A Case-Driven Multi-Omics Analysis for Longitudinal Ibrutinib Response Evaluation of Patients With Chronic Lymphocytic Leukemia
Funding: Danish Cancer Society (R246-A14603 and R281-16686), Helen Rudes Fond (60988), The OUH PhD Fund (R90-A4022), Dagmar Marshalls Fond, Agnes og Poul Friis Fond, Tornøes og Høyrups Fond (2304), Marie og Børge Kroghs Fond, Torben og Alice Frimodts Fond, Danish Lymphoma Group, Grosserer M. Brogaard og hustrus Mindefond Odense (5523-TL-ne), Folketingsmand J. Christensen og hustru K. Christensens Fond and Brødrene Hartmanns Fond (A37628).
ABSTRACT
Patients with chronic lymphocytic leukemia (CLL) undergoing ibrutinib treatment often experience incomplete response, yet the molecular level underlying clonal inertia remains to be explored. We investigated the molecular and clinical dynamics of CLL during 16 months of ibrutinib monotherapy by analyzing blood samples from two patients who continued having CLL cells in the peripheral blood during treatment. At diagnosis, the clonal burden within the B cell compartment was found to be 55% (pt1) and 86% (pt2) for the dominant clones. At 16 months following treatment these clones still constituted 66% and 89%, respectively. Utilizing multi-omic methodologies at the DNA and RNA levels, including single-cell transcriptomics, we aimed to establish a comprehensive framework for multi-omics analysis for longitudinal ibrutinib response evaluation. The presented study revealed genomically stable disease during ibrutinib treatment, but with intensified expression of genes involved in pathways related to apoptosis, cellular stress response, and canonical NF-κB signaling from diagnosis to 16 months of treatment.
1 Introduction
Chronic lymphocytic leukemia (CLL), the most common leukemia in adults, typically progresses slowly but remains incurable. Treatment options include Bruton tyrosine kinase (BTK) inhibitors, chemo-immunotherapy, stem cell transplantation, and cellular immunotherapy in advanced stages of the disease.
A notable aspect of BTK inhibitors, such as ibrutinib, is prolonged lymphocytosis. This persistent residual lymphocytosis does not indicate a lack of response or disease progression [1, 2] and does not impact survival rates [3]. Unlike other treatments [4-6], complete remission is infrequent with ibrutinib monotherapy [7]. Typical response assessments outside clinical trials include physical examination, complete blood cell count, and differential count [8]. Measurable residual disease (MRD) assessments are desirable for patients enrolled in clinical trials but otherwise not generally recommended [8]. The most commonly used MRD assessment methods include multicolor flow cytometry and polymerase chain reaction using allele-specific oligonucleotides, where CLL-specific markers and IGH rearrangements are used as molecular handles for disease evaluation [9]. In contrast to other therapies where achieving undetectable MRD status correlates with improved progression-free survival and overall survival, MRD assessment in patients with CLL on ibrutinib monotherapy is not informative [3, 10-13].
The mechanisms driving ibrutinib responsiveness and clonal inertia in persistent lymphocytosis remain unknown, necessitating a deeper understanding at the molecular level. BTK inhibition disrupts key survival and proliferation signals in B cells, leading to lymphocyte redistribution into the peripheral blood. However, the persistence of clonal B cells despite effective therapy raises questions about their biological stability and adaptive mechanisms. Investigating these processes at the molecular level is critical for understanding why most patients on ibrutinib achieve only partial responses and for identifying factors contributing to long-term disease control. The relevance is highlighted by the challenges faced by patients with high-risk CLL, as well as the patients who experience ibrutinib intolerance (25%), disease progression (20%), and Richter transformation (10%), where early detection is essential [14, 15]. Additionally, understanding clonal inertia in persistent lymphocytosis at the molecular level is highly relevant in the current discussion of safe ibrutinib pausing, dose reduction or discontinuation, and prolonged overall survival.
We present a case-driven genomic study, implementing a multi-omics technique for longitudinal surveillance of patients with CLL receiving ibrutinib monotherapy, leading to an in-depth profiling of the molecular characteristics of the B cells during ibrutinib treatment. We show that clonal lymphocytosis persists after 16 months of treatment. The clones are genomically and transcriptionally stable, with the exception of gene expression involved in apoptosis upregulated during treatment.
2 Methods
At baseline and follow-up (8 and 16 months after the start of treatment), B cells were analyzed from two male patients with CLL. Both patients had ibrutinib administered as first-line monotherapy and were included in the CLL17 clinical trial (EudraCT: 2019–003854-99) with an age at diagnosis of 59 and 70 years. Pt 1 was diagnosed with Binet stage B and CLL-IPI 3 but progressed to Binet stage C shortly after and initiated therapy. Pt 2 was diagnosed with Binet stage A and CLL-IPI 7 and started therapy five years after diagnosis when the disease progressed with enlarged lymph nodes and palpable spleen.
Both patients showed immunophenotypical profiles of CLL. Both were CD19+CD20dimCD5+CD200hiCD10−CD79b−. Patient 1 was kappadimCD23+, while patient 2 was lambdadimCD23+.
This methodological approach laid the groundwork for subsequent, larger cohort analysis, including patients in ibrutinib and rituximab-venetoclax therapy [16]. Absolute lymphocyte counts and multiparametric flow cytometry on peripheral blood were performed at all time points, with bone marrow included at baseline and after 16 months of treatment. The LST Euroflow panel [17, 18] antibodies were used to assess the frequency of CD19+ B cells out of total lymphocytes.
2.1 IGH Sequencing
Clonotype characterization was performed on 100–200 ng DNA from peripheral blood mononuclear cells (PBMCs) using the LymphoTrack IGH FR1 assay for MiSeq or S5/PGM (Invivoscribe, San Diego, CA, USA) and sequenced using MiSeq (Illumina, San Diego, CA, USA) with a V3 flow cell or using Ion GeneStudio S5 Prime systems (Thermo Fisher Scientific, Waltham, MA, USA) with Ion 530 chip, averaging 0.6 million (M) reads. The LymphoTrack Dx (v2.4.8, Invivoscribe) and MRD (v2.0.2, Invivoscribe) software were used to analyze the frequency of clonal cells out of total rearranged B cells. The VDJ gene usage was confirmed by NCBI IgBLAST [19].
2.2 Whole Exome and Bulk Transcriptome Sequencing
Apart from IGH clonotyping, all sequencing was performed on the NovaSeq 6000 Sequencing System (Illumina). Whole-exome sequencing was based on the Twist Exome 2.0 hybridization and target enrichment kit (Twist Bioscience, South San Francisco, CA, USA), and transcriptome sequencing using the Illumina Stranded mRNA Prep (Illumina, San Diego, CA, USA). These analyses were performed on cells isolated from PBMCs by negative selection using the Pan B or Pan T (for exome control counterpart) cell isolation kits (Miltenyi Biotec, Bergisch-Gladbach, Germany). The purity was assessed by flow cytometry using antibodies against CD45 (clone: MHCD4530, Life Technologies), CD3 (clone: SK7, BD Bioscience), CD56 (clone: C5.9, Cytognos), CD19 (clone: J3-119, Beckman Coulter), CD20 (clone: 2H7, Biolegend), CD5 (clone: L17F12, BD Bioscience), Kappa/Lambda (Biolegend), and LIVE/DEAD fixable near IR (780) viability kit (Invitrogen) to exclude dead cells. CD19-positive cells consisted of 87% (range: 71%–96%) of the total cells. Whole-exome sequencing (WES) was performed obtaining an average of 90 M reads per sample, resulting in a mean sample coverage of 366X, while full-transcript mRNA sequencing averaged 63 M reads per sample.
Whole exome sequencing data was mapped with BWA [20], while RNA-seq reads were aligned using STAR2 [21]. Exome and transcriptomic variant calling was performed with GATK 4.3 [22]. SnpEff (GRCh38.p13, dbNSFP v4.1a) [23] and SNPsift (dbNSFP v4.1a) [24] were used for downstream somatic variant annotation and filtration. Furthermore, annotation from external data sources gnomAD (v3.1.2) [25], ClinVar (Dec 11, 2022) [26], and COSMIC (v.98) [27] were implemented.
2.3 Single-Cell Sequencing
B cells were isolated as in the previous section, and fixed using the Chromium Next GEM Single Cell Fixed RNA Sample kit (10x Genomics, Pleasanton, CA, USA). Libraries were created using the Chromium Next GEM Single Cell Fixed RNA Hybridization and Library kit (PN-1000475, 10x Genomics), according to the manufacturer's instructions. Sequencing was performed on NovaSeq 6000 (Illumina).
2.4 Computational Processing and Statistics
Sequence processing was performed on the UCloud interactive HPC system (University of Southern Denmark, eScience Center, DK) with minor exceptions. R (v.4.3.1), Wolfram Language (Mathematica v.13.3, Wolfram Research, Champaign, IL, USA), and GraphPad Prism (v.10.1.2, GraphPad Software, Boston, MA, USA) were used for statistical analysis and computations. Single-cell reads and data were processed using the 10x cell ranger multi pipeline (Cell Ranger 7.2, 10x), RStudio (version 4.3.1) with Seurat (version 5.03) [28], SeuratObject (5.0.1) [29], LoupeR (1.0.2), cluster (2.1.6) [30], ggplot2 (3.5.0) [31], and future (1.33.2) [32] packages.
Single-cell RNA data were randomly down-sampled to 10 000 cells in R, excluding barcodes from identified T and NK cells (~1%). The maximum threshold for transcripts mapping to mitochondrial genes for each cell was 5%. Differential expression was assessed using the non-parametric Mann–Whitney U test. Markers of the highest differential expression were investigated further using gene set enrichment analysis (GSEA, Broad Institute) [33] based on Gene ontology (GO) to identify changes overlapping specific Biological Processes (hypergeometric test, Bonferroni-corrected, n = 7647, Molecular Signatures Database (MSigDB), Human MSigDB v2023.2.Hs, Oct. 2023). All gene-set markers were collated cell-wise to confirm and compare expressional distributions. Single-cell gene set enrichment analyses were complemented by bulk transcriptome sequencing for all samples. The Tyrosine-protein kinase transmembrane receptor ROR1 was used as a confirmation marker for identifying CLL cells [34].
2.5 Computational Processing and Statistics
Sequence processing was performed on the UCloud interactive HPC system (University of Southern Denmark, eScience Center, DK) with minor exceptions. R (v.4.3.1), Wolfram Language (Mathematica v.13.3, Wolfram Research, Champaign, IL, USA), and GraphPad Prism (v.10.1.2, GraphPad Software, Boston, MA, USA) were used for statistical analysis and computations.
2.6 Ethical Approval
The study adhered to the Declaration of Helsinki, and ethical approval was obtained from the Regional Ethics Committee for the Region of Southern Denmark (approval number S-20160069). Both patients gave informed consent.
3 Results
3.1 Clinical Representation
Both patients presented with unmutated IGHV and a del (13q14) (Figure S1). Additionally, Pt 2 exhibited del (11q) and a TP53 mutation (chr17:7673764 C>T, GRCh38). Therapeutic intervention with continuous ibrutinib monotherapy resulted in a partial response 16 months post-initiation based on biochemical evaluation, flow cytometry, and CT scans. The copy number variations and the TP53 mutation remained detectable in follow-up samples using whole exome sequencing.
Baseline CT scans before therapeutic intervention show widespread bilateral lymph node conglomerates of the neck and axillae of both patients (Figure 1A). Patient 1 also displayed splenomegaly and lymph node conglomerates in the mesentery. At baseline, Pt 2 presented with conglomerates in the mediastinum, iliac, and parailiac regions. After 16 months of treatment, follow-up scans of both patients showed partial response with a significant reduction in all sites, with the splenomegaly of Pt 1 being resolved. Furthermore, both patients were without B symptoms.
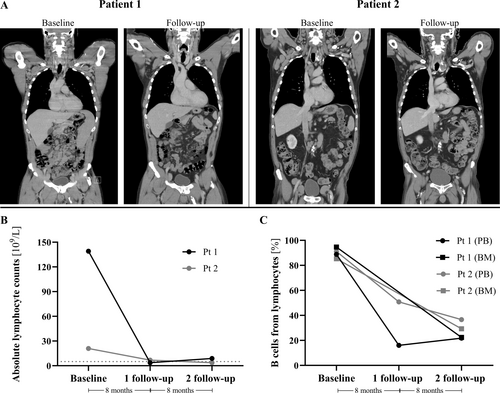
The therapeutic intervention reduced absolute lymphocyte counts to 3.67–6.86 × 109/L at 8 months and 3.66–8.87 × 109/L at 16 months follow-up after ibrutinib initiation (Figure 1B, Table 1). CD19+ cells, assessed by flow cytometry, constituted around 90% of total lymphocytes in blood at baseline for both patients. At 8 months follow-up, CD19+ cells were reduced to 16% in Pt 1 and 51% in Pt 2, followed by a slight increase to 22% in Pt 1 and a reduction to 37% in Pt 2 at 16 months. At 16 months follow-up, the CD19+ cells in the bone marrow were reduced to 22% and 29% of total lymphocytes, respectively (Figure 1C).
| Patient 1 | Patient 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Diagnosis | Baseline | 8 months | 16 months | Diagnosis | Baseline | 8 months | 16 months | |
| Hemoglobin (mmol/L) | 6.5 | 6.1 | 9.8 | 9.5 | 8.3 | 8.0 | 7.6 | 7 |
| Leukocytes (WBC) (109/L) | 189 | 201 | 8.07 | 16.6 | 15.8 | 29.4 | 11.8 | 8.61 |
| Thrombocytes (109/L) | 176 | 147 | 231 | 240 | 123 | 120 | 128 | 145 |
| Neutrophils (109/L) | 3.78 | 5.83 | 3.54 | 3.66 | 3.8 | 1.53 | 3.52 | 4.28 |
| Lymphocytes (109/L) | 155 | 193 | 3.67 | 8.87 | 11.5 | 21.5 | 6.86 | 3.66 |
| Beta-2-microglobulin (mg/L) | 3.4 | 2.1 | ||||||
| Creatinine (μmol/L) | 97 | 95 | 94 | 89 | 90 | 101 | 120 | 111 |
| Lactate dehydrogenase (LDH) U/L | 143 | 169 | 137 | Hemolysis | 166 | 184 | 145 | 163 |
| C-Reactive Protein (CRP) (mg/L) | 0.7 | 0.6 | 0.6 | 0.6 | 1.3 | 2.3 | 3.4 | 1.5 |
3.2 Molecular Representation
Only minor changes were found within the clonal CLL burden within the B cell compartment. Deep sequencing of the V(D)J rearrangement at diagnosis revealed two distinct clonal IGH rearrangements in Pt 1 (IGHV3-23*04 J4*02 and IGHV1-69*06 J3*02) accounting for 54.8% and 30.4% of the rearranged B cell population. Patient 2 harbored one major clone at diagnosis, making up 86.4% of the rearranged B cells (IGHV1-69*13 J6*02). At 8-month follow-up, the dominant clone frequency in Pt 1 increased to (60.1% and 66.8% at 16-month follow-up). In Pt 2, the clonal frequency was longitudinally stable (Figure 2A, Table S1A–F).
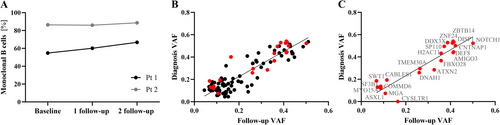
A direct correlation between putative somatic variants was found between baseline and 16 months follow-up (ρPearson = 0.91, Pt-statistics = 0.0001) with a variant overlap of 82% (132/162, > 0.05 variant allele frequency, VAF) (Figure 2B, black). No significant change in VAF from diagnosis to follow-up was observed for DNA or RNA (PU-test = 0.92 and PU-test = 0.79). Genomic lesions, transcriptionally confirmed, showed archetypical NOTCH1 (g.chr9:136496196 CAG>C, COSMIC COSV53024776) and SF3B1 (g.chr2:197402110 T>C, COSV59205318) point mutations, and ASXL1 (g.chr20:32435175 T>TA) frameshift in C-terminal exon 12/13 (Figure 2B, red, and Figure 2C). At the DNA level, allelic and chromosomal imbalances were observed in both patients, including 13q14 deletions, concordant with cytogenetics. Additional q-arm loss from 10q23 (e.g., PTEN), monoallelic deletion of ATM (11q21–q24), and the IGH locus from 14q32 were identified for Pt 2. These aberrations were collectively stable 16 months after initiating ibrutinib treatment. No apparent resistant-related mutations, e.g., in BTK or PLCG2, were detected.
As no clonal progression was indicated at the DNA or RNA level, we turned to the single-cell resolution of the B cell compartment (Figure 3A). Single-cell transcriptome analysis showed an expressional shift from baseline to follow-up with significant overlap in biological pathways related to apoptosis, cellular response to stress, and canonical NF-κB signaling transduction specific with significantly increased expression profiles at follow-up (Figure 3B,C). Bulk RNA sequencing of the coding transcriptome quantitatively confirmed this increased expression of apoptotic markers (Figure 3D).
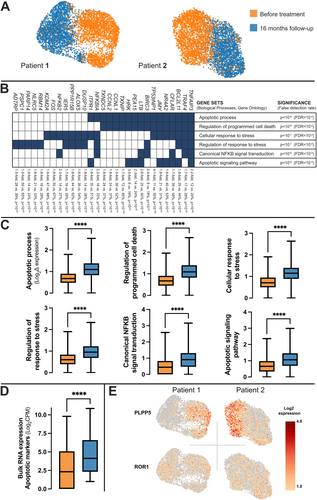
While the gene expression of the CLL-marker ROR1, was unaltered at both time points (Figure 3E), the Phospholipid Phosphatase 5 (PLPP5) gene was the most significantly altered gene (6.6-fold decrease, 46% of cells vs. 12% positivity at follow-up, PU-test < 10−100, Figure 3E). Furthermore, FCRL5 (−3.2x, 53% vs. 29%, p < 10−100), a negative regulator for the B cell receptor [35] acting by recruiting PTPN6/SHP1 [36, 37] and inhibiting B cell activation, was among the most significant genes from single-cell analyses. In contrast, NFKBIA (2.2x, 18% vs. 33%, p < 10−66), an NF-κB pathway inhibitor, and antiproliferative/proapoptotic TP53INP1 (4.1x, 11% vs. 35%, p < 10−100), among other relevant genes, showed highly significant increased expression at follow-up (Figure 3B).
4 Discussion
The mechanism of action of ibrutinib and other tyrosine kinase inhibitors can be distinguished by its direct inhibition downstream of the central B cell receptor pathway, where, instead of inducing CLL cell death, it causes a change in cellular turnover and tissue-specific redistribution of CLL cells into the peripheral blood. Following a noticeable rise in lymphocytes, the population of malignant cells reaches a steady state, which may take months to achieve clinically [2]. Thus, cellular stabilization seems an appropriate mechanistic label. However, several clinically relevant questions remain unanswered. Among these, whether all the B cells are clonally and molecularly stable and how these malignant B cells could be defined, evaluated, and monitored.
Our data underscore the efficacy of ibrutinib, reflecting a significant, although partial, clinical remission in both patients—with a general absence of CLL symptoms and a reduction in absolute lymphocyte counts. Notably, Pt 1 exhibited a marked clinical response after 16 months, while Pt 2 exhibited normal lymphocyte counts. Flow cytometry confirmed the presence of monoclonal B cells in both blood and bone marrow, and subsequent analyses revealed a high and stable clonal CLL burden in all samples. Both patients continued ibrutinib therapy due to its sustained efficacy in controlling disease progression and alleviating symptoms. These observations align with early reports by Woyach et al. [38] and others [1-3], who identified lymphocytosis as a feature of ibrutinib treatment.
However, the results also raise the question of whether clonal ibrutinib-induced lymphocytosis can be considered transient at the molecular level. The persistence of CLL cells during ibrutinib treatment, characterized by stable clonal populations and stable genetic aberrations, presents significant management challenges. This cellular stability suggests that while ibrutinib effectively reduces the clinical burden of CLL, it does not necessarily impact the underlying clonal and genetic drivers of the disease, nor is any progression evident here. Therefore, it is critical to understand not only the observed lymphocytosis, as highlighted in previous studies [2], but also the implications of clonal lymphocytosis in patients with normal lymphocyte counts.
A magnetic negative selection strategy was used to isolate B cells, thereby minimizing the risk of activation or loss of malignant cells that could be associated with positive selection methods. This approach enables the isolation of both normal and malignant B cells. Clonality assessment through IGH VDJ analyses was thus performed to confirm that the dominant clone consistently accounted for the majority of the total rearranged B cells in the follow-up samples from both patients. Additionally, to distinguish malignant cells from healthy B cells, single-cell analyses were conducted using ROR1 expression, a CLL-specific marker and exome sequencing incorporated a T-cell control to exclude germline mutations.
We observe an unchanged clonal architecture and persistence of specific genetic alterations present at baseline, such as mutated TP53, NOTCH1, and del(13q14). However, a change in the expression of several genes involved in apoptosis, stress response, and the canonical NF-κB signaling pathways was observed (Figure 3B–E). These modulations are potentially a direct effect of ibrutinib treatment, as also indicated by Herman et al. [39].
Interestingly, SF3B1 and ASXL1, associated with clonal hematopoiesis of intermediate potential [40], were found at a low mutational burden in the B cell compartment of Pt 2. Although the variants are putative consensus drivers, with SF3B1 c.2098A>G, p.K700E, being associated with hematological malignancies of both myeloid and lymphoid lineages and one of the most frequently mutated genes in CLL, and ASXL1 mutation mediating a C-terminal frameshift, it is intriguing that these aberrations do only constitute a limited fraction of the cells. This is in line with the suggestion by Wan et al. that SF3B1 mutations predominantly occur as subclonal events [41] with potential implications for progression. However, in our study, this mutation is present in the CD3+ control subset, albeit a lower frequency (5%), and thus left open for further interpretation.
A significant change in PLPP5 was observed. Although PLPP5 poses a potential candidate for tracking treatment response, its role is still not completely understood. In a lymphocytic context, the expression of PLPP5 is high in normal antibody-secreting cells [42, 43] and multiple myeloma [44]. Interestingly, PLPP5 is described as a common driver in several cancers, including breast, pancreatic, and small-cell lung cancer [45], and in a recent study also in triple-negative breast cancer [46]. Silencing PLPP5 in breast cancer cell lines has led to strong inhibition of tumor growth and increased apoptosis [47], making it a possible therapeutic target. Our results show a reduction of the PLPP5 gene expression at follow-up, and with partial response in the patients our results support that the observed reduction is indicative of a reduction of CLL cells. In contrast, genes like the Tumor Protein p53 Inducible Nuclear Protein 1 (TP53INP1), which has been shown to regulate apoptosis through Cyclin-Dependent Kinase inhibitor 1A (CDKN1A) and TP53 [48], have a higher expression at late follow-up (Figure 3C). Still, the molecular persistency of the CLL cells is clear, and the expression of CLL-specific genes, such as ROR1 [34], show comparable levels at baseline and follow-up (Figure 3E).
Although the response to ibrutinib treatment is generally high [14, 15], advanced research into the molecular mechanisms behind clonal lymphocytosis is necessary to understand the underlying development of resistance to ibrutinib and early molecular transformations into more aggressive cancer. Furthermore, the altered expression profiles can be a valuable factor to monitor during treatment or ibrutinib discontinuation as these potentially provide information about early disease progression. Studies have also shown that high-risk patients with del(17p) and TP53 mutations have shorter progression-free survival than patients without these genetic abnormalities [49, 50]. These high-risk mutations are associated with more aggressive disease and poorer response to treatments, highlighting the need for closer monitoring in this subset of patients. Using these multi-omic approaches to study the clonal inertia of high-risk CLL patients may help us understand the underlying cause of the poorer prognosis of these patients.
In conclusion, after 16 months of treatment with ibrutinib, the patients retain partial clinical remission with no B symptoms present. However, we show that the CLL burden in the B cell compartments remains high in both patient cases, with no indications of clonal evolution, progression, or clearance of lymphocytosis at the molecular level. Collectively, striking clonal concordance and stability are evident, genomically and transcriptionally. However, our data potentially points to altered cellular turnover and apoptotic processes. Thus, we extend the early findings that lymphocytosis is a feature of ibrutinib [2]. In addition, we show that clonal lymphocytosis persists, with genes especially involved in apoptosis upregulated under ibrutinib treatment. Our methods provide a solid foundation for further research into the molecular dynamics of CLL and may inform more effective, personalized treatment strategies in the future.
Author Contributions
The study was designed by S.R.V., M.H.H. and C.G.N. The initial draft of the manuscript was written by S.R.V. The project was supervised by C.G.N., M.H.H., K.D., H.F. and K.J.-J. Laboratory procedures were performed by S.R.V and M.T. assisted in the next-generation sequencing of the samples. Data analysis was carried out by S.R.V. and O.C. Bioinformatics analysis was performed by M.H.H. Patient material and information from clinical records were provided by M.B.M., M.K.N., K.J.-J. and H.F. All authors have read and approved the manuscript.
Acknowledgments
We thank the patients for participating in the study. We thank Sara Kamuk Dahlmann for laboratory assistance and Vickie Svane Kristensen for proofreading the manuscript. We are grateful for financial support from the Danish Cancer Society (R246-A14603 and R281-16686), Helen Rudes Fond (60988), The OUH PhD Fund (R90-A4022), Dagmar Marshalls Fond, Agnes og Poul Friis Fond, Tornøes og Høyrups Fond (2304), Marie og Børge Kroghs Fond, Torben og Alice Frimodts Fond, Danish Lymphoma Group, Grosserer M. Brogaard og hustrus Mindefond Odense (5523-TL-ne), Folketingsmand J. Christensen og hustru K. Christensens Fond and Brødrene Hartmanns Fond (A37628).
Ethics Statement
The study adhered to the Declaration of Helsinki, and ethical approval was obtained from the Regional Ethics Committee for the Region of Southern Denmark (approval number S-20160069).
Consent
The authors have nothing to report.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Supporting data set is available at https://doi.org/10.7910/DVN/DKCAKM.




