Interests shape how adolescents pay attention: the interaction of motivation and top-down attentional processes in biasing sensory activations to anticipated events
Abstract
The voluntary allocation of attention to environmental inputs is a crucial mechanism of healthy cognitive functioning, and is probably influenced by an observer's level of interest in a stimulus. For example, an individual who is passionate about soccer but bored by botany will obviously be more attentive at a soccer match than an orchid show. The influence of monetary rewards on attention has been examined, but the impact of more common motivating factors (i.e. the level of interest in the materials under observation) remains unclear, especially during development. Here, stimulus sets were designed based on survey measures of the level of interest of adolescent participants in several item classes. High-density electroencephalography was recorded during a cued spatial attention task in which stimuli of high or low interest were presented in separate blocks. The motivational impact on performance of a spatial attention task was assessed, along with event-related potential measures of anticipatory top-down attention. As predicted, performance was improved for the spatial target detection of high interest items. Further, the impact of motivation was observed in parieto-occipital processes associated with anticipatory top-down spatial attention. The anticipatory activity over these regions was also increased for high vs. low interest stimuli, irrespective of the direction of spatial attention. The results also showed stronger anticipatory attentional and motivational modulations over the right vs. left parieto-occipital cortex. These data suggest that motivation enhances top-down attentional processes, and can independently shape activations in sensory regions in anticipation of events. They also suggest that attentional functions across hemispheres may not fully mature until late adolescence.
Introduction
In a complex sensory environment, top-down selective attention mechanisms operate to enhance the processing of relevant information and to suppress the processing of irrelevant and potentially distracting inputs (Broadbent, 1957; Kastner & Ungerleider, 2000; Foxe et al., 2005; Foxe & Snyder, 2011). The effectiveness with which these attentional processes are deployed is undoubtedly impacted by the motivational content of the information to be acted upon. For example, functional magnetic resonance imaging (fMRI) studies using monetary incentives have shown that rewards enhance activations in regions of the attentional network (Small et al., 2005; Engelmann et al., 2009; Smith et al., 2011). Of course, many of the motivating factors in daily life are non-monetary in nature, such as a person's level of interest in the subject matter, and these will also have a significant and lasting impact on attentional processes. In educational research, for example, the level of interest in the subject matter has long been regarded as a motivating factor that promotes attention and learning processes during development (Hidi, 1990), but the neural bases of these processes have not yet been extensively studied. The current study aimed to investigate how motivation impacts the neural mechanisms of top-down attentional biasing in typically developing (TD) adolescents. We manipulated motivation by varying the ‘level of interest’ that a given participant expressed in the stimulus materials to be acted upon.
A common approach to the study of top-down spatial attention is to use covert cued attention tasks, in which an informational cue serves to direct attention toward a particular spatial location, stimulus feature or sensory modality (Posner et al., 1980; Snyder & Foxe, 2010). In turn, the enhanced amplitude of sensory evoked potentials to the cued stimulus or feature is a well-established neurophysiological marker of the influence of biased attention on post-stimulus sensory processing (Mangun & Hillyard, 1988; Eimer, 1996; Hillyard & Anllo-Vento, 1998; Foxe & Simpson, 2005; Frey et al., 2010). In these cued designs, top-down processes are deployed to shape neural receptiveness in sensory regions in anticipation of the to-be-acted-upon stimulus (Foxe et al., 1998; Kastner et al., 1999; Worden et al., 2000; Talsma et al., 2005; Banerjee et al., 2011). Anticipatory spatial attention has been shown to enhance a family of broadband event-related potential (ERP) components contralateral to the to-be-attended location, including the so-called anterior directing attentional negativity (ADAN) (Nobre et al., 2000; Kennett et al., 2007), early directing attention negativity (EDAN), late directing attentional positivity (LDAP) (Harter et al., 1989), and biasing-related negativity (BRN) (Grent-'t-Jong & Woldorff, 2007). These components are mainly observed over frontal and parieto-occipital regions, and are thought to reflect both early and late phases of the anticipatory biasing of cortices associated with attended stimulus properties (Dale et al., 2008). Additionally, oscillatory alpha-band (~8–14 Hz) power increases have been observed over the parieto-occipital cortex contralateral to a to-be-ignored location, and are taken to reflect the anticipatory suppression of retinotopically organised representations of sensory space (Worden et al., 2000; Kelly et al., 2006; Thut et al., 2006; Rihs et al., 2007; Banerjee et al., 2011; Belyusar et al., 2013). Together, alpha-band and broadband ERP responses provide insight into the temporal dynamics of anticipatory top-down attentional control at the millisecond level. In turn, the effectiveness with which these anticipatory mechanisms are deployed is associated with the selective modulations that are subsequently observed during post-stimulus sensory-perceptual processing (Thut et al., 2006; Kelly et al., 2009).
Although there is evidence that top-down spatial attentional capacities improve during development (Smith & Chatterjee, 2008), the neural bases of these processes have not been studied nearly as extensively in TD children as in adults. In an early study of the neural bases of anticipatory spatial attention in children, ERPs following a spatial cue and in anticipation of target stimuli were examined in 6- to 9-year-olds (Harter et al., 1989). As compared with a following study with adults (Harter & Anllo-Vento, 1991), the electrophysiological data in children showed patterns over posterior scalp regions that were generally consistent with adults, with some differences in the frontal components. However, the behavioral data in children showed unexpected superior performance for stimuli presented to the right hemifield, a pattern that was not seen in the adult study. Also, eye-tracking was not performed in these early attention studies, introducing the possibility that participants might have engaged in systematic eye movements toward target locations, an issue that is likely to be especially acute in young children. In addition, there were significant differences in the experimental paradigms between these two studies, complicating the interpretation of possible developmental effects. Therefore, these findings will bear replication.
The impact of motivational signals on prefrontal regions in the attentional control network as well as on earlier sensory processing regions has been amply demonstrated in human neuroimaging work. For example, fMRI was used during a cued spatial attention task to examine the influence of monetary incentives on attention-related brain activations in adults with modulations observed in key prefrontal areas of the attention network in addition to limbic regions (Small et al., 2005). Further, there were greater top-down attentional modulations in the visual cortices during incentive conditions compared with a non-incentive condition. In support of this, other fMRI work showed that the learned monetary value of a visual stimulus directly influenced its neural representation throughout the visual hierarchy, including early visual regions (Serences, 2008).
However, motivational factors appear to have different effects on top-down attention during adolescence as compared with adulthood. In a developmental study using fMRI, the influence of monetary reward on sustained attentional processes was mapped from early adolescence through adulthood (Smith et al., 2011). Participants performed a continuous performance task in which serial streams of letters were presented, and they were informed that responses to a target letter would be rewarded. A feedback bar increased with hit rate, indicating increases in winnings. Adolescents showed faster reaction times for rewarded vs. non-rewarded targets, an effect not observed in adults. With increasing age, decreases in activity in the inferior temporal cortex and posterior cingulate (regions associated with reward and saliency processing) were observed. Additionally, there were linear age-related increases in the activation of brain areas involved in sustaining attention. Thus, developmental changes during adolescence appear to impact the relative roles of reward and attentional systems on performance. It has been proposed that, during adolescence, an earlier maturation of limbic regions as compared with prefrontal areas (necessary for cognitive control) may be a basis for greater risk-taking behaviors (Casey, 2008). Later phases of adolescence have been associated with greater efficiency in cognitive control, due to maturation of prefrontal brain regions (Yurgelun-Todd, 1997; Rubia et al., 2000). By this reasoning, one might expect motivation to have greater impact on attentional processes in adolescents relative to adults.
Despite the use of monetary rewards in research on motivation, there is evidence that these may not be the most appropriate incentive for studies in children. For example, in a study examining the efficacy of a wage-payment model as an incentive for children to participate in research, children below 9 years of age showed a distinct inability to comprehend the role and value of money. A neuroimaging study examining neural sensitivity to absolute and relative anticipated reward in 12- to 15-year-olds vs. adults showed that activity in the ventral striatal regions of the reward network in anticipation of these types of monetary rewards increased as a function of age (Vaidya et al., 2013). The level of interest in items or events may serve as a more effective motivational factor for children and adolescents (Hidi, 1990). The current study therefore examined the neural mechanisms of top-down spatial attention in TD adolescents, investigating the role of motivation in modulating these mechanisms by manipulating the degree of interest that a given participant expressed in the stimulus materials under scrutiny. High and low interest stimulus classes were designated through the pre-experimental assessment of the interests of a given participant. High-density electroencephalography (EEG) was recorded during a blocked cued spatial attention task in which adolescents anticipated either high or low interest stimuli. Both broadband and oscillatory EEG measures of anticipatory spatial attention were assessed in the context of these manipulations of motivation.
Materials and methods
Participants
Data from 24 TD male adolescents between the ages of 12 and 15 years were included in this study from an initial cohort of 29 recruited participants. Two of the excluded participants had very poor signal-to-noise ratios in their EEG data and three additional participants showed an inadequate distinction in their level of interest between high and low interest items on the pre-screening interests survey (see 2.2 section below). Hence, 24 participants remained in the sample (mean age, 15.07 years; SD, 0.96). An all male population was recruited because this study was intended as foundational work for planned studies of attentional mechanisms in children with an autism spectrum disorder (ASD), a developmental disorder that is substantially more prevalent in males (Baio, 2014). Participants were recruited through the Human Clinical Phenotyping Core of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center at the Albert Einstein College of Medicine and through flyers posted in the Bronx community nearby. Children provided written assent, with their parent or guardian giving written informed consent. Head trauma, seizures, attention deficit disorder, psychiatric, learning, or developmental disorders, or having a first-degree relative with a developmental disorder constituted exclusionary criteria, which were assessed through a screening survey completed by parents. Children were administered the Wechsler Abbreviated Scales of Intelligence, and children with a full-scale IQ below 85 were excluded. All participants had normal or corrected-to-normal vision and normal hearing. A modest fee was provided for participation ($12/h). All materials and procedures were approved by the institutional review boards of The Albert Einstein College of Medicine and The City College of the City University of New York. Ethical guidelines were in accordance with the tenets of the Declaration of Helsinki.
Survey measures
Prior to performing the main computerised task while EEG was being recorded, participants completed a survey measuring their level of interest in item classes. This was in order to obtain a more quantitative measure of the interests of children in the study, as previous work on interests and attention in children used common stimulus sets that were not based on the interest levels of individual participants in the sample (Sasson et al., 2008). The survey in the current study was constructed from 110 items, of which the first 106 items asked which item in a pair of items the participant preferred. The items from the first 110 questions in the survey are shown in Table 1. Items 1–5 were selected as high interest items for the TD sample based on parental anecdotes of the interests of 39 TD boys (aged 12–15 years) who expressed initial interest in completing the pre-study ‘interests’ survey. Items 6–10 were selected through the review of restricted interests reported in existing Autism Diagnostic Observation Schedule and Autism Diagnostic Interview data for 60 potential participants with ASD (aged 11–16 years) in the laboratory database. Items 11–15 were included in the survey as potential low interest items for both TD and ASD children, as these items were not stated as interests by parents of the TD children or in the reviewed ASD clinical diagnostic measures.
| (1) Sports | (6) Anime | (11) Flowers |
| (2) Video-games | (7) Maps | (12) Furniture |
| (3) Cartoons | (8) History | (13) Buildings |
| (4) Movies | (9) Trains | (14) Clothing |
| (5) Dinosaurs | (10) Numbers | (15) Mammals |
To obtain the interest scores for each item, the number of selections of each item as ‘preferred’ was summed to result in an interest score for each surveyed item. The sample of TD children in the current study showed higher interest in video-games and sports than in anime and flowers, for example. On an individual participant basis, interest scores were verified to ensure that the scores for high interest items were at least double those of low interest items. As above, three children were not included in the sample due to an inadequate distinction in the level of interest between high and low interest items.
The last four questions in the interests survey asked children to detail the specific items that they liked most, to list the things that they liked that did not appear on the survey, how much it bothered them when they were interrupted while doing things that they liked (a lot, a little, not at all), and if they had family members or friends who shared their interests. These items were included to obtain a more specific account of the interests of each child, and to ensure that the participants did not exhibit an atypical pattern of restricted interests. In addition to the interests survey, all participants completed the Subthreshold Autism Trait Questionnaire to rule out autism traits in the current TD sample (Kanne et al., 2012). The average Subthreshold Autism Trait Questionnaire quotient in the current TD sample was 19.63 (SD, 6.46), similar to the values of TD children in the existing literature (Kanne et al., 2012).
The parents of participants completed the Yale Special Interests Survey on the development of their child's interests (A. Klin and F.R. Volkmar, unpublished data), as described in Klin et al. (2007). In the instructions for the survey, the difference between a special interest and an ordinary hobby was described, and parents were questioned as to whether their child expressed a special interest during pre-school, elementary school, and adolescent phases of development. If they did observe a special interest during a phase of development, they were asked several questions about the intensity of the interest and whether it hindered their child's social interactions. The results of this survey allowed us to determine whether any TD children showed atypical developmental patterns related to their interests that might exclude them from the study. The advantage of this measure was to ensure that the TD group did not exhibit any patterns of restricted and circumscribed interests that are often observed in individuals with ASDs (Szatmari et al., 1989; Boyd et al., 2007).
Stimulus apparatus and procedure
The participants were seated in a darkened, double-walled, electrically shielded, soundproof booth (Controlled Acoustical Environments, Industrial Acoustics Company, Bronx, NY, USA) and fixated on a point displayed continuously on the center of an LCD computer monitor placed 80 cm in front of them (Viewsonic VP2655wp, 55 × 65 cm). Stimuli were delivered using Presentation software (Neurobehavioral Systems, Albany, CA, USA). The gaze direction was monitored using an Eyelink 1000 infra-red eye-tracking camera (SR Research Ltd, Ottawa, Ontario) to ensure that participants maintained central fixation. The participants placed their chin on a chinrest to stabilise their head, as head stability was advantageous for stable eye tracking recording. Horizontal electro-oculogram data were also analysed to ensure that there were no effects of systematic eye movements.
Figure 1A shows the sequence of events in each trial. During a trial, a fixation cross was present in the center of the screen (1.4° visual angle; RGB: 255, 255, 255) on which participants were required to fix their gaze. The background of the screen was black (RGB: 0, 0, 0). Each trial started with a gray arrow cue (1.6° visual angle; RGB: 127, 127, 127) presented at central fixation, which pointed leftward or rightward for 600 ms, and participants were instructed to covertly pay attention to the side of the display toward which the arrow pointed without moving their eyes. The cue was followed by a 1000 ms cue–target interval, after which a visual stimulus appeared to the left or right of fixation for 100 ms (6.2° visual angle; average RGB: 120, 120, 120, centered at 7° from fixation). After a 20 ms delay, a mask consisting of scrambled, unidentifiable versions of a non-target image (chosen from all stimulus categories) appeared at both left and right stimulus locations for 100 ms. The images for masks were scrambled by generating a random phase structure of the same size as the image, and adding it to the original phase of the image. Masks were used to increase the task difficulty. Following the mask, a 1000 ms fixed inter-trial interval occurred before the next trial, during which only the fixation cross was present on the screen. Participants pressed a response button as quickly as possible if they detected the target picture at the attended location. See Supporting Information Table S1 for a breakdown of stimulus probabilities for all conditions, and Supporting Information Fig. S1 for the stimulus probabilities at the cued and uncued locations. Unilateral left, unilateral right, and bilateral stimuli occurred equiprobably (33.3%). Twenty percent of these stimuli were targets. The stimulus probabilities were developed based on existing literature, which indicates that endogenous attentional mechanisms are more fully engaged when there is greater probability that a stimulus will be presented at the cued location (Posner et al., 1980; Vossel et al., 2006). Therefore, three times more unilateral stimuli were presented in the attended vs. unattended conditions. An equal number of bilateral stimuli were administered across the attended and unattended trials. Due to the difference in probability of unilateral stimuli across the attended and unattended trials, special steps were taken to analyse visual evoked potentials after the cue–target interval, which are described in the 2.4 section below.
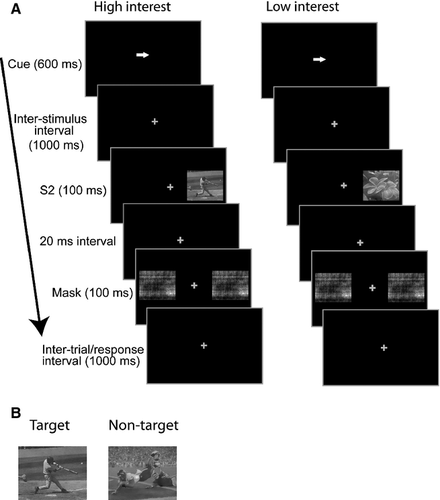
The stimulus classes used in the experiment were based on the aforementioned ‘interests’ survey results, and included video-games, sports, anime, and flowers. Each stimulus set was administered in a separate block. At the start of each block, instructions appeared with a picture of the target image. All target and non-target images were grayscale. The target was a particular image in each stimulus class (e.g. a man kicking a soccer ball in the sports block, see Fig. 1B for sample target and non-target images used in sports blocks). The luminance of the stimuli was equated using the SHINE toolbox for matlab (Willenbockel et al., 2010). Four blocks of 100 trials were administered for each stimulus class. The block duration was approximately 4.7 min (plus breaks). Targets were automatically changed in a subsequent block for a particular stimulus class when performance was greater than 70% in order to increase the task difficulty. Thus, as participants became practiced in the detection of a particular target, targets were changed to avoid ceiling effects in behavioral performance. For each stimulus class, only one target image was used per block, and there were five total possible target images in each stimulus class for the full experiment. Also, for each stimulus class, there were 15 non-targets that differed from the targets, and non-targets were randomly selected from this set on a trial-by-trial basis. There was no overlap in the image sets used for targets and non-targets to control for any potential exogenous orienting to non-target images that might have been assigned as a target in a previous block. In practice blocks prior to the experimental blocks, participants were permitted to move their eyes to look at stimuli. This was in order to acquaint participants with the local features of the stimuli, as they were presented in the periphery and were difficult to distinguish without one block of practice per stimulus class.
Electroencephalography recording and analysis
Continuous EEG was acquired through the BioSemi ActiveTwo electrode system from 168 Ag-Cl electrodes, digitised at 512 Hz. With the Biosemi system, every electrode or combination of electrodes can be assigned as a reference, which is performed purely in the software after acquisition. The Biosemi system replaces the ground electrodes that are used in conventional systems with two separate electrodes, i.e. the common mode sense and driven right leg passive electrode. These two electrodes create a feedback loop, thus rendering them as references. For more information on the Biosemi system conventions, please visit the website (http://www.biosemi.com/). The EEG data were processed using the Field-Trip toolbox (Donders Institute for Brain, Cognition, and Behavior, Radboud University Nijmegen, Nijmegen, The Netherlands; (http://fieldtrip.fcdonders.nl/) for matlab (MathWorks).
The raw data were re-referenced to the average reference for analysis after acquisition. For the anticipatory ERP analysis, a low-pass filter of 45 Hz and a high-pass filter of 0.5 Hz were applied to the continuous data (fourth-order digital Butterworth, zero-phase) to allow for the examination of slow and sustained anticipatory components (e.g. BRN). The data were epoched (400 ms before to 1300 ms after the cue onset for anticipatory responses, and 200 ms before to 600 ms after the subsequent potential target stimuli) and then averaged offline. There was an average of 258 trials per condition for the anticipatory analyses. For the post-stimulus ERP analysis, a low-pass filter of 45 Hz and a high-pass filter of 1.15 Hz were used, with the goal of examining visual evoked potential components (e.g. C1, P1, N1 and P3). For all analyses, trials with eye blinks and trials during which gaze was greater than 2° from central fixation were rejected on the basis of eye-tracking data.
For both anticipatory and post-stimulus analyses, channels with voltage values >120 μV were interpolated by the use of linear, distance-weighted interpolation. Trials in which four or more electrodes had voltage values >120 μV were rejected. The average number of trials per condition for the post-stimulus ERP analyses is discussed below. For both ERP analyses, the baseline was defined as the mean voltage over the 100 ms period preceding the onset of the cue (S1) or the potential target stimulus (S2).
The temporal spectral evolution method was used to focus analysis on alpha-band activity during the anticipatory period (Vanni et al., 1997; Foxe et al., 1998). To derive temporal spectral evolution waveforms, the data were first bandpass filtered between 8 and 14 Hz (fourth-order digital Butterworth, zero-phase). The instantaneous amplitude of the complex-valued analytic signal was then derived by the Hilbert transform (Le Van Quyen et al., 2001). The frequency and temporal resolution were determined wholly by the filtering step and were not altered by the Hilbert transformation. This procedure resulted in all positive-valued data. Because the average change of alpha-band power after the presentation of the cue was of interest, the data were rebaselined before the trials were averaged. Thus, any change from zero (baseline) would indicate a change in alpha power. The use of a narrow bandpass filter introduced an artifact near the edge of the epoch, so the baseline window for this procedure was reset to 100–0 ms to avoid including the edge artifact in the baseline calculation. Thus, voltage values in the epoch represented an absolute change in alpha-band power from baseline levels. Data were ultimately grand-averaged across subjects for the purposes of illustration.
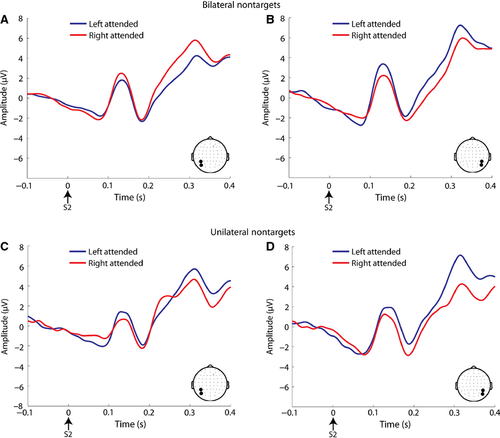
Two different analyses were performed to examine post-stimulus visual evoked potentials. As mentioned in the 2.3 section above, there was a greater number of unilateral stimuli presented in the attended vs. unattended conditions. However, the numbers of bilateral stimuli were equal across these conditions. To account for these differences in stimulus probabilities, the following measures were taken. In a first analysis, only bilateral non-target stimuli were analysed for the left attended and right attended conditions (average of 85 trials per condition: high interest attend left, high interest attend right, low interest attend left and low interest attend right). In a second analysis, only unilateral non-targets were assessed. Due to the greater number of unilateral stimulus trials in attended conditions, the number of trials in the attended conditions was equated with the number of trials in the unattended conditions. To do this, the number of trials in the unattended condition was calculated, and the same number of trials was randomly selected from the attended condition for each subject. There was an average of 67 trials in each condition for the unilateral analysis (left attended, left unattended, right attended and right unattended). The number of trials was not considered sufficient to examine high and low interest conditions separately for the unilateral stimuli.
Statistical analyses
Behavioral data
Repeated-measures anovas were used to examine the sensitivity index (d-prime) and reaction time. Based on the signal detection theory (Green & Swets, 1964), d-prime combines the information from successful target detections (hits), incorrect classifications of non-targets as targets (false alarms), and misses and correct rejections of targets. The factors used in the anova were target location (left, right) and motivation (high or low interest). As typically performed in an endogenous attention paradigm, participants were instructed to only respond to targets at the cued location. For the reaction time to targets, only trials with responses between 200 and 1000 ms were included in order to reduce the effect of outliers or anticipatory responses.
Anticipatory event-related potential data
To test for broadband ERP attention effects during the anticipatory period (i.e. LDAP, ADAN, and BRN), repeated-measures anovas with the factors of cue direction (left, right), motivation (high vs. low), and region of interest (ROI) (left or right hemiscalp) were used. See Table 2 for a description of the attention-related anticipatory components tested, and an outline of the time periods and ROIs used for these analyses. The mean amplitude was computed across these time windows and ROIs. The ROIs and time windows were chosen based on the extensive existing literature detailing these components (Harter et al., 1989; Nobre et al., 2000; Dale et al., 2008; Kelly et al., 2010; Murray et al., 2011).
| Component | Description | Time (post-cue) (ms) | ROI |
|---|---|---|---|
| ADAN | Frontal contralateral negativity | 380–420 | AF7, F5, AF8, F6 |
| LDAP | Posterior contralateral positivity | 600–660 | PO3, PO4 |
| BRN | Posterior sustained contralateral negativity | 1500–1600 | P7, P9, P8, P10 |
| IFN | IFN | 1200–1400 | AF7, F5, AF8, F6 |
Exploratory analyses: motivation effects during the anticipatory period
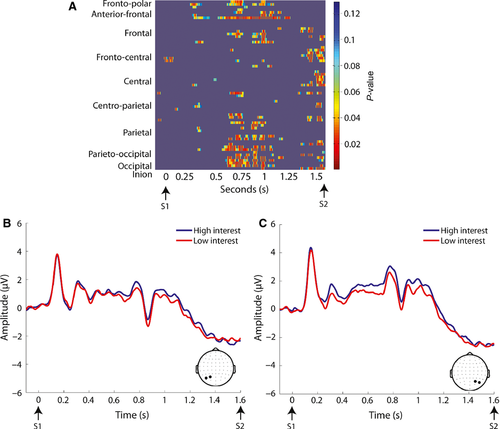
The aforementioned methods consisted of more conservative approaches to the analysis of high-density ERP data in order to limit the number of statistical tests performed, with the spatio-temporal properties of the componentry delimiting the tests. This conservative approach raised the likelihood of missed effects in assessing the independent effects of motivation on anticipatory ERPs. Considering the richness of the current data matrix, it is entirely probable that other periods and brain regions would be modulated by motivational factors that may be missed with traditional analyses (i.e. type II errors). An exploratory analysis was therefore performed as a means of fully exploring the richness of the data set and as a hypothesis-generating tool for future research. A simple method was employed for testing the entire data matrix for possible effects, termed statistical cluster plots (Molholm et al., 2002; Murray et al., 2002). Data were averaged across the cue direction conditions, and cluster plots were derived by calculating point-wise, paired, two-tailed t-tests between the high and low interest conditions. The results were then arrayed on a single grid, with scalp regions (electrode positions) plotted on the y axis and post-stimulus time plotted on the x axis, thus providing a snapshot overview of significant differences between conditions across scalp regions over time. In the present data, periods of significant difference were only plotted if an alpha criterion of 0.05 was exceeded and then only if this criterion was exceeded for at least 20 consecutive data points (~40 ms) (Guthrie & Buchwald, 1991).
Thereafter, activations across time windows within these resulting time periods (700–800, 800–900, 900–1000, and 1000–1100 ms post-cue) and from scalp regions within the resulting ROIs were computed. Using these values, repeated-measures anovas with the factors of motivation and ROI were performed to examine the main effects and interactions.
Effects of attention on anticipatory alpha-band activity
A repeated-measures anova was performed with the factors of motivation, cue direction, and ROI to examine the effects of attention on anticipatory alpha-band activity. The existing literature has established that alpha modulations increase toward the end of a cue–target interval over parieto-occipital channels (e.g. Worden et al., 2000). Thus, the average activity from 1400 to 1500 ms over parieto-occipital ROIs was used for this analysis (Murphy et al., 2014). Additionally, paired-samples t-tests were performed across cue left and cue right conditions for activations within the right parieto-occipital ROI to examine alpha-band spatial attentional modulations in the right hemisphere alone. This was performed in the light of prior attention work indicating that alpha-band activity is most prominent over the right parieto-occipital cortex (Foxe et al., 1998; Foxe & Snyder, 2011).
Measuring attentional and motivational effects on subsequent visual sensory processing
For bilateral stimuli, repeated-measures anovas with the factors of motivation, attention, and ROI were performed for the C1 (60–80 ms post-S2), P1 (110–150 ms), N1 (180–220 ms), and P3 (280–320 ms) components. As above, the factor of motivation was not examined for unilateral stimuli, due to insufficient trials. Therefore, for unilateral stimuli, repeated-measures anovas were performed for the aforementioned components, with the factors of attention, stimulus location, and ROI. The ROIs and time periods were chosen based on prior work detailing the spatio-temporal dynamics of these components (Foxe & Simpson, 2002; Kelly et al., 2008; Frey et al., 2010, 2014).
Results
Eye-tracking data
The participants maintained highly accurate fixation to the central fixation cross (within 0.52° along the horizontal median). The median eye gaze deviation along the horizontal meridian was 0.49° (SE, 0.08) in the cue left condition and 0.52° (SE, 0.08) in the cue right condition. The standard deviation of gaze along the horizontal meridian was 0.30° (SE, 0.13) in both the cue left and cue right conditions.
Behavioral performance data
All of the main effects and interactions meeting statistical significance (P < 0.05) are reported here and in the subsequent sections. In addition, the main effects and interactions in the trend range (P < 0.09) are reported in the following sections for completeness, but will not be further discussed.
For d-prime, there was a significant main effect of motivation (F1,23 = 12.27, P = 0.002), a significant main effect of stimulus location (F1,23 = 14.26, P = 0.001), and a significant motivation × stimulus location interaction (F1,23 = 17.63, P < 0.001). These findings were driven by higher d-prime scores for high vs. low interest stimuli, and higher d-prime scores in the right hemifield. For reaction time, there was a marginal main effect of motivation (F1,23 = 3.84, P = 0.062). See Table 3 for a summary of the behavioral data.
| Measure | Low interest stimulus left | High interest stimulus left | Low interest stimulus right | High interest stimulus right |
|---|---|---|---|---|
| d-prime | 2.64 ± 0.14 | 3.06 ± 0.14 | 3.17 ± 0.06 | 3.20 ± 0.06 |
| Reaction Time (ms) | 609.3 ± 12.2 | 591.8 ± 13.9 | 600.0 ± 13.1 | 585.7 ± 12.9 |
Anticipatory event-related potential data
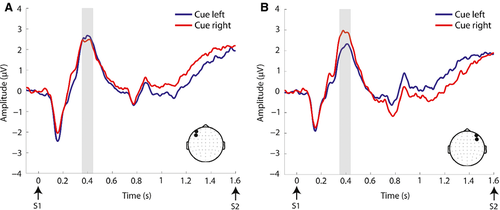
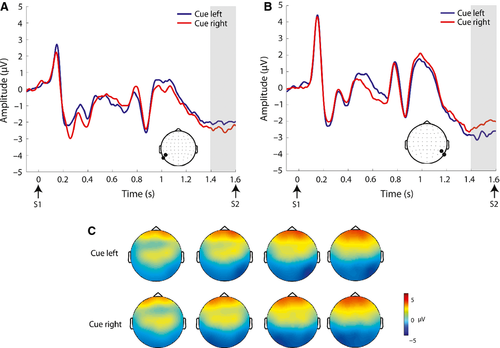
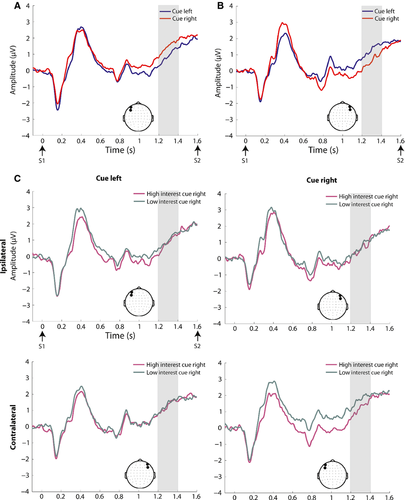
Table 4 outlines the statistical findings for each of the pre-defined broadband ERP components examined during the anticipatory period. There was no evidence for an EDAN over posterior electrodes, and this component was not investigated further. The ADAN (Fig. 2), BRN (Fig. 3), and LDAP (Fig. 4) were observed. For the ADAN, an interaction of cue × ROI was observed (F1,23 = 10.87, P = 0.003), driven by greater negative-going amplitude contralateral to the attended hemifield. There was no main effect of motivation or interaction between cue and motivation in this time frame. For the LDAP, an interaction of cue × ROI was also observed (F1,23 = 12.07, P = 0.002), driven by a more positive-going amplitude contralateral to the attended hemifield. There was also a motivation × ROI interaction in this time frame (F1,23 = 5.96, P = 0.023). Follow-up paired t-tests showed that this interaction was driven by greater amplitude over the right ROI for the high vs. low interest condition (t23 =2.49, P = 0.021). Motivation and cue did not interact. For the BRN we observed an interaction of cue × ROI (F1,23 = 21.93, P = 0.000), driven by greater, negative-going amplitude contralateral to the attended hemifield. There was no main effect of motivation or interaction between cue and motivation in this time frame. Additionally, a frontal negativity ipsilateral to the attended location [hereafter termed ipsilateral frontal negativity (IFN)] (Fig. 5) was observed in the time period from 1200 to 1400 ms post-cue. This observation was followed up with an anova for this time period for data over the frontal scalp (see Fig. 5 for location of ROIs) with factors of cue, motivation, and ROI. This yielded a significant cue × ROI interaction (F1,23 = 7.89, P = 0.010), which was driven by greater negative-going amplitude ipsilateral to the attended hemifield. Although motivation did not interact with cue, there was a marginal main effect of motivation for the IFN (F1,23 = 3.31, P = 0.082), driven by greater (negative-going) amplitude for the high interest vs. low interest condition.
| Component | Attention effects | Motivation effects |
|---|---|---|
| ADAN | Interaction of cue × ROI (F1,23 = 10.87, P = 0.003), driven by greater (negative-going) amplitude contralateral to the attended hemifield | None observed |
| LDAP | Cue × ROI interaction (F1,23 = 12.07, P = 0.002), driven by greater (positive-going) amplitude contralateral to the attended hemifield | Motivation × ROI interaction (F1,23 = 5.96, P = 0.02). Follow-up paired t-tests showed that this interaction was driven by greater amplitude over the right ROI for the high vs. low interest condition (t23 =2.49, P = 0.021) |
| BRN | Cue × ROI interaction (F1,23 = 21.93, P = 0.000), driven by greater (negative-going) amplitude contralateral to the attended hemifield | None observed |
| IFN | Interaction of cue × ROI (F1,23 = 7.89, P = 0.010), driven by greater (negative-going) amplitude ipsilateral to the attended hemifield | Marginal main effect of motivation (F1,23 = 3.31, P = 0.082) driven by greater (negative-going) amplitude for the high interest vs. low interest condition |
Anticipatory alpha-band data
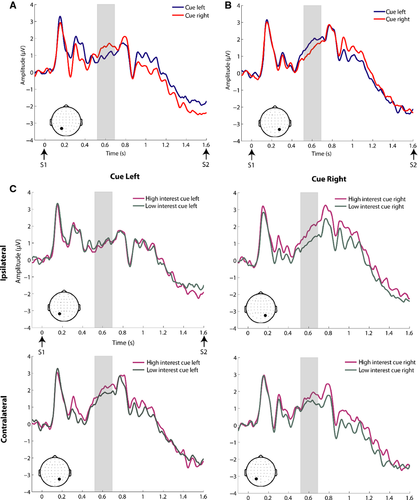
See Fig. 6 for findings in the alpha band during the anticipatory period. Results from the motivation × cue × ROI anova of anticipatory alpha-band activations showed a main effect of ROI (F1,23 = 13.02, P = 0.001) and an interaction of cue × ROI (F1,23 = 6.45, P = 0.018). The main effect of ROI was driven by higher overall alpha power over the right parieto-occipital ROI (see Fig. 6 for ROIs). However, the interaction of cue × ROI was not based on increased alpha-band activity contralateral to the to-be-ignored location, as seen in adults (Worden et al., 2000; Kelly et al., 2006). Instead, there was higher alpha power over the right vs. left ROI for both cue left and cue right conditions, although this difference was less pronounced for the cue right condition (see Fig. 6C). Paired t-tests of cue left and cue right activations (with interest conditions collapsed) revealed significantly higher alpha power for the cue left vs. cue right condition over the right parieto-occipital ROI (t23 = 2.30, P = 0.031). Scalp topographies collapsed across cue conditions also showed a peak over the right parieto-occipital cortex (Fig. 6D), with greater power in the high vs. low interest condition, but this difference did not reach significance.

Exploratory analysis of motivational effects on anticipatory activity
Statistical cluster plots comparing low and high motivation conditions during the S1–S2 interval were generated to capture any effects that might have been missed in our planned analyses, and to serve as a hypothesis-generating tool for future research (Molholm et al., 2002; Murray et al., 2002). This revealed a sustained period in which there was greater activation in the high motivation compared with the low motivation condition in the cue–target interval spanning from 700 to 1100 ms (see Fig. 7), and focused largely over the parieto-occipital scalp. This was followed up with repeated-measures anovas for successive 100 ms periods for ROIs over the right and left parieto-occipital scalp (see Fig. 7). The high motivation condition was of greater amplitude for all four time periods (700–800 ms, F1,23 = 9.54, P = 0.005; 800–900 ms, F1,23 = 9.48, P = 0.005; 900–1000 ms, F1,23 = 8.67, P = 0.007; 1000–1100 ms, F1,23 = 9.28, P = 0.006). Although there was a main effect of ROI for all time periods due to greater activity over the right hemisphere (700–800 ms, F1,23 = 6.53, P = 0.005; 800–900 ms, F1,23 = 11.88, P = 0.002; 900–1000 ms, F1,23 = 4.53, P = 0.044; 1000–1100 ms, F1,23 = 5.37, P = 0.030), motivation did not interact with ROI.
Attentional and motivational effects on subsequent visual sensory processing
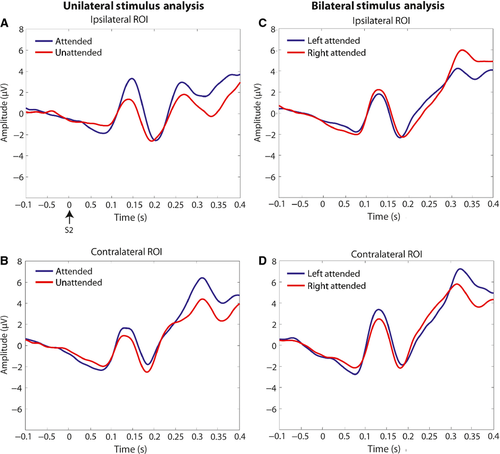
Bilateral non-target analysis
For the bilateral non-target analysis, see Fig. 8A and B for visual evoked potential (VEP) waveforms, and Fig. 9A for scalp topographies. For the C1 amplitude, there was a significant interaction of attended location × ROI (F1,23 = 14.73, P = 0.001). This was driven by greater negative-going C1 amplitude at the ROI contralateral to the attended location. For the P1, the same interaction was found (F1,23 = 26.98, P < 0.001), driven by greater positive-going amplitude at the ROI contralateral to the attended location. For the N1, there was an interaction of attended location × ROI (F1,23 = 7.20, P = 0.013), but it was driven by greater negative-going amplitude ipsilateral to the attended location. For the P3, the same interaction (F1,23 = 68.37, P < 0.001) was driven by greater positive-going amplitude at the ROI contralateral to the attended location. There were no significant interactions with the factor of motivation, and main effects of motivation were not considered as differences in features across high and low interest stimuli may have influenced the amplitudes of these components (Taylor, 2002).
Unilateral non-target analysis
For the unilateral non-target analysis, see Fig. 8C and D for VEP waveforms, and Fig. 9B and C for scalp topographies. For C1 amplitude, there were significant main effects of attention (F1,23 = 6.63, P = 0.017) and ROI (F1,23 = 16.71, P < 0.001). The main effect of attention was based on greater negative-going amplitude for the attended vs. unattended condition, and the main effect of ROI was driven by greater negative-going amplitude at the right vs. left parieto-occipital ROI. For the P1, there was a significant main effect of attention (F1,23 = 7.19, P = 0.013), driven by greater positive-going amplitude for the attended vs. unattended condition across ROIs. For the N1, there was a main effect of ROI (F1,23 = 9.23, P = 0.006), which was driven by greater negative-going amplitude over the right parieto-occipital ROI. A significant interaction of stimulus location × ROI was also observed (F1,23 = 7.06, P = 0.014), with greater negative-going amplitude ipsilateral to the stimulus location. For the P3, there was a main effect of attention (F1,23 = 29.10, P < 0.001), with greater positive-going amplitude for the attended vs. unattended condition. There was also an interaction of attention × ROI (F1,23 = 29.10, P < 0.001), based on greater amplitude over the contralateral ROI for the attended condition.
P1 latency effects
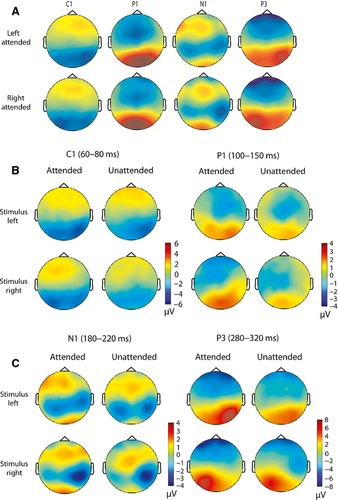
There were no a-priori predictions regarding latency effects as a function of attention for visual evoked potentials following the S2 (stimulus after the cue). However, post-hoc examination of the visual evoked potentials to the S2 suggested that P1 latency might be increased in the attended compared with unattended condition over the hemisphere ipsilateral to stimulation. To follow up on this finding, P1 latency was examined in both unilateral and bilateral S2 conditions separately, to account for differences in stimulus inputs to cortical hemispheres across these conditions. This post-hoc examination showed that P1 latency was increased in the unilateral attended vs. unattended condition over the ipsilateral ROI (see Fig. 10A and B, detailed statistical findings are given below. However, there was no difference in latency as a function of attention over the ipsilateral ROI for bilateral S2s (see Fig. 10C and D).
To perform a post-hoc analysis of the P1 latency effects for unilateral stimuli, maximum activation within the P1 time period (100–180 ms) and within the left and right parieto-occipital ROIs (Fig. 9) was computed and the corresponding time point was obtained for each condition at the participant level. The resulting values were then averaged across stimulus location conditions for the contralateral and ipsilateral ROIs separately. A repeated-measures anova (attention × ROI) was performed, and a main effect of attention (F1,23 = 11.46, P = 0.003) and an interaction of attention × ROI (F1,23 = 17.21, P < 0.001) were observed. The main effect of attention resulted from an overall increased P1 latency in attended vs. unattended conditions. To further examine the attention × ROI interaction, paired-samples t-tests were performed, which revealed significant increases in P1 latency for attended vs. unattended conditions, but ipsilateral to the stimulus location (t23 = 4.37, P < 0.001). Additionally, a comparison of P1 latency for attended stimuli alone over ipsilateral vs. contralateral ROIs showed that latency was selectively increased at the ipsilateral ROI (t23 = 3.08, P = 0.005). There were no significant differences in P1 latency across attended and unattended conditions contralateral to the stimulus location (i.e. the sensory regions receiving input from the attended field). Also, there was no significant difference in P1 latency for unattended stimuli alone over ipsilateral vs. contralateral ROIs.
Sensory processing of the cue stimuli
An issue raised during the review process centered on the use of a block design here, such that high and low interest categories were always presented in separate blocks rather than in an interleaved fashion. As we will return to below, this was motivated by the need to make the task tractable to pediatric populations. However, it raises the possibility of state arousal changes across blocks. If arousal were a major factor here, a simple prediction would be that the sensory evoked responses to the cue stimuli should show modulation as a function of category. In a post-hoc analysis, we tested for this possibility. Paired t-tests revealed no significant differences in mean amplitude during the C1 (t23 = −0.691, P = 0.496), P1 (t23 = −0.887, P = 0.384), N1 (t23 = −0.345, P = 0.733), or P3 (t23 = −1.206, P = 0.240) time frames over the parieto-occipital scalp regions between high and low interest conditions (see Supporting Information Fig. S2). Note that we employed exactly the same time windows and ROIs as were used to analyse the S2 sensory VEPs for attention effects (see above).
Discussion
Influences of motivation on anticipatory attentional processes in adolescents
The level of interest in aspects of the environment, e.g. in a given object or event, or the subject matter of a magazine article, clearly influences the effectiveness with which one deploys and maintains attention. Thus, the individual with a high level of interest in football but low interest in botany will undoubtedly be more engaged during a soccer match than an orchid show. As such, the level of interest and ‘motivation’ are inextricably linked. To date, most of the studies that have considered how motivation influences cognitive processes have focused on monetary rewards, which are readily manipulable and clearly an excellent incentive under many circumstances (Small et al., 2005; Smith et al., 2011; Morie et al., 2014). However, other more common motivating factors in daily life, such as one's level of interest in a given topic, can be equally motivating. In fact, manipulations of the interest value of stimulus materials may have more direct relevance during the study of children, for whom the value of money is often not as readily evident (Bagley et al., 2007; Vaidya et al., 2013). Further, when studying individuals from different socio-economic or cultural backgrounds, fixed monetary rewards can have considerably different valences (Heinrichs, 2006; Zammar et al., 2010). What is more, monetary rewards may be particularly unsuited to studying motivation in certain clinical populations. Here we set out to establish the role that the level of interest in a to-be-attended stimulus class plays in the effective allocation of attention in boys between the ages of 12 and 15 years.
The anticipatory mechanisms of top-down attention in adolescents
To date there have been very few studies examining the neurophysiology of cued spatial attention in children and adolescents, so we begin by describing these data. We identified several classic anticipatory broadband ERP components that have been associated with top-down spatial attentional biasing in adults, including the ADAN, LDAP, and BRN. The ADAN is a frontal negativity (~350–500 ms post-cue) that has been associated with the initiation of anticipatory shifts in spatial attention (Harter et al., 1989). The LDAP is a late and tonic posterior positivity (~550–800 ms post-cue) thought to reflect the enhancement of visual cortical excitability in anticipation of upcoming stimuli (Jongen et al., 2007; Murray et al., 2011). The BRN is a posterior negativity contralateral to the attended location (~900 ms to beyond the presentation of a subsequent target), and is thought to represent specific sustained baseline shifts that bias target-specific brain areas to enhance perceptual sensitivity (Grent-'t-Jong & Woldorff, 2007). We observed top-down attentional modulation of all of the aforementioned components in adolescents contralateral to the attended location, in a manner that resembled what is classically observed in adults, pointing to the fact that these anticipatory spatial attentional mechanisms are already quite mature by mid-adolescence.
In addition to this previously described family of anticipatory responses, we also observed a late negativity over the frontal cortex, ipsilateral to the attended hemifield (which we termed the IFN; from ~1 s post-cue to the arrival of the subsequent stimulus). To our knowledge, this ERP component has not been previously reported in the literature. Unlike other anticipatory negativities, like the earlier frontal EDAN observed in adults (~250–400 ms post-cue) that occurs contralateral to the cued (attended) region, the IFN occurred over cortices contralateral to the to-be-ignored (unattended) region of space. In light of evidence for topographic maps in frontal eye field regions (Silver & Kastner, 2009), it is possible that adolescents employ a frontally-based mechanism for the suppression of the to-be-ignored spatial representations. The mechanism underlying the IFN may be similar to that of the PD component recently reported by Gaspar & McDonald (2014), which is thought to reflect the suppression of salient distractors during visual search. However, the PD was shown to occur relatively early (~250 ms after the visual search displays onset) and over the parietal cortices in healthy adults, as compared with the later and more frontally focused IFN that we observe here in adolescents. In adults, these suppression mechanisms may be performed by more posterior regions during an earlier stage of processing, with less reliance on frontal mechanisms. Of course, it is worth mentioning that an obvious limitation of the current study is the lack of an adult cohort to make direct comparisons with, and it is certainly possible that the IFN is specific to the task that we used here.
The current results also indicated notable divergence from what is commonly observed in adults when engaged in similar cued spatial attention tasks, suggesting that some top-down spatial attention processes might not yet be fully mature by early to mid-adolescence. For example, we did not observe the EDAN (~250-300 ms) (Hopf & Mangun, 2000; Nobre et al., 2000; Praamstra & Kourtis, 2010; Murray et al., 2011). The contralateral EDAN has been associated with the processing of directional cues and the initiation of anticipatory shifts of attention, and is considered a reliable early marker of the top-down control of spatial attention in the frontal cortices (Nobre et al., 2000; Kennett et al., 2007). The current results would appear to suggest that this process may not yet have emerged in early adolescence. Such an interpretation is complicated by the fact that an EDAN response has been previously reported in children between 6 and 9 years of age (Harter et al., 1989). However, eye tracking measures were not used in that early study, raising the possibility that systematic eye movements may have occurred towards stimulus locations and that overt attentional mechanisms were engaged.
Additionally, although we observed increases in alpha power in the cue–target interval in our sample, these did not show adult-like patterns of lateralisation. In adults, lateralised alpha-band modulations contralateral to the uncued location are associated with the anticipatory, top-down suppression of the to-be-ignored hemispace (Worden et al., 2000; Kelly et al., 2005; Gomez-Ramirez et al., 2009; Banerjee et al., 2011). To the best of our knowledge, lateralised alpha-band activity has not been previously examined in children in a cued spatial attention task [but see Murphy et al. (2014) for robust alpha-band modulations in children during an inter-sensory attention task]. Given that we did not observe such lateralised alpha-band modulations in our cohort, it is possible that the suppression of to-be-ignored spatial locations via highly directed retinotopic shifts in alpha-band activity in the parieto-occipital cortices is a later developing mechanism of top-down control of spatial attention. Indeed, studies of cognitive control processes have shown that older adolescents have greater attentional capacity and processing speed than younger children in several standardised neuropsychological measures (Anderson et al., 2001), pointing to an extended developmental trajectory for these mechanisms. The attentional control system may continue to be shaped by crucial developmental milestones through mid-adolescence, and highly efficient attentional control functions may be attained only in later adolescence and early adulthood.
Here instead we saw that the modulation of alpha power as a function of spatial attention was stronger for the cue left vs. the cue right condition, and that the activity in the cue left condition was greatest over the right partieto-occipital scalp. This pattern, which represents a major divergence from the lateralised alpha-band modulations seen in adults, may be considered in the light of the behavioral data, i.e. behavioral performance (d-prime) was enhanced for stimuli presented to the right vs. the left visual hemifield [see Harter et al. (1989) for a similar finding in younger children]. In contrast, in cued spatial attention tasks in adults, a benefit for stimuli presented to the right visual hemifield is not generally observed (Posner et al., 1980; Worden et al., 2000; Banerjee et al., 2011). Thus, one possibility is that covertly attending to the left hemifield is inherently more difficult for children than covertly attending to the right hemifield. Consequently, immature posterior spatial attention mechanisms are brought online to a greater extent when the child has to selectively attend to the left side of space. However, here it is the major right parietal attentional control centers, which span both left and right hemispaces (Vallar & Perani, 1986; Muri et al., 2002; Foxe et al., 2003; Mesulam, 2004), that are relied upon due to the still-developing attentional architecture across the parietal hemispheres.
Motivation effects on anticipatory processes
The current data reveal that performing a spatial target detection task with high interest items indeed leads to significantly better performance than when the same task is conducted with low interest items. The impact of motivational context was readily observed on the parieto-occipital processes associated with anticipatory top-down spatial attention. Namely, when the to-be-attended items were of high interest, ongoing processes within the time frame of the LDAP were significantly larger than when they were of low interest over the right parieto-occipital cortex (see Fig. 4). Exploratory analysis further supported the presence of greater activity in the anticipatory period, for a relatively sustained period of time. This was focused over the bilateral parieto-occipital scalp, and although it appeared larger over the right hemisphere, this did not differ significantly across motivation conditions. These data suggest that motivational systems function in conjunction with top-down attentional mechanisms to shape anticipatory activations, with modulations seen over scalp regions that are consistent with the involvement of neural substrates involved in both attentional control and sensory processing. However, the influence of motivation through level of interest in a task on anticipatory spatial attention processes in adults remains unclear, and thus further investigation of these processes across development is warranted.
Intracranial work in non-human primates has supported the idea of a ‘salience map’ in the lateral intraparietal area of the intraparietal sulcus, which integrates the spatial location and reward value of anticipated stimuli (Gottlieb, 2007). In an fMRI study, activations in the intraparietal sulcus and other regions of the attentional network were more positively correlated with the speed of attentional shifts to food targets (calculated from reaction time data) when participants were hungry vs. satiated (Mohanty et al., 2008), supporting the idea that a salience map in the intraparietal sulcus is dynamically modulated by the current motivational relevance of stimuli to guide spatial attention. Here, increased activation over the parieto-occipital regions in anticipation of high interest stimuli was observed and behavioral accuracy (d-prime) was enhanced, in agreement with the notion that the motivational relevance of anticipated stimuli is encoded by parietal salience maps, thus shaping top-down spatial attention control at the behavioral and neurophysiological levels.
Motivational and attentional effects in the processing of subsequent visuo-spatial stimuli
Influence of motivation on visual evoked potentials
Surprisingly, motivation did not interact with visuo-spatial attention effects, as assessed for the VEPs to imperative stimuli. Given the strong influences of motivation during the anticipatory period, it was expected that motivation would influence attentional modulations following the presentation of imperative stimuli. The influence of motivation on early perceptual processes remains unclear, as there is little neurophysiological work in this area. In fMRI studies of TD adults using cued spatial attention tasks, modulations of activity in the visual striate and extrastriate regions by motivation were reported (Small et al., 2005; Mohanty et al., 2008). However, the temporal resolution of fMRI is insufficient to assess whether these effects occurred during early processing, or later, during re-entrant feedback (Baines et al., 2011). One ERP study of TD adults examined the influence of motivation on early perceptual processes, and the results showed that motivation modulated VEPs starting from the N1 component (Baines et al., 2011). These findings suggested that motivation did not influence very early perceptual processes in TD adults (indexed by the C1 and P1 components) in V1 and extrastriate regions. Although there is little developmental work in this area, it is possible that the motivational enhancement of post-stimulus visual evoked potentials emerges in late adolescence or early adulthood, when attentional control capacities are mature, and activations in paralimbic regions that drive involuntary attentional processes are reduced (Smith et al., 2011).
Attentional modulations of visual evoked potentials: bilateral and unilateral stimuli
For bilateral stimuli, VEPs were modulated by spatial attention over the contralateral parieto-occipital regions, as commonly seen in adults (Hillyard & Anllo-Vento, 1998; Kelly et al., 2008). Attentional modulation was seen during the time frames of the C1, P1, and P3 components. The modulation of C1 might be considered of particular interest, as there is ongoing debate as to whether spatial attention influences early sensory processes reflected in the C1 (Clark & Hillyard, 1996; Martinez et al., 1999; Kelly et al., 2008). Whereas we have reliably demonstrated such modulation using a design in which C1 activity was clearly attributable to V1 (Kelly et al., 2008), other studies have not shown such modulations (Clark & Hillyard, 1996; Martinez et al., 1999). The current data are consistent with the former account, and indicate that spatial attention influences sensory processing as early as V1 in an adolescent cohort. For unilateral stimuli, attention enhanced C1 and P1 amplitudes, but these effects were seen across the ROIs, which may be attributed to paradigmatic factors, which will be discussed below. The N1 amplitude was not increased contralateral to the attended location for bilateral or unilateral stimuli. Previous work in healthy adults similarly showed that the N1 effect was abolished for bilateral stimuli in a task involving similar probabilities of bilateral and unilateral stimuli as in the current study (Luck et al., 1990). Also, the N1 attention effect for unilateral stimuli was increasingly attenuated according to the proportion of bilateral stimuli in a task (Heinze et al., 1990; Luck et al., 1990). The N1 effect has been tied to the orienting or engaging of attention to a task-relevant stimulus. This process is thought to be reduced with the inclusion of bilateral trials (Luck et al., 1990), in which a stimulus is always present at the attended location. Finally, although C1 and P1 components were modulated contralateral to the attended stimulus location in bilateral trials and across hemispheres in unilateral trials, P3 amplitude was modulated contralateral to the location of spatial attention for both unilateral and bilateral stimuli.
The findings from the analysis of unilateral stimuli differ from a previous study in children (6–9 years old), which showed that P1 and N1 were modulated by spatial attention over ROIs contralateral to attention in a manner similar to adults (Harter et al., 1989). However, in the study of Harter et al. (1989), only unilateral stimuli were used, and targets were equiprobable at attended and unattended locations (50% cue validity). A cue validity of 80% was used in the current work to effectively drive top-down attention (Posner et al., 1980; Vossel et al., 2006). Therefore, participants in the current work probably learned to expect a stimulus at the attended location. Such paradigmatic factors may have contributed to the differences observed across these studies, but further work will be necessary to determine if these disparities can be attributed to developmental or experimental factors.
Attentional modulation of P1 latency: exploratory analyses
After inspecting the group-averaged data, we observed what appeared to be potential effects on P1 timing. We therefore performed exploratory post-hoc analyses to further investigate this issue but, of course, effects uncovered in this manner will bear replication and should be treated as hypothesis generation tools in the interim. We observed that the latency of the P1 component was differentially modulated by attention following unilateral stimuli, i.e. the P1 component in the attended condition showed increased latency over the ipsilateral ROI (see Fig. 10A). In contrast, there was no difference in P1 latency across attended and unattended conditions over the contralateral ROI (see Fig. 10 B). This P1 latency effect was not observed in the bilateral stimulus analysis (see Fig. 10 C and D). In the unilateral condition, sensory information was only presented to one hemifield, and this information was transferred from the contralateral to the ipsilateral hemifield. However, the P1 delay cannot be attributed merely to the collosal transfer of visual information (Brown et al., 1994; Ipata et al., 1997), as it was selective to the attended condition. Thus, it is possible that, during mid-adolescence, top-down spatial attentional mechanisms may delay the transfer of attended information from the contralateral to the ipsilateral hemisphere. In the bilateral condition, both hemispheres were engaged in processing contralateral inputs, which may have reduced the attentional modulation of information transferred across hemispheres. In contrast, a P1 latency shift for attended unilateral stimuli was not observed in younger children when cue validity was equal across attended and unattended conditions (Harter et al., 1989). Further, examination of these effects across stages of development through the use of different degrees of cue validity will reveal if this process is truly driven by top-down attention, and whether it is unique to a particular period of development.
Study limitations
A potential limitation of the current study was that low and high interest stimulus classes were presented in a block design, such that participants always knew which stimulus class was to be engaged with over a given experimental block. This raises the possibility that there could have been significant differences in the arousal level of participants between blocks. It is certainly well established that early sensory ERPs are affected by the arousal level (Eason & Harter, 1969). Although motivational effects may influence overall arousal, experimental controls (e.g. eye-tracking measures, online assessment of behavioral performance) ensured that children were engaged in the task at all times. In turn, when we tested the early sensory evoked components elicited by the cue stimuli, which would presumably have been modulated had arousal levels been significantly different between conditions, we found no such modulation of these visual evoked components (i.e. C1, P1 or N1).
A potential design improvement would be to use trial-by-trial cuing where the cue not only indicates the location to be attended, but also which stimulus class is to be anticipated. For example, the color of the arrow cue could relay the motivational salience of upcoming stimuli. This type of design would certainly be feasible in neurotypical adults and would obviously be of scientific interest. However, it would also require considerably greater processing resources on the part of participants to extract the dual meanings of each cue stimulus, which would limit its application in younger children and individuals with intellectual and developmental disabilities. The current task was expressly designed to translate well to such populations in future work where we hope to assess the role of interest in modulating attention in children with an ASD.
Conclusions
The current findings indicate that signals from motivational and top-down attention systems are integrated in the brain, and motivational state can crucially impact top-down attention control functions. Additionally, the current work revealed crucial differences in attentional functions across cortical hemispheres in adolescents as compared with adults. Although top-down attentional control may be taken for granted in daily life, individuals with neurological disorders, such as ASDs, exhibit impairments in this crucial cognitive mechanism (Ciesielski et al., 1990, 1995; Wainwright-Sharp & Bryson, 1993; Haist et al., 2005; Keehn et al., 2010). They are also often more motivated to attend to stimuli within restricted and circumscribed interests (e.g. trains), which impairs social functioning (Boyd et al., 2007; Sasson et al., 2008). Future work may assess how increasing motivation through stimuli of high interest may be used to regulate attentional control deficits to social and non-social information in individuals with ASD.
Conflict of interest statement
All authors declare that they have no conflicts of interest, financial or otherwise, that would bias the results reported here.
Acknowledgements
This work was primarily supported by a grant from the U.S. National Science Foundation (NSF) to J.J.F. and S.M. (BCS1228595). The authors would like to thank Mr Gregory Peters for help with data collection. Additional support for the work of J.J.F. and S.M. derives from a grant from the U.S. National Institute of Mental Health (NIMH RO1 MH085322). Participants in this study were recruited and evaluated at The Human Clinical Phenotyping Core, a facility of the Rose F. Kennedy Intellectual and Developmental Disabilities Research Center (IDDRC), which is funded through a center grant from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD P30 HD071593). S.B. was supported by a Ruth L. Kirschstein National Research Service Award (NRSA) pre-doctoral fellowship from the National Institute of Mental Health (MH097310A).
Abbreviations
-
- ADAN
-
- anterior directing attentional negativity
-
- ASD
-
- autism spectrum disorder
-
- BRN
-
- biasing-related negativity
-
- EDAN
-
- early directing attention negativity
-
- EEG
-
- electroencephalography
-
- ERP
-
- event-related potential
-
- fMRI
-
- functional magnetic resonance imaging
-
- IFN
-
- ipsilateral frontal negativity
-
- LDAP
-
- late directing attentional positivity
-
- ROI
-
- region of interest
-
- TD
-
- typically developing VEP, visual evoked potential




