Raichle, 2001; Raichle et al., 2001; Chee & Choo, 2004; Gould et al., 2006; Mason et al., 2007). Interestingly, it has often been reported that this network is suppressed when a cognitive task is performed (Shulman et al., 1997; Mazoyer et al., 2001), resulting from task-induced deactivation. One interpretation associates the DMN to task-unrelated thought (i.e. mind-wandering; Mason et al., 2007; Christoff et al., 2009), and that somehow suppression of this network contributes to task performance, at least for perceptual non-semantic tasks (Binder et al., 1999). Furthermore, DMN deactivation has been associated with task difficulty (McKiernan et al., 2003). However, the concept of task difficulty has been thoroughly dissected by Gilbert et al. (for extensive discussion on that matter, see Gilbert et al., 2012), and they concluded that DMN suppression could be process dependent.
Altered functioning of the DMN has been associated with a wide variety of neurological and psychiatric disorders, all highlighting different degrees of dysfunction between the DMN and frontostriatal regions. Multiples studies have reported altered functional connectivity between these two sets of regions in clinical conditions, such as depression (Bluhm et al., 2009; Ma et al., 2012), epilepsy (Rektor et al., 2013), schizophrenia (Salvador et al., 2010; Waltz et al., 2013), Huntington's disease (Werner et al., 2014) and Parkinson's disease (Kelly et al., 2009; Delaveau et al., 2010). The type of alteration observed in these disorders ranges from reduced functional connectivity between networks or hypo/hyper-connectivity within each network, suggesting a specific interaction between the DMN and frontostriatal network in normal functioning. In healthy individuals, frontostriatal and DMN interaction has also been characterized during executive tasks implicating attentional loads and working memory, both reporting a negative correlation (Tomasi et al., 2009; Rieckmann et al., 2011). However, it is still unclear how the DMN contributes functionally to the frontostriatal pathway.
Several neuroimaging studies have shown the involvement of frontostriatal regions during set-shifting (Berman et al., 1995; Rogers et al., 2000; Monchi et al., 2001, 2006; Nagahama et al., 2001). Using functional magnetic resonance imaging (fMRI), Monchi et al. (2001) showed a significant increase of activation in area 47/12 of the ventrolateral PFC, area 9/46 of the dorsolateral PFC and the caudate nucleus during the planning of a set-shift, while the posterior PFC and the putamen were observed during the execution of the set-shift. More recently, we have shown that activity in these regions increases with the number of consecutive set-shifts performed, while activity decreases with the execution of a repeated rule for increasing number of trials (Provost et al., 2012). Moreover, we showed an increased frontostriatal activity after execution of a large number of consecutive set-shifts, while the inverse pattern was observed after the execution of a same rule repeated for a long period of time. However, to date, we have not investigated the effect of the frontostriatal activity with regards to the structures associated with the DMN while set-shifting or maintaining rule is required for consecutive trials.
The aim of the present study was to investigate how the frontostriatal pathway and the DMN interact with each other. More specifically, we hypothesized that these two sets of regions would be inversely correlated (i.e. negative correlation) during the performance of a set-shifting task. In particular, unlike frontostriatal regions, we expected that the involvement of DMN-associated regions, especially the mPFC, would augment as the same rule is performed for consecutive trials, while DMN suppression would correlate with the number of consecutive trials where a set-shift (or change of rule) is required. To assess this effect, we used a task that was developed in our laboratory (Provost et al., 2012), in which a target item needed to be associated with a reference item according to a specific rule given by the task.
Materials and methods
Subjects
Fifteen subjects (seven males, eight females), 20–29 years old (mean age: 23.9 years; SD: 2.8), participated in this study. They were all right-handed, as assessed with the Edinburgh Handedness Inventory (Oldfield, 1971), and had no history of psychiatric or neurological disorders. All participants gave their informed, written consent, and this study was reviewed and approved by our local ethics committee at the Montreal Geriatric Institute Research Center (CRIUGM), ‘the Comité Mixte de l'Ethique à la Recherche of the Regroupement Neuroimagerie Québec (CMER-RNQ). This committee follows the guidelines of the Tri-Council Policy Statement of Canada, the civil code of Quebec, the Declaration of Helsinki, and the code of Nuremberg.
Cognitive task
The present fMRI study used a mixed-design paradigm in order to look at the specific change in the blood oxygen level-dependent (BOLD) signal during the selection process. A longer description of the cognitive protocol can be found in Provost et al. (2012). The task was developed using the stimulus presentation software ‘Media Control Function’ (Digivox, Montreal, Canada). The stimuli were presented via an LCD projector onto a mirror placed in front of the participant in the scanner. On the screen, the same four reference cards were displayed throughout the experiment, showing, respectively, one red triangle, two green stars, three yellow crosses and four blue circles (Fig. 1). On each trial, a different test card was presented in the middle of the screen and the participants were required to pair the test card with one of the reference cards based on a shared attribute (color or shape or number). The rule of classification was presented to the participant before each trial, unlike the classical Wisconsin Card Sorting Task. A letter cue (‘N’ = number, ‘S’ = shape, ‘C’ = color or ‘I’ = identical) was presented at the beginning of each trial to specify the rule for classification. The letter cue ‘I’ (identical) indicated that the test card would be the same as one of the reference cards and the subject would have to classify on the basis of identity. The subjects indicated their selection by using a magnetic resonance-compatible button box that was placed in their right hand for which the left button moved a cursor under the reference cards from left to right, while the right button was used to validate the subject's selection.
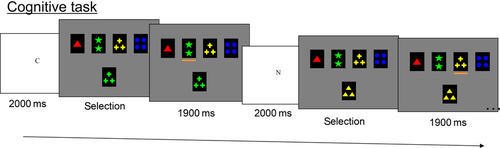
Each trial consisted of two distinct events: the indication of the rule to use; and the matching period. The rule indication was presented for 2000 ms, while the length of the matching periods depended on the subject's response time and provided the de-synchronization necessary between the stimuli and frame acquisition in order to perform event-related analysis. After a selection was made, the screen froze for 1900 ms before the beginning of another trial.
Four conditions were presented during the scanning session. In the ‘CONTINUOUS SHIFT’ condition, a different rule of classification was provided on each one of 12 consecutive trials. In the ‘SAME RULE’ condition, participants matched the test card with one of the reference cards according to the same rule for 12 consecutive trials. The rule was selected randomly by the computer program and all rules were used equally often during the experiment. In the ‘ALTERNATING’ condition, a set-shift was required after a few numbers of trials of the same rule. The task was programmed in such a way that a set-shift would occur after two, three or four trials of performing the same rule. In the ‘CONTROL’ condition, the test card was the same as one of the reference cards and the subjects were required to match it with its twin.
Each participant was properly trained by completing three blocks of the four conditions randomly presented, and they all reached a level of 95% of correct answers before the scanning session. A block is defined as a set of the four conditions randomly presented.
MRI acquisitions
Scanning was performed in the 3T Siemens Trio Magnetom MRI Scanner at the Functional Neuroimaging Unit of the CRIUGM. The session started with a scout for positioning the subject. This was followed by a T1-weighted acquisition for anatomical localization. Then, it was followed by four runs of T2*-weighted functional acquisitions. Each functional run lasted 10.5 min and consisted of 252 frames, each acquired at every 2.5 s. Each frame contained 36 slices placed along the anterior commissure/posterior commissure with a matrix of 64 × 64 pixels, an isotropic voxel size of 3.4 × 3.4 × 3.4 mm3. The FA was 90° and the TE 30 ms.
Data analysis
The methods for data analysis were the same as those in Monchi et al. (2001, 2006) using the fmristat analysis software developed by Worsley et al. (2002) http://www.math.mcgill.ca/keith/fmristat/ combined with the minc tools. The first two frames in each run were discarded. Images from each run were first realigned to the third frame for motion correction, and were smoothed using a 6-mm full-width half-maximum isotropic Gaussian kernel. The statistical analysis of the fMRI data was based on a linear model with correlated errors. The design matrix of the linear model was first convolved with a difference of two gamma hemodynamic response functions timed to coincide with the acquisition of each slice. The correlation structure was modeled as an autoregressive process. At each voxel, the autocorrelation parameter was estimated from the least squares residuals, after a bias correction for correlation induced by the linear model. The autocorrelation parameter was first regularized by spatial smoothing and was then used to ‘whiten’ the data and the design matrix. The linear model was re-estimated using least squares on the whitened data to produce estimates of effects and their standard errors. The resulting effects and standard effect files were then spatially normalized by non-linear transformation into the standard proportional stereotaxic space of Talairach & Tournoux (1988) using the algorithm of Collins et al. (1994) and the ICBM152 atlas as an approximation. Anatomical images were also normalized to the same space using the same transformation. In a second step, runs, sessions and subjects were combined using a mixed-effects linear model. A random-effects analysis was performed by first estimating the ratio of the random-effects variance to the fixed-effects variance, then regularizing this ratio by spatial smoothing with a Gaussian filter. The amount of smoothing was chosen to achieve 100 effective degrees of freedom (Worsley, 2005). Statistical maps were thresholded at P < 0.05 correcting for multiple comparisons using the minimum between a Bonferroni and random field correction. This corresponds to a t-statistics equal to or above 4.3 (based on peak intensity, Bonferroni correction) or a cluster size larger than 420 mm3 (based on cluster size, random field correction), and only those peaks are reported here unless mentioned otherwise.
An event-related analysis was performed considering only the selection period that corresponded to the time period that started with the presentation of the stimuli and ended with the selection of one reference card indicating the choice of the participant. Two contrasts were generated from the statistical analysis for the comparison between trials, and two statistical maps were produced for the correlation analysis. We examined both positive and negative peaks of the following contrasts: (i) CONTINUOUS SHIFT minus the CONTROL condition; and (ii) SAME RULE minus CONTROL condition. For the correlation analysis, we looked at the effect of trial position on the BOLD signal within the CONTINUOUS SHIFT and SAME RULE conditions (not contrasted with the control condition), separately. In this analysis, a covariate was added assigning different weights according to the trial position within a given block. Both positive and negative are reported for the correlation analyses. Due to our question of interest, that is to study the pattern of activation of the frontostriatal and DNM-associated regions with regards to long periods of set-shifting or maintaining the same rule, the trials from the ‘ALTERNATING’ condition will not be discussed in the present manuscript and were removed from our current analysis.
Results
Behavioral data
All 15 participants completed the task. Behavioral data were analysed using spss 17.0 for Windows. The mean percentage of errors for all conditions together was at 1.4%. More specifically, we observed 3.4% of errors in the CONTINUOUS SHIFT condition, 0.8% in the SAME RULE condition and 1.1% of errors in the CONTROL condition. We found a significant difference in the number of errors in the CONTINUOUS SHIFT vs. the SAME RULE condition (t4213 = 2.19, P < 0.05).
The average reaction time for the CONTINUOUS SHIFT condition was 1515 ms (SD = 645 ms), 1425 ms (SD = 633 ms) for the SAME RULE condition, and 1325 ms (SD = 593 ms) for the CONTROL condition.
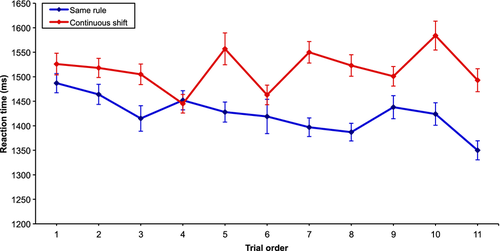
We examined the difference in the reaction times for the three conditions. There were significant reaction time differences among all conditions (F2 = 43.54; P < 0.001; CONTINUOUS SHIFT vs. SAME RULE, t4281 = 4.63, P < 0.001; CONTINUOUS SHIFT vs. CONTROL, t4261 = 10.03, P < 0.001; SAME RULE vs. CONTROL, t4270 = 5.32, P < 0.001). Furthermore, we plotted the reaction time for both experimental conditions for each of the 12 trials, and we can observe that only the end of each condition drives the significant difference (Fig. 2). Note that the first trial of the SAME RULE and CONTINUOUS SHIFT conditions was removed to avoid trials with potential rule repetition from the previous condition as for the CONTINUOUS SHIFT condition, or potential set-shift from the previous condition in the case of the SAME RULE condition. A negative correlation between the reaction time and trial order was observed in the SAME RULE condition (r = −0.53; P < 0.05), while no correlation was found in the CONTINUOUS SHIFT condition (r = −0.32; P > 0.05).
fMRI data
A whole-brain analysis was performed for the contrasts of interest. For the fMRI analysis, trials with errors were removed. In conditions in which a set-shift needed to be performed, the mPFC, the cingulate cortex, as well as the medial and superior temporal cortex (major components of the DMN) showed a decrease in activity, and this negative correlation increased following the trial order, while we observed significant increase of activation in the ventrolateral and dorsolateral areas of the PFC as well as in the striatum, and an increase in their activity as trial number increases. Similarly, a significant increase of activity was observed in the mPFC, the cingulate cortex and the superior temporal cortex, while a significant decrease of activity in the dorsolateral PFC and the caudate nucleus was observed as the number of trials increased in the SAME RULE condition.
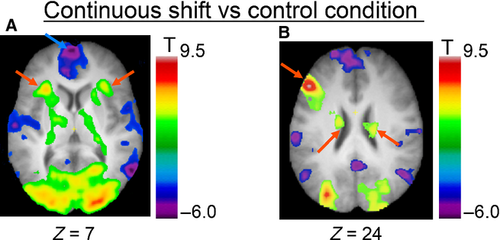
CONTINUOUS SHIFT vs. CONTROL condition
We found significantly increased activity in the left dorsolateral PFC (area 9, 9/46), the ventrolateral PFC (area 47/12), the supplementary motor area bilaterally, the left posterior PFC, the left fusiform area, cerebellum and the middle occipital cortex bilaterally, the right posterior cingulate cortex and middle temporal cortex, and the left superior parietal lobule (Fig. 3; Table 1). Subcortically, we observed significant activation in the caudate nucleus and ventrolateral nucleus of the thalamus bilaterally, and the left postero-lateral nucleus of the thalamus. On the other hand, we observed a significant decrease in activity in the left frontopolar cortex, the medial superior frontal cortex bilaterally, the right posterior PFC, the left anterior cingulate cortex, bilateral insula, superior temporal cortex, middle temporal cortex and parahippocampal cortex, the left precentral cortex, and the bilateral middle temporal cortex and lateral parietal cortex.
| Anatomical areas | Stereotaxic coordinates | ||||
|---|---|---|---|---|---|
| x | y | z | t-values | Cluster size | |
| Positive peaks | |||||
| Dorsolateral PFC (area 9, 46/9) | |||||
| Left | −48 | 30 | 24 | 9.47 | > 10 000 |
| Ventrolateral PFC (area 47/12) | |||||
| Left | −28 | 22 | 8 | 5.91 | > 10 000 |
| Right | 32 | 28 | 6 | 4.67 | 1544 |
| Paracingulate/supplementary motor area | |||||
| Left | −4 | 16 | 50 | 4.85 | 3864 |
| Right | 10 | 16 | 48 | 4.76 | 3864 |
| Posterior PFC (area 6, 8) | |||||
| Left | −42 | 4 | 34 | 7.47 | > 10 000 |
| Left | −50 | 16 | 32 | 6.56 | > 10 000 |
| Caudate nucleus (body) | |||||
| Left | −16 | −10 | 26 | 4.93 | > 10 000 |
| Right | 18 | −16 | 26 | 5.18 | 6912 |
| Right | 26 | −30 | 12 | 4.73 | 6912 |
| Thalamus | |||||
| Left | −14 | −10 | 10 | 4.64 | > 10 000 |
| Left | −20 | −22 | 16 | 5.25 | > 10 000 |
| Right | 20 | −14 | 18 | 4.88 | 6912 |
| Inferior parietal lobule | |||||
| Left | −42 | −42 | 42 | 5.61 | > 10 000 |
| Fusiform area | |||||
| Left | −44 | −44 | −8 | 5.27 | > 10 000 |
| Cerebellum – declive | |||||
| Left | −36 | −58 | −18 | 4.95 | > 10 000 |
| Middle occipital gyrus (area 18 and 19) | |||||
| Left | −42 | −66 | −4 | 8.2 | > 10 000 |
| Right | 20 | −94 | 14 | 7.28 | > 10 000 |
| Posterior cingulate cortex (area 31) | |||||
| Right | 18 | −62 | 10 | 5.75 | > 10 000 |
| Middle temporal gyrus | |||||
| Right | 30 | −68 | 18 | 4.95 | > 10 000 |
| Superior parietal lobule (area 7) | |||||
| Left | −26 | −68 | 48 | 9.44 | > 10 000 |
| Cerebellum – posterior lobe | |||||
| Right | 6 | −78 | −22 | 6.37 | > 10 000 |
| Cuneus | |||||
| Right | 2 | −88 | 12 | 5.71 | > 10 000 |
| Inferior occipital gyrus (area 18) | |||||
| Left | −34 | −88 | 4 | 8.45 | > 10 000 |
| Right | 22 | −96 | 4 | 7.27 | > 10 000 |
| Negative peaks | |||||
| Frontopolar cortex (area 10) | |||||
| Left | −2 | 64 | 6 | 5.85 | > 10 000 |
| Superior frontal gyrus (medial area 9) | |||||
| Left | −6 | 54 | 28 | 5.2 | > 10 000 |
| Left | −20 | 38 | 54 | 4.39 | > 10 000 |
| Right | 12 | 42 | 50 | 5.95 | > 10 000 |
| Posterior PFC (area 8) | |||||
| Right | 6 | 52 | 42 | 4.98 | > 10 000 |
| Right | 18 | 32 | 48 | 4.71 | > 10 000 |
| Anterior cingulate cortex (area 32) | |||||
| Left | −4 | 44 | 30 | 4.64 | > 10 000 |
| Subcallosal gyrus | |||||
| Left | −10 | 12 | −12 | 4.22 | 688 |
| Insula | |||||
| Left | −40 | 6 | −10 | 4.42 | > 10 000 |
| Right | 44 | −28 | 22 | 4.46 | 7432 |
| Superior temporal gyrus (area 22) | |||||
| Left | −60 | −2 | 0 | 5.49 | > 10 000 |
| Right | 54 | −2 | 18 | 5.02 | 3176 |
| Middle temporal gyrus (area 21) | |||||
| Left | −50 | −6 | −18 | 5.05 | > 10 000 |
| Right | 52 | −10 | −16 | 4.75 | 3176 |
| Parahippocampal cortex/amygdala | |||||
| Left | −28 | −8 | −16 | 4.6 | > 10 000 |
| Right | 24 | −6 | −16 | 5.32 | 3104 |
| Precentral gyrus (area 6) | |||||
| Left | −62 | −16 | 40 | 5.66 | > 10 000 |
| Postcentral gyrus | |||||
| Left | −62 | −20 | 22 | 4.99 | > 10 000 |
| Right | 66 | −16 | 26 | 4.43 | 7432 |
| Posterior cingulate cortex/paracentral lobule (area 31) | |||||
| Left | −2 | −28 | 50 | 8 | > 10 000 |
| Right | 2 | −50 | 30 | 5.7 | > 10 000 |
| Middle temporal gyrus (area 37) | |||||
| Right | 60 | −62 | 6 | 5.87 | > 10 000 |
| Lateral parietal cortex | |||||
| Left | −54 | −68 | 34 | 5.84 | 7960 |
| Right | 56 | −62 | 32 | 5.98 | > 10 000 |
| Right | 58 | −50 | 46 | 4.92 | > 10 000 |
- PFC, prefrontal cortex.
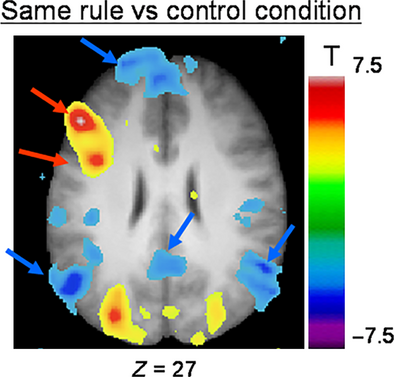
SAME RULE vs. CONTROL condition
We found significant activation in the left dorsolateral PFC (area 9/46), the left lateral premotor cortex (area 6), the fusiform cortex bilaterally, the right posterior parietal cortex, as well as the cerebellum and the occipital cortices (Fig. 4; Table 2). In terms of deactivation, we observed significant activity in the left mPFC, the medial superior frontal cortex bilaterally, the bilateral lateral PFC, the left postcentral cortex, and the posterior insula, posterior cingulate cortex and lateral parietal cortex bilaterally.
| Anatomical areas | Stereotaxic coordinates | ||||
|---|---|---|---|---|---|
| x | Y | z | t-values | Cluster size | |
| Positive peaks | |||||
| Dorsolateral PFC (area 46/9) | |||||
| Left | −48 | 30 | 28 | 7.57 | > 10 000 |
| Lateral premotor cortex (area 6) | |||||
| Left | −40 | 4 | 34 | 6.88 | > 10 000 |
| Cerebellum (anterior lobe – culmen) | |||||
| Left | −36 | −52 | −18 | 5.32 | > 10 000 |
| Fusiform area | |||||
| Left | −38 | −56 | −14 | 5.51 | > 10 000 |
| Right | 40 | −60 | −10 | 5.09 | > 10 000 |
| Inferior occipital gyrus (area 19) | |||||
| Left | −42 | −64 | −2 | 6.65 | > 10 000 |
| Right | 40 | −78 | −2 | 5.22 | > 10 000 |
| Superior parietal lobule (area 7) | |||||
| Left | −24 | −66 | 48 | 8.27 | > 10 000 |
| Cerebellum – posterior lobe (declive) | |||||
| Right | 6 | −78 | −18 | 4.39 | 584 |
| Middle occipital gyrus (area 19) | |||||
| Left | −36 | −88 | −4 | 8.55 | > 10 000 |
| Right | 30 | −88 | 6 | 5.97 | > 10 000 |
| Inferior occipital gyrus (area 18) | |||||
| Left | −30 | −90 | −2 | 8.26 | > 10 000 |
| Right | 30 | −86 | −6 | 4.86 | > 10 000 |
| Cuneus (area 18) | |||||
| Left | −10 | −98 | 6 | 5.37 | > 10 000 |
| Right | 16 | −98 | 8 | 4.8 | > 10 000 |
| Negative peaks | |||||
| Medial PFC | |||||
| Left | −6 | 64 | 6 | 4.11 | > 10 000 |
| Left | −6 | 50 | 30 | 4.06 | > 10 000 |
| Medial superior frontal cortex | |||||
| Left | −10 | 48 | 38 | 3.99 | > 10 000 |
| Right | 10 | 42 | 50 | 4.34 | > 10 000 |
| Lateral PFC | |||||
| Left | −20 | 22 | 62 | 3.87 | 424 |
| Right | 32 | 20 | 50 | 3.8 | 704 |
| Postcentral cortex | |||||
| Left | −50 | −18 | 20 | 4.34 | 2640 |
| Posterior insula/parietal opercula | |||||
| Left | −42 | −26 | 24 | 4.16 | 2640 |
| Right | 48 | −28 | 22 | 3.98 | 440 |
| Posterior cingulate cortex/paracentral lobule | |||||
| Left | −2 | −32 | 50 | 6.25 | 1024 |
| Right | 4 | −52 | 36 | 3.94 | 1792 |
| Lateral parietal cortex (area 39) | |||||
| Left | −54 | −68 | 32 | 5.4 | 6792 |
| Right | 50 | −66 | 36 | 5.34 | 6632 |
- PFC, prefrontal cortex.
Correlation analysis for CONTINUOUS SHIFT condition
We observed a significant positive correlation between the level of BOLD signal and increasing trial position in the CONTINUOUS SHIFT condition, in the left dorsolateral PFC (area 46), ventrolateral PFC bilaterally (area 47/12) and left posterior PFC (area 8), as well as the left inferior frontal junction (area 6, 8, 44; Fig. 5; Table 3). In the more posterior regions, we found activation in the left occipito-temporal junction, the left cuneus, the left posterior parietal cortex, as well as in the right cerebellum and bilateral occipital cortices. Subcortically, significant activation was found in the right thalamus and in the left caudate nucleus. No frontal or subcortical activity correlated significantly with a decrease in trial position. On the other hand, significant anticorrelation was observed in the left mPFC, the right amygdala, the superior temporal sulcus and the posterior cingulate cortex bilaterally, and the right middle temporal and posterior parietal cortices bilaterally.
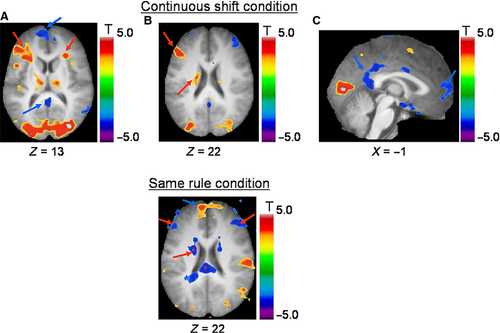
| Anatomical areas | Stereotaxic coordinates | ||||
|---|---|---|---|---|---|
| x | y | z | t-values | Cluster size | |
| Positive peaks | |||||
| Dorsolateral PFC (area 46) | |||||
| Left | −46 | 34 | 18 | 4.13 | 7312 |
| Ventrolateral PFC (area 47/12) | |||||
| Left | −26 | 20 | 14 | 3.92 | 7312 |
| Right | 28 | 26 | 12 | 4.03a | 392 |
| Posterior PFC (area 8) | |||||
| Left | −52 | 18 | 32 | 4.46 | 7312 |
| Inferior frontal junction (area 6/8/44) | |||||
| Left | −42 | 6 | 38 | 4.73 | 7312 |
| Thalamus | |||||
| Right | 16 | −14 | 18 | 4.2 | 576 |
| Caudate nucleus | |||||
| Left | −18 | −8 | 22 | 3.45 | 1032 |
| Left | −18 | −20 | 20 | 4.11 | 1032 |
| Occipito-temporal junction | |||||
| Left | −42 | −64 | −8 | 5.58 | 1768 |
| Cuneus (area 17) | |||||
| Left | −16 | −70 | 8 | 4.41 | 552 |
| Superior parietal lobule (area 7) | |||||
| Left | −26 | −72 | 52 | 7.01 | 34 272 |
| Left | −26 | −64 | 42 | 5.86 | 34 272 |
| Left | −26 | −86 | 32 | 4.86 | 34 272 |
| Cerebellum | |||||
| Right | 6 | −78 | −24 | 4.34 | 288 |
| Occipital cortex (area 18) | |||||
| Left | −2 | −82 | 6 | 5.2 | 34 272 |
| Right | 32 | −82 | 12 | 5.33 | 34 272 |
| Negative peaks | |||||
| Medial PFC | |||||
| Left | 2 | 68 | 8 | 3.60a | 184 |
| Amygdala | |||||
| Right | 22 | −2 | −14 | 4.81 | 1120 |
| Superior temporal sulcus | |||||
| Left | −52 | −8 | −16 | 3.86a | 216 |
| Right | 48 | 12 | −24 | 3.89a | 192 |
| Posterior cingulate cortex | |||||
| Left | −2 | −44 | 10 | 3.89 | 496 |
| Right | 2 | −36 | 38 | 3.76 | 424 |
| Middle temporal cortex (area 21) | |||||
| Right | 62 | −54 | 4 | 3.96 | 800 |
| Inferior parietal lobule | |||||
| Right | 50 | −66 | 42 | 4.03 | 672 |
- PFC, prefrontal cortex.
- a Significant by cluster analysis.
Correlation analysis for the SAME RULE condition
We observed a significant positive correlation between the level of BOLD signal and increased trial position (as the time since the last set-shift increases) in the mPFC and the premotor cortices bilaterally, left insula, the superior temporal cortex bilaterally, the right cingulate cortex, the postcentral cortex bilaterally, the right retrosplenial cortex and the lateral occipital cortex bilaterally (Fig. 5; Table 4). Significant activation correlated (i.e. negative correlation) with decreasing trial position (as the time since the last set-shift decreases) in the left dorsolateral PFC (area 9/46), the left cingulate and postcentral cortices, the posterior parietal cortex bilaterally, as well as in the left precuneus and the right cerebellum. Subcortically, the right putamen and the caudate nucleus bilaterally were also found to be significantly deactivated.
| Anatomical areas | Stereotaxic coordinates | ||||
|---|---|---|---|---|---|
| x | y | z | t-values | Cluster size | |
| Positive peaks | |||||
| Medial PFC/anterior cingulate cortex (area 10/32) | |||||
| Left | −8 | 60 | 22 | 3.90a | 368 |
| Left | −10 | 50 | −6 | 4.07 | 488 |
| Right | 10 | 60 | 2 | 3.44a | 48 |
| Posterior inferior frontal cortex (area 44/6) | |||||
| Left | −54 | 0 | 6 | 5.04 | 6088 |
| Right | 52 | −4 | 4 | 4.23 | 3504 |
| Insula | |||||
| Left | −40 | 0 | −12 | 4.31 | 6088 |
| Superior temporal cortex (area 21)/Sylvian fissure | |||||
| Left | −60 | −2 | 2 | 4.39 | 6088 |
| Right | 58 | −18 | 14 | 4.69 | 3504 |
| Cingulate cortex | |||||
| Right | 10 | −16 | 44 | 3.94a | 208 |
| Postcentral gyrus | |||||
| Left | −48 | −24 | 16 | 4.15 | 6088 |
| Right | 60 | −20 | 20 | 4.29 | 3504 |
| Retrosplenial cortex | |||||
| Right | 20 | −54 | 12 | 3.86a | 192 |
| Lateral occipital cortex | |||||
| Left | −48 | −82 | 6 | 4.32 | 456 |
| Right | 38 | −82 | 12 | 4.48 | 512 |
| Negative peaks | |||||
| Dorsolateral PFC | |||||
| Left | −50 | 30 | 26 | 3.88 | 2360 |
| Right | 44 | 38 | 24 | 3.96a | 360 |
| Putamen | |||||
| Right | 24 | 8 | 12 | 4.44 | 856 |
| Caudate nucleus (body) | |||||
| Left | −18 | 2 | 26 | 4.05 | 1256 |
| Right | 18 | −2 | 28 | 3.9 | 640 |
| Cingulate cortex | |||||
| Left | −2 | −30 | 28 | 4.92 | 2144 |
| Postcentral gyrus | |||||
| Left | −46 | −46 | 50 | 5.78 | 20 616 |
| Posterior parietal cortex | |||||
| Left | −32 | −62 | 48 | 6.79 | 20 616 |
| Right | 38 | −60 | 46 | 4.97 | 2688 |
| Precuneus | |||||
| Left | −2 | −74 | 56 | 4.8 | 20 616 |
| Cerebellum – lobule VI | |||||
| Right | 10 | −82 | −20 | 4.22 | 968 |
- PFC, prefrontal cortex.
- a Significant by cluster analysis.
Discussion
The present study investigated the relationship between the frontostriatal regions and the regions associated with the DMN in the context of a set-shifting task. In our experimental task, one condition included 12 consecutive trials in which a set-shift had to be performed, one condition in which no set-shift was required for 12 consecutive trials, and a control condition where participants had to match the test card with its twin. The contrast involving the CONTINUOUS SHIFT vs. the CONTROL conditions revealed significant increased activation in the dorsolateral PFC, the ventrolateral PFC and the caudate nucleus, while the mPFC, the middle and superior temporal cortices, the posterior cingulate cortex and lateral parietal cortex, commonly associated with DMN, were significantly deactivated as previously reported (Provost et al., 2012). In the contrast SAME RULE minus CONTROL condition, we still observed significant activation in the dorsolateral PFC, but not the ventrolateral PFC and caudate nucleus, while significant decreased activity was recorded in the mPFC, posterior cingulate cortex and lateral parietal cortex. The correlation analysis allowed us to observe the specific regions that increase or decrease with regards to trial position. In other words, it gives us information on which regions will be more activated (or deactivated) as one moves along within a specific condition. The correlation analysis of the CONTINUOUS SHIFT condition showed a significant increase of activity in the frontostriatal regions, namely the dorsolateral PFC, the ventrolateral PFC and the caudate nucleus, while a negative correlation was observed in the DMN-associated regions such as the mPFC, the superior and middle temporal cortices, the posterior cingulate cortex and the lateral parietal cortex, indicating that these regions deactivated more as you move along in the CONTINUOUS SHIFT condition. In the correlation analysis of the SAME RULE condition, significant increased activity was observed in the mPFC, the superior temporal cortex and cingulate cortex, while the dorsolateral PFC and caudate nucleus were significantly deactivated. These results support the idea that the frontostriatal regions are specifically anticorrelated with regions associated with the DMN.
The contrasts used in this experiment indicated the presence of a deactivation in the DMN but, interestingly, the correlation analysis of the two experimental conditions revealed a pattern in which the frontostriatal and the DMN regions react in an opposite fashion. The ventrolateral PFC was not significantly deactivated in the correlation analysis of the SAME RULE condition. However, we explain this result by the nature of our task. The ventrolateral PFC was shown to be involved in the active retrieval process, which is a selection process that proceeds by actively comparing the target held in mind with the presented stimuli to allow a selection according to a bias and reducing the ambiguity between stimuli (Petrides, 2002). Even though the same rule is applied repeatedly, the selection process needs to be maintained across all trials. In the CONTINUOUS SHIFT condition, participants had to search the proper stimulus according to the new rule that is being given on each trial. Similarly, in the SAME RULE condition, even if the rule is similar to the previous trials, the stimulus had to be found according to rule given. Therefore, even if the rule does not change, the ambiguity between stimuli remains on each trial. Also, spatial position could not account for this change as the position of the target was randomized each time. However, in the CONTINUOUS SHIFT condition, as the rule is changing after each trial, there could be interference between the different rules as the number of trials increases leading to the need of a greater control in the selection of the target.
The most striking result of our study is that the recruitment of the DMN can increase even if a cognitive task is performed, such as in the SAME RULE condition. As the execution of the rule becomes more familiar, the DMN start disinhibiting itself as the correlation analysis of the SAME RULE condition highlighted. This possibly indicates that the measures of DMN activity might be useful in determining the familiarity with a given process in the context of a task that needs to be learned. It has been shown in the past that task difficulty could affect the DMN (McKiernan et al., 2003) but, in our case, task difficulty does not reside in the selection process itself but in the interference induced by previous trials. Indeed, in terms of selection demand, both conditions can be seen as equivalent. Although we observed significant reaction time differences between our experimental conditions, we would argue, here, that it more likely reflects rule interference between trials reducing familiarity with the rule to apply, than complexity resulting from the selection process to successfully execute the pairing, as it was previously suggested by Gilbert and colleagues (Gilbert et al., 2012). We cannot rule the effect of task difficulty on our results because familiarity is somehow interconnected with the concept of task difficulty; as an individual is getting more familiar with a task, it is quite reasonable to assume that the level of difficulty is reduced. It is important to reiterate the fact that the experimental conditions were unknown to the participants and at no time participants could have guessed that one condition was starting over another. Participants still had to acknowledge the rule to perform, compare the target card with the four reference cards, and ultimately select the correct reference card. We could speculate that during the SAME RULE condition, participants were spending less resource in consolidating the rule to apply as the condition evolved, and that could have the effect of reallocating resources to attentional processes toward self-reflection.
Numerous studies have shown the deactivation of the DMN while performing a cognitive task (McKiernan et al., 2003; Mason et al., 2007; Leech et al., 2011). It has been proposed that the DMN could be disengaged to allow a redistribution of resources to the task-related regions during a cognitive task (Gusnard & Raichle, 2001; Raichle et al., 2001). However, this interpretation has been challenged by a study of Hampson et al. (2006), which suggested that the DMN could actively participate in the performance of a cognitive task. In their study, functional connectivity revealed that the mPFC and posterior cingulate cortex correlate negatively with working memory task performance, but also that the connectivity between these regions strengthens at rest suggesting a possible monitoring role in cognition. It was proposed that some regions of the DMN could be sensitive to the cognitive task at hand (Mayer et al., 2010). Indeed, they identified common regions as well as specific regions that deactivated during attentional and working memory tasks. They found that the left superior and inferior frontal gyri, the mPFC bilaterally, the left middle and bilateral temporal gyri, left angular gyrus and right posterior insula were specifically negatively correlating with the attentional task. On the other hand, the right posterior cingulate cortex and precuneus, the supramarginal gyrus, the temporal areas, the anterior and mPFC, and the inferior frontal gyrus were found to be more sensitive to the working memory task. Our results include most of these regions, suggesting correlation with both processes even if our task relies more on attentional processes than working memory. In fact, to successfully execute our task, a fair amount of resources should be directed toward attentional processes, which in turn will direct focus to the proper reference card. However, at the same time, we cannot neglect that the rule to apply needs to be encoded within working memory whether it is the CONTINUOUS SHIFT or the SAME RULE conditions. There could be regions that could specifically depend on the type of material to process (Binder et al., 1999) or according to the types of operations to be performed (Gilbert et al., 2012). In our case, we argue that the brain changes observed rely more on the set-shifting process during the switching condition and the level of familiarity during the same rule condition.
Parallels could also be drawn with implicit learning experiments in which individuals learn a motor sequence without conscious learning. Indeed, several neuroimaging studies have reported the contribution of the striatum as well as the PFC during the implicit learning of these sequences (Destrebecqz et al., 2005; Meier et al., 2013). A study by Badgaiyan et al. (2007) have highlighted the contribution of the PFC and striatum in a serial reaction time protocol involving a 12-item sequence. In these types of tasks, participants cannot recall any sequences even though their performance increases significantly showing an implicit learning. In our task, we can speculate that participants may have tried to learn implicitly the order of the rule presentation during the continuous shift condition contributing to the increasing activity observed in the frontostriatal pathway. Hence, the inverse pattern of activity observed in the correlation analysis of the same rule condition could rely on the same processes, that is, as participants executed the condition, a sequence of the rule presentation is easily implicitly learned decreasing the frontostriatal pathway. At the same time, familiarity with the task increases resulting in the activation of the structures associated with the DMN. Therefore, the interaction between these two networks could depend, on one hand, on level of ambiguity introduced by the continuously changing information, and the level of familiarity when a similar event occurs continuously. Considering that these networks operate each on specific and yet not necessarily contradictory processes, it is still plausible that they interact in a complementary fashion.
It has been clearly established that the dopaminergic system plays a crucial role in striatal functioning, and that dysfunction of the dopamine pathway could have an incidence on prefrontal activity (Taylor et al., 1986). The findings of the present study are in line with an increasing number of studies revealing that the DMN can be modulated by striatal activity and nigrostriatal dopamine integrity (van Eimeren et al., 2009; Nagano-Saito et al., 2009; Dang et al., 2012; Cole et al., 2013). In particular, a recent study has pointed out that during a card-sorting task, patients with Parkinson's disease showed a different pattern of deactivation of the DMN (van Eimeren et al., 2009). This modification of the DMN could reflect the dysfunction of the frontostriatal network usually associated with Parkinson's disease. In another study, using positron emission tomography, Braskie et al. (2011) revealed that the level of dopamine synthesis was affecting the modulation of the DMN activity, and that age-related deficits could be explained by this difference in the level of dopamine. It has even been reported that increased caudate nucleus volume leads to enhanced striatal-DMN connectivity in expert chess players (Duan et al., 2012).
Conclusion
The present manuscript helps to further clarify the relationship between regions associated with DMN and the execution of a goal-directed task soliciting executive processes such as set-shifting. The DMN has been shown to be required at rest, and that it would deactivate itself when a goal-directed task was performed. In our study, we were able to show that as the frontostriatal regions increase their activation during a set-shifting task, an anticorrelation was observed with the regions of the DMN. More importantly, the regions associated with the DMN regained their activity when no set-shifts were performed for an extended period. Specifically, as the task became more familiar (i.e. repetition of the same rule as opposed to switching continuously from one another), regions in the DMN increased activity like one would observe during a ‘resting state’ condition. We speculate that, even if the task is demanding (i.e. match a new test with one of the reference cards according to a specific rule), it seems that familiarity of a task could reduce the amount of resource devoted to the cognitive and reallocate them to regions that we can observe activating during rest, whether it is to re-direct attention to our self-referential environment or to direct our attention to unrelated thinking. The function of the DMN is still unclear, but hopefully our results can be added to the corpus of knowledge showing that familiarity is a factor to consider that could influence the DMN.
Acknowledgements
The authors would like to thank all the participants, the staff of the functional Neuroimaging Unit at the CRIUGM for practical help as well as support. This work was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to O.M. (No. 327518).
Abbreviations
-
- BOLD
-
- blood oxygen level-dependent
-
- DMN
-
- default-mode network
-
- fMRI
-
- functional magnetic resonance imaging
-
- mPFC
-
- medial prefrontal cortex
-
- PFC
-
- prefrontal cortex




