Biological Findings and Clinical Outcomes in Patients Treated With R-CHOP Plus High-Dose Methotrexate as First-Line Therapy in Large B-Cell Lymphoma With Testis Involvement
Funding: The authors received no specific funding for this work.
ABSTRACT
Primary testicular lymphoma (PTL) is a rare occurrence of diffuse large B-cell lymphoma (DLBCL) that accounts for 1%–2% of all cases. Nodal DLBCL with testis involvement (DLBCL-T) and PTL are associated with poor prognosis, with high incidence of central nervous system relapse. Fifteen patients (median age 60 years) with PTL (n = 5) or DLBCL-T (n = 10) received high-dose methotrexate + R-CHOP. Overall, complete response (CR) rate was 73% and overall response rate 86%. With a 3.9-year median follow-up, 100% of patients with PTL had CR and none relapsed. On the contrary, 55% of DLBCL-T patients achieved CR among which only one was still in remission at the end of follow-up. Molecular parallels between PTL and Primary CNS Lymphoma (PCNSL) suggest shared origins, urging further research for tailored treatments and enhanced understanding of these lymphomas' biology.
1 Introduction
Primary testicular lymphoma (PTL) is a rare occurrence of diffuse large B-cell lymphoma (DLBCL) that accounts for 1%–2% of total cases [1] and the testis is the third most frequent site of localized extranodal disease [2]. PTL is described separately from other DLBCL in the WHO 2016 classification [3] and classified as primary-large B cell lymphoma of immune-privileged sites in the 5th edition of WHO classification [4, 5]. Nodal DLBCL with testis involvement (DLBCL-T) and PTL are associated with poor prognosis, with high incidence of central nervous system (CNS) relapse [6, 7], and the IPI score is poorly correlated with clinical evolution [8]. CNS relapses are mostly parenchymal and can occur up to 15 years after the initial treatment of DLBCL-T or PTL [8, 9]. CNS prophylaxis in DLBCL is highly discussed [10]. High-dose methotrexate (HD-MTX) is currently recommended for CNS prophylaxis [11-13], although recent retrospective studies show variable efficacy of HD-MTX in this setting [14-17]. However, these studies included a minority of DLBCL-T/PTL and CNS diffusing chemotherapy including HD-MTX was associated with increased survival in DLBCL-T/PTL patients [18]. There is currently no recommendation specifying the best sequence between HD-MTX and standard chemotherapy like R-CHOP (concomitant or successively) or the number of cycles in DLBCL-T/PTL. HD-MTX is associated with non-negligible toxicities and the risk of delaying chemotherapy [19, 20].
In this study, we retrospectively analyzed the frontline use of HD-MTX associated with chemotherapy (R HD-MTX CHOP) for CNS prophylaxis in PTL or DLBCL-T patients. The aim of the study is to describe the efficacy and tolerance of this association in this setting.
2 Material and Methods
Patients treated with R-CHOP and HD-MTX in first-line for DLBCL-T or PTL in our hospital between 2012 and 2020 were selected. HD-MTX was intercalated at day 0, after Rituximab perfusion and before CHOP administration at day 1. Data were collected retrospectively from histological findings of DLBCL on orchidectomy pieces and from pharmaceutical database among a total of 561 patients who received HD-MTX (over 1 g per m2) for a hematologic malignancy during the study period. Patients were excluded if they had received any prior treatment for DLBCL.
In accordance with the Helsinki declaration, all patient provided written informed consent for participation in the study. The study protocol was approved by the institutional review board of Cochin Hospital (IRB CLEP N° 2023 0126165822).
Patients were diagnosed DLBCL-T if they presented lymph nodes involvement or extra nodal involvement other than testis at diagnosis. Age-adjusted international prognostic index (aaIPI) was used to stratify patient risk at baseline [21]. All lymphomas were assessed and classified according to the lymphopath recommendations [22]. GCB/non-GCB status was determined according to the Hans classification. Ambiguous position emission tomography (PET) scanner were reviewed by a nuclear radiologist. CNS involvement was detected by systematic lumbar puncture prior to treatment with cytological and immunophenotypic analysis. None of the patients underwent brain MRI, as there were no clinical signs of neurological involvement.
Progression-free survival (PFS) was defined as the time from treatment initiation to lymphoma progression or death from any cause. Response to treatment was assessed by PET scan according to the Lugano criteria [23]. Overall survival (OS) was defined as the time from diagnosis to death. DNA extracted from tissue samples was analyzed by targeted amplicon-based Next-Generation Sequencing (NGS) of 43 genes (ARID1A, B2M, BCL2, BIRC3, BRAF, BTK, CARD11, CCND1, CCND3, CD58, CD79A, CD79B, CDKN2A, CDKN2B, CIITA, CREBBP, CXCR4, EP300, EZH2, FOXO1, GNA13, ID3, IRF4, KLF2, MEF2B, MYC, MYD88, NFKBIE, NOTCH1, NOTCH2, PIM1, PLCG2, PRDM1, SF3B1, SOCS1, STAT6, TCF3, TNFAIP3, TNFRSF14, TP53, TRAF2, TRAF3, XPO1). Coding exons of selected genes were screened by paired-end sequencing reactions of 150-bp reads on a MiSeq platform (Illumina, Paris, France). The bioinformatic analysis included the trimming of raw NGS reads (FASTQ), mapping, variant calling, variant annotation, and filtering. Somatic variant curation was performed as recommended in cancer [24].
Summary statistics, namely median, or percentages were used to describe patient characteristics across treatment groups. Categorical variables are summarized as counts and/or percentages. Continuous variables are summarized as “parametric” (means and standard deviations) or “non-parametric” (medians and interquartile ranges). For demographic description, a Chi2 test is performed for categorical variables and an ANOVA test is performed for continuous variables. All analyses were performed using R software (v4.1.1).
3 Results
3.1 Patient Outcomes
Between May 2012 and August 2020, a total of 15 patients received HD-MTX in association with R-CHOP for DLBCL involving testis in our hospital. Among them, 5 patients were treated for PTL (patients 1–5) and 10 patients for DLBCL-T (patients 6–10). Individual patient characteristics at baseline are described in Table 1. Median age at diagnosis was 60 years (41–87). DLBCL-T patients had more extranodal sites involvement and a higher aaIPI. The controlateral testis was also involved in 1/5 PTL and 3/10 DLBCL-T, i.e. about a third of all patients.
| DLBCL-T (n = 10) | PTL (n = 5) | p | ||
|---|---|---|---|---|
| Age (Median) | 59.28 (14.71) | 61.66 (8.00) | 0.744 | |
| Age > 60 | 4 (40.0) | 3 (60.0) | 0.855 | |
| Extranodal sites > 2 | 8 (80.0) | 0 (0.0) | 0.017 | |
| Kidney | 2 (20.0) | 0 (0.0) | 0.788 | |
| Adrenal gland | 2 (20.0) | 0 (0.0) | 0.788 | |
| Controlateral testis at diagnosis | 3 (30.0) | 1 (20.0) | 1.000 | |
| CNS at diagnosis | 2 (20.0) | 1 (20.0) | 1.000 | |
| aaIPI | 1.40 (0.70) | 0.20 (0.45) | 0.004 | |
| CNS-IPI | 2.70 (1.70) | 1.20 (0.84) | 0.089 | |
| Histological subtype a | Non-GC | 7 (70.0) | 5 (100.0) | 0.494 |
| GC | 2 (20%) | 0 | ||
| High grade lymphoma | 1 (10%) | 0 | ||
| Double expressors | Positive | 5 (50.0) | 1 (20.0) | 0.472 |
| Negative | 3 (30.0) | 3 (60.0) | ||
| NA | 2 (20.0) | 1 (20.0) | ||
| Double hit/triple hit | Positive | 1 (10.0) | 0 (0.0) | 0.741 |
| Negative | 6 (60.0) | 3 (60.0) | ||
| NA | 3 (30.0) | 2 (40.0) |
- a Two patients did not have available information for histological subtype according to the Hans algorithm.
Patients received a median of 4 (range 2–4) administrations of HD-MTX concomitantly with conventional R-CHOP chemotherapy (6 or 8 courses if patients in CR after 4). All patients except 2 received 3 g/m2 of methotrexate. The remaining two patients (patients 7 and 10) received respectively 1 and 1.5 g/m2 of methotrexate with R-mini-CHOP due to age. Two patients received four additional cycles of intrathecal methotrexate (patients 2 and 6) and one patient received controlateral testicular radiotherapy (30 Gy, patient 15). Median follow-up was 3.89 years for the whole cohort.
Overall response rate was 86%, with 73% (11 patients) experiencing complete remission during first-line therapy. Two patients achieved partial remission (patient 9 and 10) after 4 cycles and two patients experienced progression (patient 6 and 11).
The most common treatment-related adverse events were febrile neutropenia reported in five patients (33%). One patient had grade 4 thrombocytopenia with intracranial hemorrhage. Other grade 1–2 toxicities included mucositis, nausea, diarrhea, hepatotoxicity, and paresthesia. Two patients had delayed administration of chemotherapy (patient 10 and 11) due to infection or altered general condition. Another patient (patient 7) could not receive additional chemotherapy after 4 cycles due to infectious complications and subsequent progression. Overall, the treatment toxicity seems acceptable in this setting in accordance with the high age of our cohort (approximately 50% of the patients were 60 years or older).
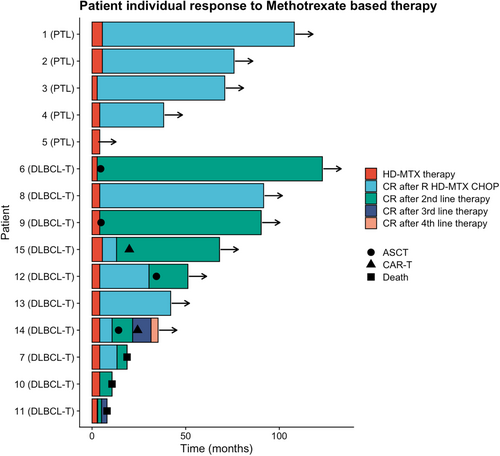
Among patients treated for PTL, none experienced relapse with a median follow-up of 6.08 years in this subgroup. Among DLBCL-T, four patients were primary refractory and four relapsed after initial complete response. Two patients experienced CNS relapse including one patient with doubt on CNS involvement at diagnosis (presence of small lymphocytes on cytology in the absence of kappa/lambda imbalance on immunophenotyping), with a median of 10.7 months from diagnosis to CNS relapse. Other sites of relapse included adrenal glands, muscle, skin, lungs, bone, thyroid gland, and lymph nodes. Two patients died of progressive disease during follow-up. Eight patients received a second-line chemotherapy allowing four of them to receive an autologous stem cell transplant. Patient 6 (progressive disease) and patient 9 (partial response) were switched after 4 cycles to RDHAX as a bridge to ASCT. Two patients received CAR T-cells in third line therapy. Individual evolution is reported on Figure 1.
3.2 Lymphoma Biology
All five PTL had non-GC phenotype, whereas 7 of 10 DLBCL-T were non-GC and one of them (patient 11) had high grade lymphoma (Table 2). Myc expression was positive in 2 among 10 assessed patients, both were double expressors. Bcl-2 and Bcl-6 expressions were assessed for all patients except one. Bcl-2 was positive in all 13 assessable patients. Bcl-6 expression was positive in 6 patients. MYC, BCL-2, and BCL-6 rearrangements were assessed for 10 patients. MYC, BCL-2, and BCL-6 rearrangements were present in 1 patient harboring triple hit lymphoma (patient 11), whereas no other patients had double hit lymphoma or significant rearrangements. Polysomy of MYC, BCL-2, and/or BCL-6 was found in 2 patients. PDL1 expression and was observed in one patient on eight analyzed patients (patient 10).
| Patient N | Type of lymphoma | Age | Stage | Extranodal sites kidney/adrenal involvement | aaIPI | CNS-IPI | Controlateral testis | LNH subtype |
|---|---|---|---|---|---|---|---|---|
| CNS involvement | ||||||||
| 1 | PTL | 70 | IIE | None | 1 | 2 | No | non-GC |
| 2 | PTL | 69 | IE | None | 0 | 1 | Yes | non-GC |
| 3 | PTL | 54 | IE | None | 0 | 0 | No | non-GC |
| 4 | PTL | 53 | IV | Small lymphocytes without detectable clonality | 0 | 2 | No | non-GC |
| 5 | PTL | 60 | IE | None | 0 | 1 | No | non-GC |
| 6 | DLBCL-T | 56 | IV | Bone marrow, CSF, adrenal gland, kidney | 2 | 4 | No | non-GC |
| 7 | DLBCL-T | 81 | IV | None | 1 | 2 | No | GC |
| 8 | DLBCL-T | 49 | IE | None | 1 | 1 | No | non-GC |
| 9 | DLBCL-T | 49 | IV | Kidney, larynx | 2 | 4 | No | GC |
| 10 | DLBCL-T | 87 | IV | Lung, adrenal gland | 1 | 4 | Yes | non-GC |
| 11 | DLBCL-T | 60 | IV | Pancreas, bowel, bone, kidney | 3 | 6 | No | GC |
| 12 | DLBCL-T | 41 | IV | Thyroid gland | 1 | 2 | No | non-GC |
| 13 | DLBCL-T | 48 | IV | Lung, peritoneum, bowel, prostate | 1 | 2 | Yes | non-GC |
| 14 | DLBCL-T | 62 | IV | Small lymphocytes without detectable clonality | 1 | 1 | Yes | non-GC |
| 15 | DLBCL-T | 56 | IV | None | 1 | 1 | No | non-GC |
| Patient N | Double-expressor | Myc FISH | Double/ triple hit | MYD88 L265P mutation | PD-L1 expression | IGH VDJ rearrangement, IGHV mutational status | Total R-CHOP administration | Total HD-MTX administration | Best response |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | Yes | 0 | VH4-34/JH1, SHM | 8 | 2 | CR |
| 2 | 0 | NA | NA | Yes | 0 | VH3-73/JH6, NA | 8 | 4 | CR |
| 3 | NA | NA | NA | NA | NA | NA | 4 | 4 | CR |
| 4 | 1 | 0 | 0 | Yes | 0 | Polyclonal | 6 | 4 | CR |
| 5 | 0 | 1 | 0 | Yes | 0 | VH3/JH4 (unproductive) | 6 | 4 | CR |
| 6 | NA | NA | NA | NA | NA | NA | 4 | 4 | PD |
| 7 | 0 | NA | NA | Yes | 0 | VH4-28/JH6 (unproductive) | 4 a | 2 (1 g/m2) | CR |
| 8 | 1 | NA | 0 | No | 0 | VH4-34/JH5, NA | 6 | 2 | CR |
| 9 | 0 | NA | 0 | Yes | 0 | VH4-34/JH4, SHM | 4 | 4 | PD |
| 10 | 1 | 0 | 0 | NA | 1 | VH3-9/JH6, SHM | 6 a | 2 (1,5 g/m2) | PR |
| 11 | NA | 1 | TH | NA | NA | NA | 4 | 1 | PD |
| 12 | 1 | NA | NA | NA | NA | NA | 6 | 4 | CR |
| 13 | 1 | 0 | 0 | NA | NA | NA | 6 | 4 | CR |
| 14 | 1 | 0 | 0 | NA | NA | NA | 6 | 4 | CR |
| 15 | 0 | 0 | 0 | Yes | 0 | VH3-7/JH1, SHM | 8 | 4 | CR |
- Abbreviations: CR: complete remission; CSF: cerebrospinal fluid; GC: germinal center; HD-MTX: high-dose methotrexate; IPI: international pronostic index; PD: progressive disease; PR: partial response; SHM: Somatic hypermutation; TH: triple hit.
- a Patients receiving R-mini-CHOP and HD-MTX due to frailty.
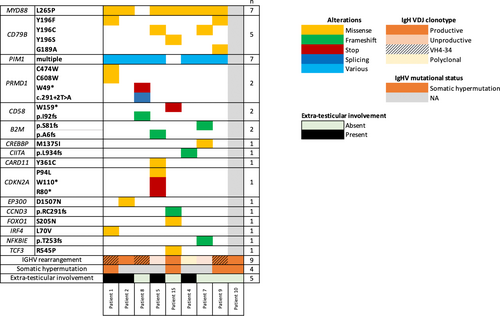
To better understand the similarity between these two entities, we performed NGS analysis using a panel targeting 43 lymphoid genes in 8/15 patients with available tissue material. Eighty-seven percent (7/8) of the patients exhibited gain of function mutation in MYD88 (MYD88 L265P) and 62% (5/8) had missense mutations in CD79B at position Y196. 7/8 patients also exhibited frequent multiple mutations in the PIM1 gene, a known target of aberrant somatic hypermutation. Of note, all patients evaluable for IGHV mutational status (n = 4) exhibited hypersomatic mutations. Non-recurrent mutations were additionally detected in various genes: PRDM1, CD58, B2M, CREBBP, CIITA, CARD11, CDKN2A, EP300, CCDND3, FOXO1, IRF4, NFKBIE, TCF3 (Figure 2). No significant differences were detected in the mutational landscape between DLBCL-T and PTL (Figure 2).
4 Discussion
PTL and DLBCL-T are rare yet serious subsets of DLBCL and are notably associated with CNS relapse. In this study, we reviewed 15 cases of DLBCL-T treated with R HD-MTX CHOP in first-line therapy. First, the treatment toxicity seems acceptable in this setting in accordance with the high age of our cohort (approximately 50% of the patients were 60 years or older). Our results are in favor of a difference in relapse and OS between PTL and DLBCL-T although we lacked power to demonstrate a statistical difference. Whilst PTL is usually associated with CNS relapse, none of our 5 PTL patients receiving HD-MTX experienced CNS relapse with a 6.08-year median follow-up. In contrast, two patients in the DLBCL-T subgroup experienced CNS relapse. In recent retrospective studies evaluating HD-MTX, specific outcome for testicular involvement is not described and no distinction between PTL and DLBCL-T is made [6, 14-16, 25]. Our study finds that HD-MTX seemed highly effective in PTL unlike DLBCL-T. Increased CNS relapse risk and overall poorer prognosis in DLBCL-T has previously been described [16] and could be related to the larger tumor burden in DLBCL-T as compared to PTL with insufficient dose-intensity for a systemic disease or to disease-specific factors. On the contrary, efficacy of HD-MTX associated to R-CHOP in PTL may be related to dose-intensity and to a higher penetration of HD-MTX through the testicular barrier and higher in-tissue concentrations than conventional chemotherapy. Results from the IELSG 30 trial (NCT00945724) evaluating intrathecal liposomal cytarabine, intraveinous methotrexate (1.5 g/m2) and controlateral testis irradiation in PTL are similar to our findings with a 5 year PFS of 88% and a 5 year OS of 92%, without any CNS relapse [26]. Our results are also consistent with a recent retrospective study showing the absence of CNS relapse at 5 years in 54 patients treated with HD-MTX for PTL [27]. A large retrospective study in DLBCL does not appear to show an increased risk of CNS relapse when methotrexate is delayed at the end of treatment (after R-CHOP) [28]. HD-MTX seems to be important in PTL but timing remains to be defined.
Our analyses of the biology of these lymphomas and data from the literature seem to point towards a common cell of origin between PTL and Primary CNS Lymphoma (PCNSL) [5]. Similarly to PCNSL, PTL is associated with a non-germinal center/activated B-cell subtype with frequent BCL6 rearrangements and MYD88/CD79b mutations in up to 75% of cases [29, 30]. In our study, 87% of patients harbored MYD88 L265P gain-of-function mutations and 62.5% of patients harbored a CD79b mutation. Moreover, our results are consistent with those of a report involving 65 patients, where MYD88, CD79B, and PIM1 mutations were among the most frequent alterations in patients with PTL or DLBCL-T [31]. In this series, there is no observable difference between the two lymphoma subtypes, except for the presence of mutations in the CD58 gene (more frequent in advanced stages) and in BTG2 (more frequent in localized stages). In our cohort, we also found two CD58 mutations in patients with extra-testicular involvement. CD58 plays a role in immune evasion and could therefore contribute to the lymphomatous spread outside immune-privileged sites [32].
Additionally, an increase in IGHV4-34 rearrangements has been previously reported in up to 70% of PCNSL and other oculocerebral lymphomas, [33-35] in favor of antigenic restriction, [36] and this rearrangement has been associated with a non-GC phenotype [37] and the presence of the MYD88 L265P mutation [34]. In our cohort, three of the 6 patients with identified IgH VDJ clonal productive rearrangement had an IGHV4-34 rearrangement suggesting an antigenic restriction bias in PTL as reported in PCNSL. Two of 3 patients with VH4-34 rearrangements had a non-GC phenotype and 2/3 patients carried the MYD88 L265P mutation.
Standard of care PCNSL currently includes HD-MTX in frontline immuno-chemotherapy [38-40]. Whether HD-MTX has a better efficacy in this cell of origin subtype has not yet been proven.
Limits of our study include its retrospective nature, a limited number of patients and the length of follow-up. Indeed, PTL is known for the occurrence of late relapses up to 15 years after initial treatment [9, 41].
Overall, this study suggests that PTL and DLBCL-T show differences both in clinical presentation and in response to conventional first-line chemotherapy with HD-MTX prophylaxis. Future studies should separate PTL and DLBCL-T into two distinct entities. HD-MTX may hold a prophylactic role for PTL relapse but further studies are needed to confirm our findings and alternative treatments should be investigated for DLBCL-T. These results also demonstrate the biological similarity between PTL and PCNSL and the need for additional studies to better understand the specific biology of this lymphoma.
Author Contributions
R.L. and L.W. conceived of the presented idea. R.L. and L.W. collected the data. R.L. and L.W. analyzed the data. B.B. and P.P. reviewed histological findings. A.C. reviewed PET-TDM imaging. E.B. and L.L. performed and analyzed NGS. L.W. and B.B. supervised the findings of the work. All authors discussed the results and contributed to the final manuscript.
Ethics Statement
This research was approved by local ethics committee.
Consent
Patients or their representatives provided written informed consent to non-interventional use of personal data.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data is available by contacting: [email protected].




