Differential contributions of de novo and maintenance DNA methyltransferases to object memory processing in the rat hippocampus and perirhinal cortex – a double dissociation
Abstract
Epigenetic mechanisms are increasingly acknowledged as major players in memory formation. Specifically, DNA methylation is necessary for the formation of long-term memory in various brain regions, including the hippocampus (HPC); however, its role in the perirhinal cortex (PRh), a structure critical for object memory, has not been characterized. Moreover, the mnemonic effects of selective DNA methyltransferase (DNMT) inhibition have not yet been investigated systematically, despite distinct roles for de novo (DNMT3a, 3b) and maintenance (DNMT1) methyltransferases. Consequently, we assessed the effects of various DNMT inhibitors within the HPC and PRh of rats using the object-in-place paradigm, which requires both brain regions. The non-nucleoside DNA methyltransferase inhibitor RG-108 impaired long-term object-in-place memory in both regions. Furthermore, intracranial administration of Accell short-interference RNA sequences to inhibit the expression of individual DNMTs implicated DNMT3a and DNMT1 in the HPC and PRh effects, respectively. mRNA expression analyses revealed a complementary pattern of results, as only de novo DNMT3a and DNMT3b mRNA was upregulated in the HPC (dentate gyrus) following object-in-place learning, whereas DNMT1 mRNA was selectively upregulated in the PRh. These results reinforce the established functional double dissociation between the HPC and PRh and imply the operation of different epigenetic mechanisms in brain regions dedicated to long-term memory processing for different types of information.
Introduction
DNA methylation has been established as a dynamic regulator of mnemonic processes in the adult rodent brain (Day & Sweatt, 2011; Baker-Andresen et al., 2013). Catalysed by DNA methyltransferases (DNMTs), methyl groups are transferred onto cytosine bases in the DNA, triggering chromatin compaction and gene silencing (Bird, 2002). The finding that DNMT inhibition immediately post-learning selectively impairs long-term memory (LTM) suggests that DNA methylation is necessary for memory consolidation (Monsey et al., 2011; Oliveira et al., 2012).
The necessity of this epigenetic modification has been demonstrated in numerous brain regions for several forms of LTM (Miller & Sweatt, 2007; Miller et al., 2008, 2010; Han et al., 2010; Monsey et al., 2011; Sui et al., 2012; Day et al., 2013; Maddox et al., 2014), including requirement in the hippocampus (HPC) for object memory (Zhao et al., 2010; Oliveira et al., 2012). Although the HPC clearly can contribute to object recognition, its exact role remains controversial, and the weight of evidence suggests that it is involved specifically in remembering spatial or contextual information (Forwood et al., 2005; Piterkin et al., 2008; Barker & Warburton, 2011). Conversely, the perirhinal cortex (PRh) is implicated in processing of object identity (Buckley & Gaffan, 2006; Murray et al., 2007; Winters et al., 2008; Brown et al., 2012). Indeed, the roles of the HPC and PRh in spatial and object mnemonic processing, respectively, are doubly dissociable in the rat (Winters et al., 2004). The contribution of DNA methylation to PRh-mediated memory has not been investigated. We have therefore systematically examined the involvement of DNMTs in HPC- and PRh-dependent memory using the object-in-place (OiP) task. Because this paradigm requires both brain areas (Barker & Warburton, 2011), regional comparisons were possible in the absence of any potential cross-task differences.
The main catalytically active DNMTs comprise two families: the de novo methyltransferases, DNMT3a and DNMT3b, preferentially methylate un-methylated DNA de novo, whereas maintenance methyltransferase, DNMT1, methylates hemi-methylated DNA (Bird, 2002). Although recent progress has been made comparing the function of DNMT1/3a in HPC-dependent memory (Feng et al., 2010), all three enzymes have yet to be systematically assessed in different brain regions associated with the consolidation of different forms of information. The present study used non-selective DNMT inhibitors, small interfering RNAs (siRNAs) and quantitative PCR to investigate the involvement of DNMT1/3a/3b in the HPC and PRh for OiP memory. We specifically used Accell siRNA as no transfection agent is required (Thermo Scientific: Dharmacon, Lafayette, CO, USA), and its viability has been demonstrated in neurons in vivo (Nakajima et al., 2012). Considering the ubiquitous involvement of DNA methylation in long- but not short-term memory (Monsey et al., 2011; Oliveira et al., 2012), we expected DNMTs to be necessary in both the HPC and PRh for OiP LTM. Furthermore, given their role in de novo methylation, we hypothesized that specifically DNMT3a and/or DNMT3b would be required for memory within the HPC, a site of adult neurogenesis. Conversely, we posited potential dissociations in the non-neurogenic PRh.
Materials and methods
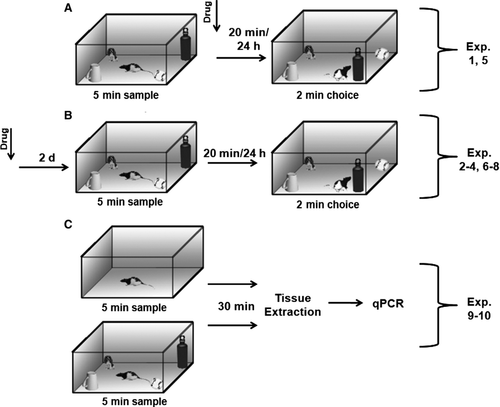
To determine the involvement of DNMT enzymes in the HPC for short- and long-term OiP memory, we employed the non-selective DNMT inhibitor, RG-108 (Fig. 1A; Exp. 1). Subsequent experiments investigated the mnemonic consequences of selective DNMT inhibition in the HPC using siRNAs (Fig. 1B; Exps 2–4). The effects of RG-108 (Fig. 1A; Exp. 5) and DNMT siRNAs (Fig. 1B; Exps 6–8) were additionally assessed in PRh. mRNA expression analyses (Fig. 1C) provided results complementary to the behavioral effects seen in both the HPC (Exp. 9) and the PRh (Exp. 10).
Subjects
The subjects were 196 adult male Long Evans rats (Charles River, QC, Canada), weighing 300–350 g at the start of testing and approximately 3 months old. They were pair housed in opaque cages and maintained on a 12-h reverse light cycle (08:00 h lights off, 20:00 h lights on). All behavioural testing occurred during the rats' waking hours (dark phase), although the testing room was illuminated by ceiling-mounted fluorescent lighting. During experimental testing periods each rat received 20 g of rodent chow to maintain an 85–90% free-feeding body weight. On experimental testing days rats were fed after testing was completed. Water was available ad libitum. All procedures adhered to the guidelines of the Canadian Council on Animal Care and were approved by the Animal Care Committee at the University of Guelph.
Surgical procedures
The majority of the subjects underwent intracranial surgical cannula implantation targeting either the dorsal HPC or PRh. Prior to surgery, rats were anesthetized using isofluorane (Benson Medical Industries, Markham, ON, Canada) and received a subcutaneous injection of meloxicam (5 mg/mL; Boehringer Ingelheim, Ingelheim am Rhein, Germany). With the incisor bar placed at −3.3 mm, rodents were secured in a stereotaxic frame (Koft Instruments, Tujunga, CA, USA). The scalp was then incised and retracted to expose the skull. Holes were drilled into the skull and 22-gauge indwelling cannula guides were implanted according to the following coordinates relative to the skull at Bregma (Paxinos & Watson, 1998): anteroposterior, −5.5 mm; lateral, ± 6.6 mm; dorsoventral, −7.0 mm for PRh placements, and anteroposterior −3.8 mm, lateral ± 2.5 mm, dorsoventral −2.5 mm for HPC placements. The guide cannulas were anchored to the skull by four jeweller screws and dental acrylic. Dummy cannulas, 0.36 mm in diameter, were placed into the guide cannulas where they remained at all times except during infusions. Dummy cannulas were cut to extend 1.1 mm past the end of the guide cannula. HPC-operated animals additionally had a dust cap screwed onto the top of the in-dwelling cannula and dummy. Post-surgery the scalp was sutured and rats were placed on a recovery heat pad for 1–2 h. All animals were given 7–10 days to recover before any behavioral testing.
Drugs and DNMT inhibitors
To broadly target the DNMTs we used the non-nucleoside DNMT inhibitor RG-108 (0.4 μg/μL in 20% DMSO/saline solution; Sigma Aldrich, St Louis, MO, USA). To target the DNMTs in a selective fashion, Accell siRNAs were chosen; specifically, Accell DNMT1, 3a and 3b siRNAs (0.2 μg/μL; Thermo Scientific: Dharmacon) were selected to target within the open reading frame. siRNA sequences are presented in Table S1. A non-targeting Accell siRNA (0.2 μg/μL) was used as a negative control (sequence 1; Thermo Scientific: Dharmacon). All doses for the above listed agents were chosen based on pilot studies or previously published results (Miller et al., 2010; Thermo Scientific). As per the manufacturer's guidelines, siRNA was resuspended in 1× Accell siRNA buffer for a working stock of 100 μm, and Accell siRNA delivery medium was added on the day of, immediately preceding infusions (Thermo Scientific: Dharmacon). Accell siRNA was specifically chosen as it has been designed to incorporate into difficult-to-transfect cells, including neurons, without the use of a transfection agent (Thermo Scientific: Dharmacon). Furthermore, this product has been shown to incorporate almost exclusively into neurons when infused broadly into the brain (Nakajima et al., 2012).
Infusions
For all behavioral experiments, within a given trial rats received bilateral infusions of one of the inhibitors or control solutions outlined above. siRNAs were administered 2 days prior to the sample phase (Fig. 1B) based on the manufacturer's instructions and previous literature (Thermo Scientific: Dharmacon; Nakajima et al., 2012), whereas RG-108 was administered immediately following the sample phase (Fig. 1A). All experiments were run with infusate as a within-subjects factor, and rats received counter-balanced infusions of inhibitor or control over multiple trials; specifically, one control and one drug infusion was administered per animal, per experimental phase (e.g. intra-HPC DNMT3a, 24-h delay). For experiments assessing the effects of RG-108, infusions were separated by 3 days, whereas infusions of siRNAs were separated by at least 5 days. Due to the nature of our within-subjects design, if a rat received the same drug–control pairing on more than two trials (e.g. multiple experimental phases run with the same animals, i.e. Exps 1a, 2, 5a, 6 and 7), the infusions of the drug/inhibitor were separated by a minimum of 6 days in the case of RG-108 and 10 days for the siRNAs. These parameters should limit any cumulative effects of the inhibitors, and the consistent differences observed between conditions in the results described below support this assumption.
During infusions, the dummy cannulas were removed to allow insertion of the 28-gauge infusion cannulas. Two 1-μL Hamilton syringes were inserted into propylene tubing holding infusion cannulas and fastened to a Harvard Apparatus (Hilliston, MA, USA) precision syringe pump set to deliver infusions at a rate of 0.5 μL/min for 2 min. The internal cannulas were left in for an additional 1.5 min to allow for diffusion of the infusate. Following infusions, internal cannulas were removed and the dummy cannulas were reinserted. Mock infusions during habituation sessions proceeded in an identical fashion, but no solution was administered.
Apparatus and stimuli
The open field was constructed from white, opaque corrugated cardboard (100 × 100 × 46 cm). Before each trial began the rat was placed in a tall, bottomless, cardboard tube situated in the center of the open field. The trial began when the tube was lifted and the rat was exposed to the objects. This procedure ensured that the rats did not begin exploration before the trial began, i.e. looking at the objects as the rat is placed into the open field.
A video camera was mounted on a tripod above the open field to record all trials. Duplicate copies of objects made from plastic, ceramic, glass and aluminum were obtained. The height of the objects ranged from 10 to 20 cm, and they varied with respect to their visual and tactile qualities. All objects were affixed to the floor of the apparatus with a reusable adhesive putty to prevent them from being displaced during testing, and objects sat against the back wall of each arm. As far as could be determined, the objects had no natural significance for the rats, and they had never been associated with a reinforcer. Before being placed in the apparatus, objects were always wiped with 50% ethanol.
Object-in-place procedure
All of the behavioral/infusion experiments adhered to the following procedures. More specific experimental details are outlined below.
All rats experienced two 5-min habituation sessions on successive days, to an empty open field. Behavioral testing occurred at least 24 h following the last habituation trial. Each trial consisted of an infusion, plus a sample and choice phase separated by a retention delay. For all OiP experiments, rats were first tested with a retention delay of 24 h to assess the hypothesis that DNMT inhibition would disrupt LTM. If an impairment was observed, the same rats were subsequently tested with a short-term retention delay (20 min). If, as hypothesized, no impairment was seen with the short-term delay, the same rats were once again tested with the 24-h delay to ensure that the absence of short-term memory deficit could not be explained by an order or practice effect. This 24 h–20 min–24 h retention delay cycle was used for Experiments 1, 2, 5 and 8. In the event that no long-term impairment was seen in the initial 24-h testing phase (i.e. Experiments 3, 4, 6 and 7), no subsequent trials were run. A new set of four distinct objects was used for each trial for a given group of rats (i.e. no rat experienced the same set of objects on more than one trial), and all experimental trials were fully counterbalanced; specifically, the side of the open field on which objects were displaced (right or left) was counter-balanced, as was the object arrangement. The four objects were placed in the corners of the open field, approximately 5 cm from the walls. Each rat was individually brought into the experiment room in a transfer cage. To prevent any ‘pre-exposure’ to the objects before the phase began, rats were placed in a cardboard tube located in the middle of the apparatus. The sample phase began once the tube was lifted and the rats were exposed to the objects. Rats were then removed after 5 min in the apparatus and transferred back to the colony room. Following a retention delay (see details below), rats were exposed to the same four sample objects in a 2-min choice phase; however, the two objects on one side (right or left) of the apparatus had switched locations (Fig. 1A and B). All other procedures were identical to the sample phase.
The time spent exploring the objects on either side (right or left) was scored by an experimenter, blinded to the treatment conditions, viewing the rat on a video screen. A key on the computer keyboard corresponded to the left and right side of the apparatus. The corresponding key was pressed at the onset of the exploratory bout and pressed again at offset, and this timed the duration of exploration for that bout. Exploration of an object was defined as directing the nose to the object at a distance of < 2 cm and/or touching it with the nose.
Histology
Following behavioral analyses (excluding Experiments 9 and 10), rats were anesthetized by an intraperitoneal injection of 2 mL Euthansol (340 mg/mL; Schering Canada Inc., QC, Canada), and perfused transcardially with 100 mL phosphate-buffered saline (PBS, pH 7.4) followed by 250 mL of 4% neutral buffered formalin (pH 7.4; EMD, Gibbstown, NJ, USA). The brains were then removed, post-fixed in 4% formalin at 4 °C for at least 1 week, and afterwards immersed in 20% sucrose in PBS until they sank. A cryostat was used to slice coronal sections (60 μm) through the extent of HPC or PRh, and every fifth section was mounted on a gelatin-coated glass slide and stained with cresyl violet. Verification of cannula placements was carried out using light microscopy.
Behavioral training and tissue extraction for mRNA analyses
The habituations, apparatus and stimuli used in mRNA quantification experiments (Exps 9 and 10) were identical to the OiP behavioural experiments noted above. Cage-mates were assigned to either the control or the experimental group. All rats experienced the 5-min sample phase of the OiP paradigm, but half of these rats observed objects (experimental condition; exactly as described for the behavioral studies), while the other half explored an empty apparatus (control condition). Specific details for each experiment are outlined below.
The entire PRh [anterior/posterior (A/P) −2.80 to −7.04 mm], dentate gyrus (dorsal HPC; A/P −1.60 to −4.0 mm) and CA region (1–3; dorsal HPC; A/P −1.60 to −3.80 mm) tissue was collected from rats that were killed (CO2) 30 min following open field exposure with or without objects (Fig. 1C). This 30-min time point was chosen based on previous literature assessing mRNA levels of DNMTs post-learning (Miller & Sweatt, 2007; Zhao et al., 2010; Jobe et al., 2012). The dentate gyrus and CA regions were analysed separately based on our hypothesis that de novo DNMTs might be implicated in learning-induced adult HPC neurogenesis, which occurs in the dentate gyrus. The group of rats that viewed objects were exposed in an identical fashion to the OiP sample phase described above, thereby enabling us to assess the mRNA levels of DNMTs following learning (experimental group). Because all rats had been previously habituated to the apparatus as detailed earlier, the group of rats that were exposed to an empty apparatus acted as our ‘non-learning’ control group. Each tissue section was homogenized using Trizol (1 mL/100 mg; Invitrogen, Carlsbad, CA, USA) and stored at −80 °C for subsequent RNA isolation.
RNA isolation and quantitative real-time (qRT) PCR
Total RNA was extracted from individual brain areas in Trizol reagent according to the manufacturer's instructions. The RNA was then quantified (μg/μL) and assessed for purity (A260/280) using a spectrophotometer. Only samples with an A260/280 ratio between 1.7 and 2.0 were further analysed.
RNA (5 μg) was treated with DNase I and reverse transcribed with Superscript II using oligo-dT as the primer, for 75 min at 43 °C, and the generated cDNA was used for qualitative PCR assessment with platinum Taq DNA polymerase. All PCR reactions were performed in a final volume of 50 μL with an initial 2-min strand separation at 94 °C and a final 2-min elongation step at 72 °C, following the last cycle. PCR products were separated on a 1.5% agarose gel and visualized with ethidium bromide under UV light. In initial experiments, all of the DNMT isoforms were assessed qualitatively and the identity of the PCR products was confirmed by sequencing. In later experiments only qualitative detection of beta-actin was assessed to ensure cDNA integrity.
Specific DNA products were then amplified using quantitative PCR (ViiA™ 7 Real-time PCR Machine; Life Technologies, Carlsbad, CA, USA) with POWER SYBRgreen reagents containing ROX reference dye to minimize well-to-well error. All qPCR reactions were performed in a final volume of 10 μL (1 μL cDNA). Samples were analysed in triplicate for each target gene with primers designed using the primer3 program. The primer pairs for the individual DNMTs are presented in Table S2. With the exception of a missing N-terminal end on DNMT3a2, DNMT3a1 and 3a2 isoforms are identical (Chen et al., 2002); therefore, a primer sequence within DNMT3a2 that completely overlapped with DNMT3a1 was chosen to ensure that both isoforms were quantified. Relative quantification was carried out using the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT; Table S2). Each cycle began with an initial activation phase at 95 °C for 10 min followed by 50 cycles of 15 s at 95 °C and 60 s at 60 °C. Cycle threshold (Ct) values were chosen in the linear range of amplification, and quantification of target genes from experimental animals (object exploration) was compared with those of biological controls (empty-apparatus exploration) using the comparative ∆∆CT method (Schmittgen & Livak, 2008).
Statistical analyses
For all behavioral experiments with infusions, although the total 2 min of choice exploration was recorded and scored, attention was focused on the first minute, during which object discrimination is typically most sensitive (Dix & Aggleton, 1999). As such, choice control measures were also calculated using strictly the first minute of data, whereas the entire 5 min was used to calculate sample control measures (see Tables S3 and S4). A discrimination ratio (DR) was calculated for each rat per object-recognition trial, and the average DRs across conditions were used for statistical analyses. This DR is defined as – the difference in time spent exploring the novel vs. the familiar object in the first minute of choice exploration, divided by the total time spent exploring both objects in the first minute of choice exploration. This measure takes into account individual differences in the total amount of exploration time. Where appropriate, either repeated-measures or mixed-factor anovas were employed. Further planned comparison paired-samples t-tests were run to compare drug and control conditions within each experiment. In addition, for each condition, both levels of the drug manipulation were compared with a zero or chance DR using one-sample t-tests. These two statistics allow us to determine if the drug manipulation abolished memory (i.e. comparing with 0), in addition to assessing whether this is significantly different from control condition performance. All analyses were conducted using two-tailed analyses with a confidence interval of 0.05.
Control measures for each experiment included paired samples t-tests comparing both total sample exploration (full 5 min) and total choice exploration (first minute), between control and experimental conditions. Non-significant statistics here suggest no initial exploratory differences (sample exploration), in addition to the lack of obvious non-mnemonic effects of the drug (choice exploration), such as basic motivational, locomotor or sensory effects. Means and standard deviations for experimental sessions can be found in Table S3. Additionally, sample DRs were calculated to ensure no initial object or side bias, and can be found in Table S4. All control analyses were non-significant (statistics not reported) unless otherwise specified in the Results.
For mRNA analyses (Experiments 9 and 10), one-sample t-tests (reference value of 100) were used to determine any changes in mRNA expression levels of experimental animals vs. controls, as performed previously (Miller & Sweatt, 2007). Two-tailed t-tests were used for all analyses, with the exception of the conditions for which we had clear directional predictions based on our behavioral results (Experiment 9 – dentate gyrus DNMT3a; Experiment 10 – PRh DNMT1), for which one-tailed t-tests were employed. All analyses were performed using a confidence interval of 0.05.
Results
Behavioral/infusions
Histology
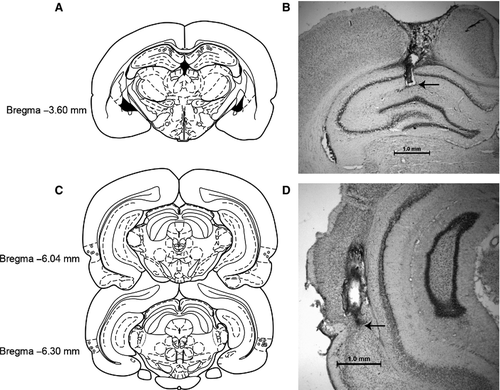
All rats included in the behavioral analyses for Experiments 1–4 had guide cannulas located bilaterally with infusion needle tips terminating in the dorsal HPC; these placements were consistently located approximately 3.60 mm posterior to Bregma (Fig. 2A and B). All rats included in the behavioral analyses for Experiments 5–8 had guide cannulas located bilaterally with infusion needle tips terminating in the PRh near the border between areas 35 and 36 (Burwell, 2001); these placements were consistently located at approximately 6.04 or 6.30 mm posterior to Bregma (Fig. 2C and D).
Further histological analysis examined the spread of the non-targeting siRNA through the dorsal HPC and PRh (Exp. S1). Specifically, 2 days following intracranial administration of Accell FAM-(6-fluroescein amidite) labeled non-targeting siRNA (green), fluorescence was detected in the dorsal HPC from approximately −2.56 to −3.60 mm A/P relative to bregma. As can be seen in Fig. S1A, the area of diffusion included both area CA1 and the dentate gyrus. Within PRh, fluorescence was detected from approximately −6.04 to −6.80 mm A/P from bregma (see Fig. S1B).
Experiment 1 – RG-108 in the HPC impairs long-term OiP memory
Experiment 1 examined the effects of the non-selective, non-nucleoside DNMT inhibitor, RG-108, within the HPC, on both long-term and short-term OiP memory. It has previously been established that DNA methylation in the HPC is necessary for long-term but not short-term memory in several mnemonic paradigms (Miller & Sweatt, 2007; Miller et al., 2008, 2010; Han et al., 2010; Monsey et al., 2011; Oliveira et al., 2012; Sui et al., 2012); therefore, we predicted that DNMTs in the HPC would also be necessary for long-term OiP memory.
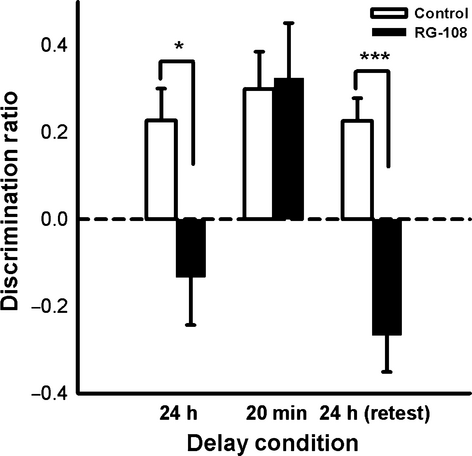
Eleven naïve rats received bilateral, immediate post-sample, intradorsal HPC infusions of RG-108 or control (DMSO), and OiP memory was tested in three successive phases: a 24-h retention delay, followed by a 20-min retention delay and then a replication of the 24-h retention delay (‘retest’). A repeated-measures anova revealed a significant main effect of both drug (F1,8 = 6.15, P = 0.035) and delay (F2,16 = 4.44, P = 0.027), as well as a significant interaction (F2,16 = 5.28, P = 0.016; Fig. 3).
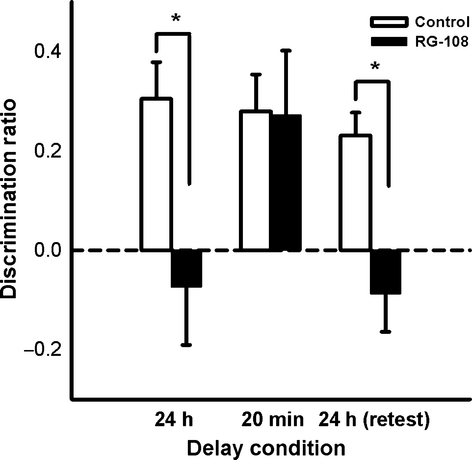
A mnemonic impairment in the RG-108 condition compared with the control condition was noted when a 24-h retention delay was employed, as demonstrated by significant a paired-samples t-test (t10 = 2.63, P = 0.025). Additional one-sample t-tests confirmed that the drug condition did not display memory above chance level (t10 = 0.63, P = 0.54), whereas the control condition did (t10 = 4.12, P = 0.002).
A paired-samples t-test indicated no statistical difference between the control and RG-108 conditions when the retention delay was shortened to 20 min (t10 = 1.22, P = 0.25). One-sample t-tests supported the lack of memory impairment, as both the control condition (t10 = 3.76, P = 0.004) and RG-108 condition (t10 = 4.553, P = 0.001), produced memory significantly higher than chance. Similarly, intact OiP memory was observed in a separate group of rats tested with a 2-h retention delay between sample and choice phases (see Exp. S2), consistent with intact short-term memory following RG-108 treatment.
The 24-h delay re-test produced comparable results to the first 24-h phase; a significant paired-samples t-test indicated a mnemonic impairment in the RG-108 condition compared with the control condition (t9 = 3.37, P = 0.008), and the RG-108 condition failed to display significant memory compared with chance (t9 = −0.92, P = 0.38), whereas the control condition did (t9 = 5.94, P < 0.001), consistent with intact short-term memory (20 min) following RG-108 treatment.
The results from Experiment 1 demonstrate that inhibition of DNMT activity in the HPC by the non-nucleoside DNMT inhibitor, RG-108, impairs OiP memory in a delay-dependent fashion. This delay-dependent impairment was also replicated following immediate post-sample intra-HPC infusions of 5-AZA-2′-deoxycytidine (see Exp. S3), a mechanistically distinct non-selective DNMT inhibitor that has been used in several similar studies (Levenson et al., 2006; Miller & Sweatt, 2007; Miller et al., 2008, 2010; Nelson et al., 2008; Han et al., 2010; Munoz et al., 2010; Monsey et al., 2011; Sui et al., 2012; Scott et al., 2013).
Experiments 2–4 – DNMT3a, but not DNMT3b or DNMT1, siRNA impairs OiP memory in the HPC
To explore further the role of DNMTs within the HPC for object memory, we examined the effects of selective inhibition of the main catalytically active DNMTs (DNMT1, 3a and 3b) on OiP memory using siRNA. qPCR analyses verified that Accell DNMT1, DNMT3a and DNMT3b siRNAs were active 2 days following intracranial administration and selective to their respective targets (Exp. S4, Fig. S4A–C).
Experiment 2
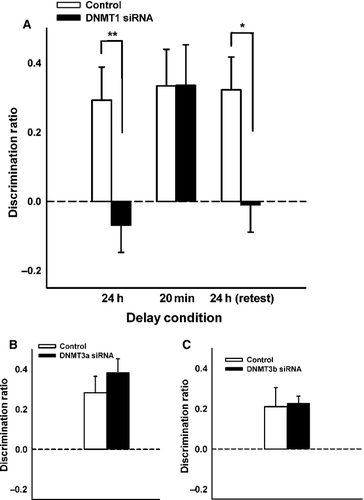
Experiment 2 tested the effects of DNMT3a inhibition within the HPC on both short and long-term OiP memory. A new group of experimentally naïve rats (n = 12) received 2-day pre-sample infusions of DNMT3a siRNA or non-targeting siRNA, counter-balanced, and memory was tested using the 24-h delay, 20-min delay, 24-h delay experimental cycle. A repeated-measures anova demonstrated a significant main effect of drug condition (F1,11 = 5.58, P = 0.008), a non-significant main effect of phase (F2,22 = 2.35, P = 0.12), and an interaction trending towards statistical significance (F2,22 = 2.93, P = 0.07; Fig. 4A). Given the delay-dependent effects of intra-HPC RG-108, planned comparisons assessed the effects of DNMT3a siRNA at each phase of delay testing.
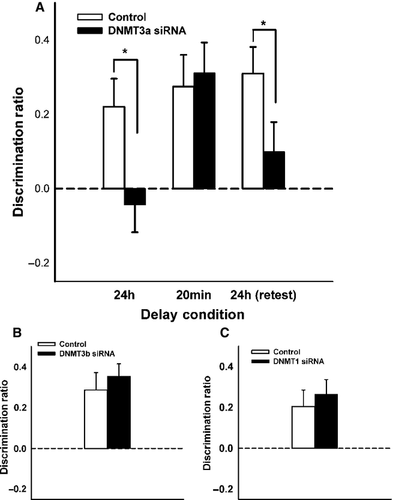
A paired-samples t-test indicated a significant difference between drug conditions in the initial 24-h delay phase (t11 = 2.57, P = 0.026), and additional one-sample t-tests indicated intact memory in only the control condition (t11 = 3.21, P = 0.008, t11 = 0.29, P = 0.78, for the control and experimental conditions, respectively).
When the delay was reduced to 20 min, DNMT3a siRNA did not impair performance, as demonstrated by a non-significant paired-samples t-test (t11 = 0.36, P = 0.73). Furthermore, memory was significantly greater than chance in both the control (t11 = 3.23, P = 0.008) and the experimental conditions (t11 = 3.77, P = 0.003). Because the siRNAs were shown to be active 2 days following administration (Exp. S4), the absence of any STM impairment indicates a lack of DNMT involvement in STM, rather than the ineffectiveness of the DNMTs at this earlier post-sample time point.
Results in the 24-h delay retest were similar to those in the initial 24-h delay phase, as groups demonstrated a significant difference from one another (t11 = 2.50, P = 0.03), and only the control condition displayed memory significantly higher than chance level (t11 = 4.33, P = 0.001, t11 = 1.24, P = 0.24, for the control and experimental conditions, respectively).
We additionally determined the effect of intra-HPC DNMT3a siRNA administration at time points closer to the sample phase. Specifically, immediate post-sample administration did not cause a mnemonic impairment (Exp. S5a), whereas 1-day pre-sample administration did (Exp. S5b); however, we employed 2-day pre-sample administration in all other siRNA experiments as it was also effective behaviorally, and more closely mimicked the parameters used in previous literature (Nakajima et al., 2012).
Experiment 3
Using similar parameters as in Experiment 2, naïve rats (n = 12) received 2-day pre-sample infusions of DNMT3b siRNA or non-targeting siRNA, counter-balanced, and long-term (24 h) OiP memory was assessed. Rats performed equally well on the OiP task following infusions of DNMT3b or control siRNA (t10 = 0.76, P = 0.46; Fig. 4B). One-sample t-tests confirmed the absence of mnemonic impairment in the DNMT3b siRNA condition (t10 = 3.70, P = 0.004) and control condition (t10 = 2.50, P = 0.031). Because no LTM impairments were noted, no further delay testing was undertaken.
Experiment 4
Under identical parameters to those in Experiments 2 and 3, 12 naïve rats performed equally well on the OiP (24-h delay) task when they received intra-HPC infusions of DNMT1 siRNA or control siRNA (t10 = −0.77, P = 0.46; Fig. 4C) in a counter-balanced manner. One-sample t-tests confirmed the lack of mnemonic impairment in the DNMT1 siRNA condition (t10 = 3.36, P = 0.006) and control condition (t10 = 5.89, P < 0.001). No further delay testing occurred given the null effects with the long-term retention delay.
The results from Experiments 2 to 4 suggest that only DNMT3a is necessary within the HPC for successful long-term OiP memory.
Experiment 5 – RG-108 in the PRh impairs long-term OiP memory
Experiment 5 examined the effects of RG-108 within the PRh on short-term and long-term OiP memory. Twelve experimentally naïve rats received bilateral, immediate post-sample intra-PRh infusions of RG-108 or control (DMSO), and were tested using the 24-h delay 20-min delay and 24-h delay retest cycle employed in Experiments 1 and 2. A repeated-measures anova indicated a significant drug by delay interaction (F2,22 = 4.08, P = 0.031; Fig. 5), in addition to a significant main effect of both drug (F1,11 = 18.65, P = 0.001) and delay (F2,22 = 8.132, P = 0.002). Similar to the effects seen in the HPC, RG-108 significantly impaired performance compared with the control condition (t11 = 2.78, P = 0.018). Furthermore, rats in this experimental condition did not remember significantly above chance (t11 = −1.21, P = 0.25), whereas in the control condition a significant one-sample t-test was noted (t11 = 3.96, P = 0.002).
The paired-samples t-test for the 20-min delay phase indicated no differences between the drug conditions (t11 = 0.18, P = 0.86), and one-sample t-tests confirmed no mnemonic impairment, as both the control condition (t11 = 2.58, P = 0.026) and the RG-108 condition (t11 = 3.48 P = 0.005) displayed significant differences from chance. Similarly, intact OiP memory was observed in a separate group of rats tested with a 2-h retention delay between sample and choice phases (Exp. S6; Fig. S6).
To ensure that the lack of impairment seen when the delay was reduced to 20 min was not a result of practice effects or other compensatory changes related to repeated drug exposure, we repeated the 24-h delay (24-h retest) following 20 min testing. Again, a significant paired-samples t-test was noted (t11 = 5.216, P < 0.001). Further chance analyses showed that the control condition demonstrated significant memory (t11 = 4.32, P = 0.001). The RG-108 condition also displayed a significant one-sample t-test (t11 = −3.15, P = 0.009); however, this was due to a familiarity preference, not a novelty preference, as is the usual standard to define rodent object memory. It is possible this is a result of a reduced level of total choice exploration (mean = 13.61, SEM = 1.56) in comparison with the control condition (mean = 17.75, SEM = 1.10; t11 = 2.30, P = 0.011).
The results from Experiment 5 demonstrate that DNMT activity is necessary in the PRh for successful long-term, but not short-term, OiP memory. Interestingly, however, whereas the RG-108 effects were replicated with a second non-selective DNMT inhibitor, 5-AZA, in the HPC (Exp. S2), within the PRh, 5-AZA had no effects (Exps S7a and S8b; Figs S7–S9). This may be related to the mechanistic differences between RG-108 and 5-AZA (see 4 and Supporting Information for further consideration).
Experiments 6–8 – DNMT1, but not DNMT3a or 3b, siRNA in the PRh impairs OiP memory
Experiment 6
Using naïve subjects (n = 12), Experiment 6 tested the effects of DNMT3a inhibition within the PRh on long-term OiP memory. Two-day pre-sample infusions of DNMT3a siRNA or non-targeting siRNA were administered, counter-balanced. Rats performed equally well on the long-term version (24-h delay) of the OiP task following infusions of DNMT3a siRNA or control siRNA (t11 = 0.79, P = 0.45; Fig. 6B). One-sample t-tests confirmed the lack of mnemonic impairment in the DNMT3a siRNA condition (t11 = 5.43, P < 0.001) and control condition (t11 = 3.46, P = 0.005). Because no LTM impairments were noted, no further delay testing was undertaken.
Experiment 7
Experiment 7 explored the effects of DNMT3b inhibition within the PRh on long-term OiP memory. Using a naïve set of rats (n = 12), DNMT3a or control siRNA infusions were administered, counter-balanced, 2 days prior to the sample phase. Rats performed equally well on the long-term (24-h delay) version of the OiP task following infusions of DNMT3b siRNA or control siRNA (t11 = 0.05, P = 0.96; Fig. 6C). One-sample t-tests confirmed the absence of mnemonic impairment in the DNMT3b siRNA condition (t11 = 2.98, P = 0.012) and control condition (t11 = 3.36, P = 0.006). No further delay testing occurred following null effects with the long-term retention delay.
Experiment 8
Experiment 8 investigated the effects of DNMT1 inhibition in the PRh on both short-term and long-term OiP memory. A new group of experimentally naïve rats (n = 12) was administered 2-day pre-sample infusions of DNMT1 siRNA or non-targeting siRNA, counter-balanced, and memory was tested using the 24-h delay, 20-min delay, 24-h delay cycle. A repeated-measures anova indicated a significant main effect of drug (F1,11 = 16.40, P = 0.002; Fig. 6A), but neither the main effect of delay nor the interaction was significant (F2,22 = 2.45, P = 0.11, and F2,22 = 1.83, P = 0.18, respectively). Given the delay-dependent effects of intra-PRh RG-108, planned comparisons assessed the effects of DNMT1 siRNA at each phase of delay testing.
A paired-samples t-test indicated a significant difference between drug conditions when a 24-h retention delay was employed (t11 = 4.03, P = 0.002). Additional one-sample t-tests revealed a mnemonic impairment in the DNMT1 siRNA condition (t11 = −0.89, P = 0.39), but not the control condition (t11 = 3.06, P = 0.011).
When the retention delay was reduced to 20 min no impairment was noted, as there was no difference between the drug conditions (t11 = 0.02, P = 0.97), and both the DNMT1 siRNA condition (t11 = 3.04, P = 0.011) and the control condition (t11 = 3.14, P = 0.009) displayed significant memory when compared with chance. A paired-samples t-test comparing the total sample exploration time between the control and experimental conditions was also significant (t11 = 3.89, P = 0.001); however, because both groups displayed average sample exploration within a reasonable range (> 40 s; Table S3), and no memory impairment was noted, this difference is not a major concern.
The 24-h delay retest results replicated the pattern of effects seen in the first 24-h delay phase; a paired-samples t-test indicated significant differences between drug conditions (t11 = 2.25, P = 0.046), and additional one-sample t-tests revealed a mnemonic impairment in the DNMT1 siRNA condition (t11 = −0.141, P = 0.890), but not the control condition (t11 = 3.399, P = 0.006).
Altogether, the results of Experiments 6–8 indicate that only maintenance DNMT1 is necessary within the PRh for successful long-term OiP memory.
In all, the behavioral/infusion experiments strongly suggest a functional double dissociation at the level of DNMT involvement in OiP memory; specifically, although DNMTs are necessary in both the HPC and the PRh, as demonstrated by the results with the non-selective DNMT inhibitor RG-108, only de novo DNMT3a seems to be required in the HPC, whereas maintenance DNMT1 is the only enzyme of the three necessary in the PRh. Subsequent experiments were run to investigate the potential for converging evidence at the level of learning-induced DNMT mRNA expression. Given the behavioral results just reported, we predicted that selective upregulations might be observed within the HPC (DNMT3a) and PRh (DNMT1).
mRNA
Experiment 9 – de novo DNMT3a and 3b, but not maintenance DNMT1, mRNA are upregulated in the dentate gyrus following object learning
The dentate gyrus of the HPC was excised from 28 experimentally naïve animals (14 control) following the molecular methods outlined above. The CA region (CA1, 2 and 3 together) of the HPC was excised from a subset of these same rats (n = 20; 10 control). Within the CA region, there were no learning-induced changes in the level of DNMT1 mRNA (t7 = 1.31, P = 0.23); however, one-sample t-tests indicated a significant decrease in the expression of both DNMT3a (t8 = −3.40, P = 0.009) and DNMT3b (t8 = −2.42, P = 0.042) mRNA levels following learning (Fig. 7). This latter finding is surprising, given that upregulation of DNMT3a and 3b mRNA within the CA1 region has been noted following contextual fear learning (Miller & Sweatt, 2007), as well as in the entire dorsal HPC following estradiol-coupled object learning (Zhao et al., 2010).
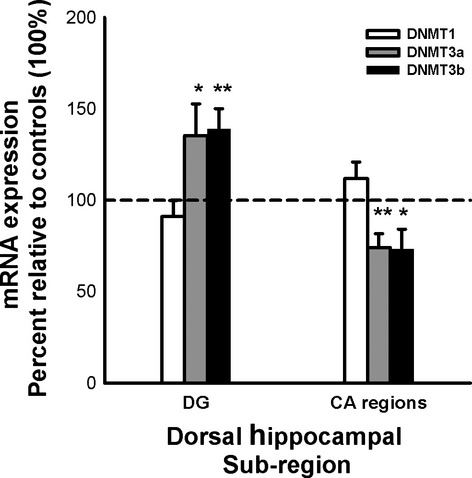
Conversely, in the dentate gyrus, one-sample t-tests demonstrated that both DNMT3a and DNMT3b mRNA were significantly upregulated post-learning (t11 = 2.04, P = 0.034, and t11 = 3.42, P = 0.006, respectively), whereas DNMT1 mRNA levels remained unchanged (t13 = −1.02, P = 0.33; Fig. 7). Our results indicate that OiP learning up-regulates only de novo DNMTs in the dentate gyrus, as predicted.
Experiment 10 – DNMT1, but not DNMT3a or 3b, mRNA is upregulated in the PRh following object learning
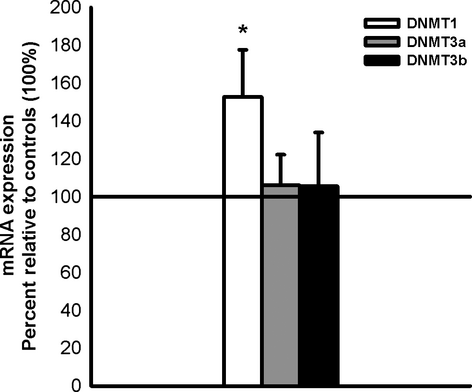
PRh was excised from 28 experimentally naïve animals (14 control) following the molecular methods outlined above. One-sample t-tests indicated that only maintenance DNMT1 mRNA was significantly upregulated post-learning (t11 = 2.11, P = 0.030), whereas both DNMT3a (t11 = 0.72, P = 0.37) and DNMT3b (t9 = 0.19, P = 0.85) mRNA levels remained unchanged (Fig. 8).
In a detailed analysis of the behavior exhibited by the experimental (viewed objects) and control (context-only) animals used in Experiments 9 and 10, no group differences were found on measures of grooming, locomotion (line crossings), number of fecal droppings, inactivity or the time spent in different areas of the apparatus (see Fig. S10A–C). Although the use of habituated context-only controls in this type of experiment is common (Miller & Sweatt, 2007; McNulty et al., 2012; Li et al., 2014), and has been used to determine mRNA changes following object learning specifically (McNulty et al., 2012), there does exist the possibility that without objects to explore, control animals might investigate the environment more, potentially enhancing hippocampal activity. As might be expected, the only differences noted in the present experiment were significantly higher wall/corner and floor exploration exhibited by the control group (see Fig. S10C); however, we do not believe this to be an indication of new learning or enhanced hippocampal activity. First, it should be stressed that all animals were habituated to the apparatus on two successive days prior to experimentation; therefore, the apparatus was familiar, drastically reducing or even eliminating new learning. Indeed, neuronal suppression following repetition and habituation (including contextual) has been demonstrated by several electrophysiological studies in various species (Desmedt & Jaffard, 1998; Kruse et al., 2004; Dong & Clayton, 2009; Glanzman, 2009), suggesting familiarization at the neuronal level. Additionally, our analysis demonstrated that exploration levels of the external environment did not differ between groups (see Fig. S10C). We defined external exploration as exploratory behavior when the rodent's nose was pointed upwards (i.e. nose > 90° angle to the floor) without looking at the floor, wall or objects, or in other words, exploration of the environment surrounding the apparatus. Although the testing environment (apparatus location, décor around room) was kept constant, changing sounds and smells might add novel components to the environment; however, both groups explored the external environment equally.
Collectively, the findings of Experiments 9 and 10 are consistent with the apparent double dissociation seen in the behavioral experiments; specifically, only de novo DNMT3a/b were up-regulated in the HPC (dentate gyrus) following OiP learning, whereas learning-induced increases in only maintenance DNMT1 were found in the PRh.
Discussion
The present study investigated the involvement of DNMTs within the HPC and PRh in OiP memory. We specifically chose the OiP paradigm as it requires both structures in order to process different aspects of this form of memory (Bussey et al., 1999, 2000; Winters et al., 2004; Barker & Warburton, 2011). Use of the OiP task thus enabled comparison across regions, potentially revealing dissociations resulting from functional differences, in the absence of confounding cross-task differences. Using intracranial, non-selective DNMT inhibitors, we demonstrated that DNMT activity is necessary in both the HPC and the PRh for long-term, but not short-term, OiP memory. The delay-dependent involvement of DNA methylation has been shown in numerous other studies (Miller & Sweatt, 2007; Miller et al., 2008, 2010; Han et al., 2010; Monsey et al., 2011; Oliveira et al., 2012; Sui et al., 2012), and the selective LTM effects we report are consistent with the epigenetic functions of the DNMTs, functions that would not be required for typical STM processes. Although DNMT involvement has been documented previously for HPC-dependent spatial and contextual memory (Miller & Sweatt, 2007; Miller et al., 2008, 2010; Feng et al., 2010; Zhao et al., 2010; Oliveira et al., 2012), this is the first account of a role for DNMTs in PRh-mediated object memory.
To further probe the specific involvement of each DNMT we used an siRNA-mediated knock-down technique. Unlike transgenic models or viral knock-out methods, transient mRNA knock-down by acute administration of siRNAs allowed the use of a within-subjects design, in addition to the ability to target particular mnemonic phases (i.e. consolidation) within our task. Accell siRNA was specifically chosen as it has been shown to successfully incorporate into neurons without the use of a transfection agent (Nakajima et al., 2012). Using this approach we have uncovered a novel dissociation – only DNMT3a was necessary in the HPC for OiP memory, whereas the PRh required only DNMT1. Because of the within-subjects design used for these experiments, the successful performance of animals in all control conditions (Exps 5–7, 9–11) and the null effects noted with short-term memory delays (Exps 5, 11) strongly suggest that the results reported here are not due to carry-over or off-target effects. Moreover, because of the need for pre-sample administration of the siRNAs, it could be suggested that the apparent LTM impairments are the result of disrupted memory acquisition rather than consolidation; however, given the lack of STM impairment, this is unlikely to be the case, as successful memory cannot be preceded by an inadequate learning phase. Furthermore, the consistent absence of significant differences in sample exploration between drug conditions provides additional evidence for the lack of non-specific effects of the siRNAs on memory acquisition or motivation to explore (see Tables S3 and S4). Importantly, we have also demonstrated the efficacy and selectivity of the DNMT siRNAs, as each DNMT siRNA induced a significant knock-down of only the targeted transcript, 2 days following administration (Exp. S4).
qPCR analyses revealed findings consistent with the infusion experiments, as mRNA levels of the de novo DNMTs were selectively upregulated in the dentate gyrus of the HPC post-learning, whereas only the maintenance DNMT1 mRNA was upregulated in the PRh. This pattern of data strongly suggests that DNMT3a and DNMT1 are essential for long-term OiP memory processing in the dentate gyrus and PRh, respectively, and that treatment with RG-108 or the selective siRNAs blocked LTM by inhibiting the actions of these enzymes and/or preventing their learning-induced upregulation.
The present results, together with past findings regarding the nature of perirhinal and hippocampal contributions to long-term object memory processing, strongly imply a functional double dissociation at the level of epigenetic mechanisms mediating HPC-dependent contextual memory and PRh-dependent memory for object identity (Bussey & Aggleton, 2002; Winters et al., 2004; Murray et al., 2007; Jo & Lee, 2010). Not only is this the first demonstration of behavioral consequences following intracranial Accell siRNA administration, but it is the first systematic investigation of specific DNMT inhibition in the domain of learning and memory, and the first demonstrated functional dissociation of its kind.
DNMT3a has previously been associated with HPC memory consolidation. Oliveira et al. (2012) demonstrated that aging-associated cognitive decline in mice was coupled with a decrease in DNMT3a mRNA levels in the dorsal HPC, and rescuing DNMT3a levels ameliorated these cognitive deficits. Additionally, increases in both DNMT3a and 3b, but not DNMT1, mRNA in the dorsal HPC have been shown following 17-β-estradiol-induced enhancements in object recognition (Zhao et al., 2010), as well as contextual fear conditioning (Miller & Sweatt, 2007). Interestingly, Miller & Sweatt (2007) reported increases in DNMT3a and 3b specifically within the CA1 region, whereas in the present study similar increases were noted in the dentate gyrus, while decreases of DNMT3a and 3b were found in CA1–3 combined. Our results suggest the necessity of a fine balance of DNMT expression within subregions of the HPC to support successful memory consolidation in the OiP task. Future studies might seek to determine subregion-specific changes in this paradigm, as well as potential changes within specific neuronal populations (e.g. granule cells). The discrepancy between the current results and those of Miller & Sweatt (2007) might be due to inherent differences between learning paradigms (contextual fear conditioning and OiP); clearly, additional research is merited to assess this potentially interesting dissociation. Regardless, what is consistent between the current results and the few other studies that have examined learning-induced DNMT expression is the modulation of only the de novo DNMTs within the HPC (Miller & Sweatt, 2007; Zhao et al., 2012). Taken together with our results, this suggests that de novo DNMTs, specifically DNMT3a, are necessary within the HPC for consolidation of spatial or contextual memory. Conversely, our study is the first to show a necessity for DNMT1 in any learning paradigm, and its involvement in PRh-mediated long-term OiP memory is consistent with the established role for this brain region in long-term object memory functions (Winters et al., 2008; Brown et al., 2012). What might drive the functional dissociation we report at the epigenetic level? We hypothesize that it could be related to the neurogenic status of each region.
Adult neurogenesis has reliably been demonstrated in two brain regions, the subventricular zone of the lateral ventricles, which provides new neurons to the adult olfactory bulb, and the subgranular zone of the dentate gyrus (Deng et al., 2010). Extensive research has implicated adult HPC neurogenesis in certain forms of learning and memory (Deng et al., 2010); learning or activity (e.g. exercise) influences HPC neurogenesis (Shors et al., 2001; Kempermann & Gage, 2002; Dupret et al., 2007; Epp et al., 2007), and blocking HPC neurogenesis can either inhibit memory consolidation (Jessberger et al., 2009) or prevent HPC memories from becoming reliant on cortical structures (Kitamura et al., 2009). Given the role of DNMT3a and DNMT3b as de novo methyltransferases, these enzymes have the potential to methylate DNA within adult-born neurons in an activity-induced manner. Indeed, there is support for the involvement of these enzymes during developmental neurogenesis. For example, juvenile DNMT3a null mice display decreased levels of neurogenesis in both the subgranular and the subventricular zones, and this appears to be the result of a decreased ability to stop further differentiation into glial cells (Wu et al., 2010, 2012). Moreover, the DNMTs appear to play different roles during olfactory development; specifically, DNMT1 is expressed at all post-stem cell stages, DNMT3b is expressed during proliferation of neurogenic progenitors, and DNMT3a is expressed in post-mitotic neurons during the transition phase from immature to mature and synaptically stable olfactory receptor neurons (MacDonald et al., 2005; Colquitt et al., 2014). MacDonald et al. (2005) postulate that de novo methyltransferases sequentially modulate the epigenetic silencing of olfactory receptors, promoting a distinctive ‘phenotype’ throughout the life of the neuron. Preliminary evidence for this hypothesis has been recently demonstrated. Colquitt et al. (2014) found that although DNMT3a knockout mice were completely void of the DNMT3a enzyme, overall levels of 5-methylcytosine and 5-hydroxymethylcytosine in mature olfactory receptor neurons were largely unchanged in comparison with wild-type mice; however, alterations were noted specifically on ‘accessible’ genes, such as immediate early genes. Furthermore, the only transcriptional perturbations evident were of these accessible or activity-regulated genes, but only following odorant stimulation, suggesting a role for DNMT3a in the plasticity of maturing olfactory receptor neurons (Colquitt et al., 2014). A similar function for de novo methyltransferases might apply in the adult dentate gyrus in response to environmental stimulation. For example, a learning episode might trigger DNMT3a and DNMT3b to aid in neuronal differentiation, neuronal survival and/or priming of the neuron for future responding (i.e. synaptic metaplasticity; Baker-Andresen et al., 2013).
Unlike the HPC, the PRh has not been shown to engage in adult neurogenesis. This may explain the lack of involvement of DNMT3a and 3b in PRh in our experiments, but what function might DNMT1 be playing? As a maintenance methyltransferase, DNMT1 is involved in the methylation of the daughter strand following DNA replication during cell division (Bird, 2002), in addition to re-methylating following DNA repair processes such as base excision repair (BER; Mortusewicz et al., 2005; Lee et al., 2012). Recent literature has suggested a key role for the BER process in mediating active DNA demethylation in the post-mitotic neurons of the adult hippocampus in response to learning (Kaas et al., 2013; Rudenko et al., 2013). More specifically, following hydroxylation and several stages of oxidation or deamination, a modified 5-methylcytosine is excised and replaced with a cytosine base through the BER pathway (Bhutani et al., 2011; Kaas et al., 2013; Rudenko et al., 2013) in a strand-specific manner (Guo et al., 2011). Because DNMT1 has been shown to re-methylate following DNA damage-induced BER, it might also re-methylate previously demethylated cytosine bases following stimulation from environmental input at a later time. Indeed, it has also been suggested that Tet3, one of the ten-eleven translocase proteins implicated in active DNA demethylation, might preserve hypomethylated states by actively preventing DNMT1-mediated re-methyation (Li et al., 2013). Thus, DNMT1 might be poised for activity-induced re-methylation. Interestingly, there appear to be cases in which DNMT1 exhibits resistance to 5-AZA, and one of these is thought to be DNA repair (Momparler, 2005). This might explain why we, and others (Munoz et al., 2010; Scott et al., 2013), have failed to find a mnemonic impairment following administration of 5-AZA into the PRh. Unlike RG-108, which is a non-nucleoside DNMT inhibitor, 5-AZA is a cytidine analog forming an adduct with DNMT enzymes bound to the DNA (Gros et al., 2012). Due to reports of instability and toxicity, the use of cytidine analogs has decreased (Gros et al., 2012), and here we report another instance where the use of 5-AZA might be inappropriate.
HPC neurogenesis is involved in many forms of HPC-dependent memory, and thus neurogenesis could enable very similar learning experiences (such as the presence of identical objects in slightly different contextual locations) to be orthogonally represented and thus distinct and resistant to interference (Sadeh et al., 2014). In contrast, neocortex represents similar stimuli using overlapping representations, apparently independently of significant adult neurogenesis, and this probably applies to the representation of specific object features in the PRh (Sadeh et al., 2014). Thus, our findings are consistent with an interpretation that implicates de novo methylation of genes in newly born adult neurons required for the complexities of HPC-dependent contextual memory processing; indeed, in the present study, OiP learning caused upregulations of DNMT3a and 3b specifically in the dentate gyrus, the site of adult HPC neurogenesis. Conversely, the nature of object identity encoding and representation within the PRh apparently requires a different epigenetic mechanism. Because a given PRh neuron may be more likely to contribute to the specific representations of multiple explicit object memories throughout its lifespan, a continual process of demethylation and re-methylation might be critical to support PRh-mediated learning and memory. We suggest that DNMT1 in the PRh plays an important role in the re-methylation of memory-related genes for this process. Future work will be required to investigate OiP learning-induced changes in methylation status of memory-related genes in the HPC/dentate gyrus and PRh to enable further specification of the mechanisms at work in the present results.
This is the first study to demonstrate involvement of DNMTs in PRh-mediated object memory and the first to reveal a functional epigenetic double dissociation in the adult brain; DNMT3a is selectively required in the HPC/dentate gyrus for successful long-term OiP memory, whereas only DNMT1 is necessary in PRh. These findings indicate that epigenetic mechanisms of LTM may not be as generalizable as implied by previous research. Indeed, epigenetic requirements for memory formation and storage may differ in relation to the specific types of information processing taxed by a given task and the brain region(s) involved, as implied here for HPC-dependent spatial memory and PRh-dependent memory for object identity The central aim of the present study was to assess the possibility of dissociable roles for the various DNMTs in LTM. Clearly, a critical next step will be to evaluate OiP learning-induced changes in the DNA methylation status of various memory-related genes (Miller & Sweatt, 2007; Miller et al., 2010), as well as the effects of selective siRNA administration on such modifications. Nonetheless, the current mRNA analyses confirm that behavior – in this case, OiP learning – which is sensitive to DNMT inhibition also upregulates DNMT enzymes necessary for the methylation process. Our data are consistent with the interpretation that DNA methylation has been co-opted to subserve learning and memory in a much more intricate fashion than originally proposed. The OiP task relies on at least two different types of information processing (spatial and object identity), which are thought to be performed by distinct brain regions (the HPC and PRh, respectively) in an interactive manner (Bussey & Aggleton, 2002; Winters et al., 2004; Jo & Lee, 2010; Barker & Warburton, 2011). The present findings strongly suggest that these putative specialized information processing capacities are regulated by distinct epigenetic mechanisms requiring different DNMT enzymes. Additional research will be required to investigate this notion directly using learning and memory tasks that rely selectively on the HPC or PRh.
Conflict of interests
The authors declare no competing financial interests.
Acknowledgements
This research was supported by a National Sciences and Engineering Research Council (NSERC) of Canada Discovery Grant to B.D.W., an NSERC grant to B.E.K. and both an NSERC and Ontario Graduate Scholarship to K.A.M. Additional thanks to Daniel Bailey and Daniel Palmer for graphical and programming support, Amina Al-lzzi for video scoring, and Craig Bailey for help with the fluorescence detection.
Abbreviations
-
- A/P
-
- anterior posterior
-
- BER
-
- base excision repair
-
- DNMT
-
- DNA methyltransferase
-
- DR
-
- discrimination ratio
-
- HPC
-
- hippocampus
-
- HPRT
-
- hypoxanthine-guanine phosphoribosyltransferase
-
- LTM
-
- long-term memory
-
- OiP
-
- object-in-place
-
- PBS
-
- phosphate-buffered saline
-
- PRh
-
- perirhinal cortex
-
- siRNA
-
- small interfering RNA




