Musculoskeletal Symptoms and Misdiagnoses in Children With Acute Myeloid Leukaemia: A Nationwide Cohort Study
Funding: The authors received no specific funding for this work.
ABSTRACT
Objectives
Childhood cancer often presents with non-specific signs and symptoms that might mimic non-malignant disorders including musculoskeletal diseases, potentially leading to rheumatic and orthopaedic misdiagnoses. We aimed to compare clinical presentation, diagnostic interval and survival in paediatric acute myeloid leukaemia (AML) with and without initial musculoskeletal symptoms.
Methods
This nationwide retrospective, cohort study reviewed medical records of 144 children below 15 years diagnosed with AML in Denmark from 1996 to 2018.
Results
Musculoskeletal symptoms occurred in 29% (42/144) of children with AML and 8% (11/144) received an initial musculoskeletal misdiagnosis, being mainly non-specific and pain-related. The children with and without musculoskeletal symptoms did not differ markedly up to the diagnosis of AML and blood counts were affected equally in both groups. However, the children with prior musculoskeletal symptoms were more likely to have elevated levels of LDH and ferritin. Furthermore, they revealed a tendency towards a longer total interval (median 53 days vs. 32 days, p = 0.07), but the overall survival did not differ.
Conclusion
AML should be considered as an underlying cause in children with unexplained musculoskeletal symptoms and abnormal blood counts. Concomitant elevation of LDH and ferritin should strengthen the suspicion.
1 Introduction
Childhood cancer is rare but remains the leading cause of disease-related mortality among children and adolescents in developed countries [1, 2]. Acute leukaemia constitutes the majority of childhood cancer and can be divided into two major subgroups: acute lymphoblastic leukaemia (ALL) accounting for 80% and acute myeloid leukaemia (AML) accounting for 15%–20% [3, 4].
Childhood cancer often presents with diverse, non-specific signs and symptoms, potentially mimicking more common, non-malignant disorders [5, 6]. The diagnostics become more challenging as laboratory tests and imaging may not detect malignancy in the initial stages [7, 8]. Consequently, these difficulties in early detection can result in diagnostic delays and mistreatment, ultimately affecting survival [2, 9, 10]. The presence of musculoskeletal complaints is known to complicate the diagnostic process mainly in children with ALL, potentially leading to rheumatic and orthopaedic misdiagnoses [5, 7, 11, 12]. The common occurrence of musculoskeletal pain among children further contributes to the diagnostic challenge [13].
The prevalence of cancer in children with musculoskeletal complaints referred to paediatric rheumatology units has previously been investigated, and a final diagnosis of cancer was found in less than 1% of cases, mainly as ALL [6, 14, 15]. In ALL, the presence of musculoskeletal symptoms is well documented, usually described as diffuse bone pain, arthralgia or arthritis in the lower limbs [16, 17]. Limited research on the initial presentation of musculoskeletal symptoms in AML indicates a lower occurrence (3–19%) compared to ALL (10–35%) [11, 12, 18-21]. Notably, the findings of musculoskeletal symptoms and misdiagnosis in AML are based on mixed cohorts with very few cases of AML and the main literature is limited to case reports [22-25]. To the best of our knowledge, no previous studies have evaluated musculoskeletal symptoms and misdiagnoses in a national, non-selected cohort.
The primary objective of this study was to identify and characterize the subgroup of children with musculoskeletal symptoms prior to the diagnosis of AML and to compare their clinical presentation, diagnostic interval and survival to the remaining children diagnosed with AML without musculoskeletal symptoms. Secondly, we aimed to describe the subgroup with a musculoskeletal misdiagnosis to identify clinically important patterns or red flags.
2 Methods
2.1 Study Design
We conducted a nationwide, retrospective cohort study including all children below 15 years of age diagnosed with AML in Denmark from January 1996 to December 2018. The study population was identified from the Danish Childhood Cancer Registry (DCCR), including all children residing in Denmark below 15 years of age diagnosed according to the International Classification of Diseases, 10th edition (ICD10) [26]. This encompassed the following AML diagnoses: acute myeloblastic leukaemia (M1–M2, C920), acute promyelocytic leukaemia (M3, C924), acute myelomonocytic leukaemia (M4, C925), other myeloid leukaemia (C927), acute monocytic leukaemia (M5, C930), acute erythroid leukaemia (M6, C940) and acute megakaryoblastic leukaemia (M7, C942).
2.2 Data Collection
The study population consisted of all children diagnosed with AML, treated at paediatric haematology and oncology departments in Denmark. A total of 176 children were identified; 32 children were excluded due to misclassification, incomplete records or age above 15 years at the time of diagnosis. Thereby, the final study cohort consisted of 144 children (Figure 1). The following data were collected by systematic chart review: age, sex, date of diagnosis, AML subtype, cytogenetic abnormalities, central nervous system (CNS) involvement, presence of myeloid sarcomas, treatment, mistreatment, comorbidities, bone marrow transplantation, relapse and mortality. Furthermore, symptoms, findings at clinical examination, laboratory values, diagnostic imaging, misdiagnoses and diagnostic intervals were collected.
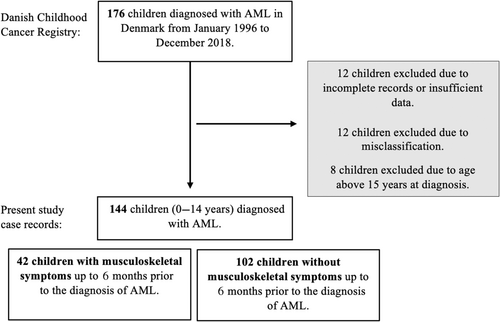
Leg pain was defined as diffuse pain in the legs, arthralgia as pain distinctly localized to one or more joints and arthritis as swelling within a joint and/or limitation of joint motion with joint pain. Musculoskeletal symptoms were defined as subjective musculoskeletal complaints occurring up to 6 months before the diagnosis of AML and later explained by the cancer. If the musculoskeletal symptoms were incorrectly attributed to another cause than cancer, it was defined as a musculoskeletal misdiagnosis.
The date of diagnosis was defined as the day a bone marrow investigation confirmed the diagnosis of AML. Cytogenetic abnormalities were categorised according to the ELN guidelines into favourable risk, intermediate risk, adverse risk and no information [27]. Laboratory values were collected both closest to first symptom and closest to diagnosis. Haematological parameters were defined as abnormal if the values were outside the age-dependent reference ranges. Additional parameters had upper limits defined as follows: uric acid >0.32 mmol/L, C-reactive protein (CRP) >8 mg/L, ferritin >120 μg/L and erythrocyte sedimentation rate (ESR) >20 mm/h. Diagnostic imaging was collected only for children with musculoskeletal symptoms.
Standardized definitions of diagnostic time intervals were utilised as illustrated in Figure S1 [28]. Two additional intervals were added: ‘The first hospital doctor interval’, representing the time from first hospital contact until the involvement of a specialist defined as a paediatric oncologist. ‘The specialist interval’, representing the duration from involvement of a paediatric oncologist until the diagnosis of AML. The specialisation of the first doctor at the hospital was recorded, with a paediatrician defined as either a resident paediatrician under the supervision of an attending paediatrician or an attending paediatrician.
2.3 Statistical Analysis
Non-normally distributed data were presented with median, interquartile range (IQR) and range. t-Tests with a bootstrapping approach (5000 replicates) were used for comparisons. Normally distributed data presented with mean, 95% confidence interval (CI) and unpaired t-tests were used for comparisons. Categorical data were tabulated by prevalence. Prevalence ratio (PR) with 95% CI was calculated for comparisons using Poisson regression models with robust variance. The Kaplan–Meier method with log-rank analysis was used to compare 1- and 5-year overall survival for children with and without prior musculoskeletal symptoms.
The statistical significance level was 5%. Statistical analyses were performed using STATA/MP version 17.
2.4 Ethics Statement
The Danish Data Protection Agency approved the study with the identification number (1-45-70-90-21). The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
3 Results
3.1 The Musculoskeletal Symptoms and Misdiagnoses
Musculoskeletal symptoms prior to the diagnosis of AML occurred in 29% (42/144), and 8% (11/144) had an initial musculoskeletal misdiagnosis (Table 1). The misdiagnoses included three cases with accidental lesions, three with reactive arthritis and three with growing pains. Further, one case was misdiagnosed with Guillain–Barré syndrome, and one case with systemic lupus erythematosus (SLE). Accidental lesions covered one case of battered child syndrome and two cases of pain associated with injuries/trauma: an ankle injury in sports and a suspected leg injury. Reactive arthritis covered three children with coxitis simplex, whereas one also had knee involvement. The remaining 31 children with prior musculoskeletal symptoms were not misdiagnosed and are referred to as musculoskeletal non-misdiagnoses.
| Musculoskeletal symptoms (n = 42) | |
|---|---|
| Musculoskeletal misdiagnosis, n | 11 |
| Accidental lesions a | 3 |
| Reactive arthritis | 3 |
| Growing pain | 3 |
| Systemic lupus erythematosus | 1 |
| Guillain–Barré syndrome | 1 |
| Musculoskeletal non-misdiagnosis, b n | 31 |
| Musculoskeletal pain | 24 |
| Arthralgia | 4 |
| Torticollis | 1 |
| Hemiparesis | 1 |
| Gait abnormalities | 1 |
| Musculoskeletal symptoms, n | 42 |
| Leg pain | 27 |
| Gait abnormalities | 9 |
| Unspecified pain | 7 |
| Arthralgia | 7 |
| Hip | 3 |
| Knee | 2 |
| Ankle | 1 |
| Pain in over extremities | 1 |
| Night-time pain | 4 |
| Arthritis | 3 |
| Back pain | 3 |
| Neck pain | 2 |
| Hemiparesis | 1 |
| Torticollis | 1 |
| Musculoskeletal symptoms as one of first presenting symptom, n | 22 |
| Leg pain | 8 |
| Arthralgia | 6 |
| Hip pain | 2 |
| Knee pain | 2 |
| Ankle pain | 1 |
| Pain in over extremities | 1 |
| Unspecified pain | 3 |
| Arthritis | 3 |
| Back pain | 1 |
| Neck pain | 1 |
- Note: Children could present with more than one musculoskeletal symptom.
- Abbreviation: n, number of observations.
- a Accidental lesions covered two injuries and one case of battered child syndrome.
- b Defined as children with musculoskeletal symptoms prior to the diagnosis of AML without a musculoskeletal misdiagnosis.
In 52% (22/42) of children with prior musculoskeletal symptoms, it was one of the first presenting symptoms, including 29% (12/42) debuting with isolated musculoskeletal symptoms. Pain in the lower limbs was the most frequent presentation with leg pain in 64% (27/42) and gait abnormalities in 21% (9/42).
3.2 The Clinical Presentation
The demographics and cancer characteristics of the children with and without musculoskeletal symptoms prior to the diagnosis of AML are compared in Table 2. The groups did not differ in age, sex, AML subtype, cytogenetic abnormalities, CNS involvement, bone marrow transplantation, sequelae, relapse or cancer-related mortality. Comorbidities were less frequent in the group with prior musculoskeletal symptoms (15% vs. 35%, PR = 0.41, 95% CI: 0.19–0.91). None of the children with musculoskeletal symptoms had Down syndrome compared to 14% (14/102) in the group without.
| Musculoskeletal symptoms (n = 42) | No musculoskeletal symptoms (n = 102) | Prevalence ratio (95% CI) | |
|---|---|---|---|
| Age (years), median (IQR) | 4.8 (2.1; 9.7) | 3.2 (1.4; 10.2) | p = 0.60 a |
| Males, n | 23 (55%) | 50 (49%) | 1.12 (0.80–1.57) |
| AML subtypes, n | |||
| Acute myeloblastic leukaemia (M1, M2) | 20 (48%) | 38 (37%) | 1.28 (0.85–1.92) |
| Acute promyelocytic leukaemia (M3) | 2 (5%) | 2 (2%) | 2.43 (0.35–16.79) |
| Acute myelomonocytic leukaemia (M4) | 6 (14%) | 20 (20%) | 0.73 (0.31–1.69) |
| Acute monocytic leukaemia (M5) | 10 (24%) | 16 (16%) | 1.52 (0.75–3.08) |
| Acute erythroid leukaemia (M6) | 0 (0%) | 6 (6%) | — |
| Acute megakaryoblastic leukaemia (M7) | 4 (10%) | 18 (18%) | 0.54 (0.19–1.51) |
| Other myeloid leukaemia | 0 (0%) | 2 (2%) | — |
| Cytogenetic abnormalities, n | 36 (86%) | 92 (90%) | 0.95 (0.83–1.09) |
| Favourable risk | 4 (10%) | 16 (16%) | 0.61 (0.21–1.72) |
| Intermediate risk | 18 (43%) | 41 (40%) | 1.07 (0.70–1.63) |
| Adverse risk | 14 (33%) | 35 (35%) | 0.97 (0.59–1.61) |
| No information | 6 (14%) | 10 (10%) | 1.46 (0.56–3.77) |
| Non-musculoskeletal misdiagnosis, n | 5 (12%) | 17 (17%) | 0.71 (0.28–1.82) |
| CNS involvement, n | 3 (7%) | 8 (8%) | 0.91 (0.25–3.28) |
| Bone marrow transplantation, n | 16 (38%) | 39 (38%) | 1.00 (0.63–1.58) |
| Comorbidity, b n | 6 (15%) | 36 (35%) | 0.41 (0.19–0.91) |
| Down syndrome | 0 (0%) | 14 (14%) | — |
| Haematological c | 1 (2%) | 4 (4%) | 0.61 (0.07–5.31) |
| Musculoskeletal d | 0 (0%) | 4 (4%) | — |
| Neurofibromatosis | 0 (0%) | 2 (2%) | — |
| Other | 5 (12%) | 14 (14%) | 0.87 (0.33–2.26) |
| Sequelae, n | 13 (32%) | 28 (28%) | 1.12 (0.65–1.94) |
| Cancer relapse, n | 13 (31%) | 29 (28%) | 1.09 (0.63–1.88) |
| Death, e n | 12 (29%) | 25 (25%) | 1.17 (0.65–2.10) |
- Note: Significant prevalance ratios are marked with bold.
- Abbreviations: AML, acute myeloid leukaemia; CI, confidential interval; CNS, central nervous system; IQR, interquartile range; n, number of observations; ‘—’, not possible to calculate prevalence ratio.
- a p value calculated by t test with a bootstrapping approach.
- b Two children had two comorbidities: Down syndrome and hypermobility and Down syndrome and preceding myeloid reaction.
- c Included four cases of primary myelodysplastic disease and one case of preceding myeloid reaction.
- d Included paresis, scoliosis and hypermobility.
- e Death related to cancer or side effects to treatment.
Non-musculoskeletal misdiagnosis occurred in 15% (22/144) of the total cohort prior to diagnosis of AML, being equally frequent among the children with and without musculoskeletal symptoms (12% vs. 17%, PR = 0.71, 95% CI: 0.28–1.82). These included various infections in 8% (12/144), idiopathic thrombocytopenic purpura (ITP) in 2% (3/144) and haemolytic–uremic syndrome in 1% (2/144). Additionally, individual cases were misdiagnosed with myelofibrosis, heart disease, constipation, skin rash and fatigue, respectively.
The clinical presentation of the children with and without musculoskeletal symptoms prior to the diagnosis of AML is compared in Table 3. In the total cohort, non-specific general symptoms were the most frequent, occurring in 96% (138/144) most often as fever followed by fatigue, infections and reduced appetite with no differences between the two groups. The children with prior musculoskeletal symptoms had a higher number of symptoms at the time of diagnosis compared to the children without (six vs. five symptoms, p = 0.002). The groups did not differ when comparing the general symptoms, gastrointestinal symptoms or objective signs overall, but the group with prior musculoskeletal symptoms had a higher prevalence of CNS symptoms (PR = 2.11, 95% CI: 1.03–4.32) and a lower prevalence of coughing (PR = 0.38, 95% CI: 0.16–0.91).
| Musculoskeletal symptoms (n = 42) | No musculoskeletal symptoms (n = 102) | Prevalence ratio (95% CI) | |
|---|---|---|---|
| General symptoms, n | 40 (95%) | 98 (96%) | 0.99 (0.92–1.07) |
| Fever | 31 (74%) | 61 (60%) | 1.23 (0.97–1.57) |
| Fatigue | 23 (55%) | 61 (60%) | 0.92 (0.67–1.26) |
| Infection | 15 (36%) | 47 (46%) | 0.78 (0.49–1.23) |
| Reduced appetite | 21 (50%) | 39 (38%) | 1.31 (0.88–1.93) |
| Bleeding tendency | 15 (36%) | 39 (38%) | 0.93 (0.58–1.50) |
| Weight loss | 13 (31%) | 20 (20%) | 1.58 (0.87–2.88) |
| Pallor | 3 (7%) | 18 (18%) | 0.40 (0.13–1.31) |
| Swollen lymph nodes | 1 (2%) | 5 (5%) | 0.49 (0.06–4.06) |
| Night-time sweats | 1 (2%) | 3 (3%) | 0.81 (0.09–7.62) |
| Other | 0 (0%) | 2 (2%) | — |
| CNS symptoms, n | 11 (27%) | 13 (13%) | 2.11 (1.03–4.32) |
| Headache | 7 (17%) | 10 (10%) | 1.70 (0.69–4.18) |
| Dizziness | 2 (5%) | 6 (6%) | 0.81 (0.17–3.87) |
| Focal neurological signs a | 6 (14%) | 0 (0%) | — |
| Cardiopulmonary symptoms, n | 11 (27%) | 45 (44%) | 0.61 (0.35–1.06) |
| Coughing | 5 (12%) | 32 (31%) | 0.38 (0.16–0.91) |
| Dyspnoea | 5 (12%) | 17 (17%) | 0.71 (0.28–1.82) |
| Other | 2 (5%) | 1 (1%) | 2.43 (0.35–16.79) |
| Gastrointestinal symptoms, n | 18 (43%) | 33 (33%) | 1.31 (0.84–2.06) |
| Abdominal pain | 9 (21%) | 16 (16%) | 1.37 (0.65–2.85) |
| Vomiting | 5 (12%) | 14 (14%) | 0.87 (0.33–2.26) |
| Diarrhoea | 4 (10%) | 9 (9%) | 1.08 (0.35–3.33) |
| Nausea | 3 (7%) | 5 (5%) | 1.46 (0.36–5.85) |
| Other | 1 (2%) | 6 (6%) | 0.40 (0.05–3.28) |
| Number of symptoms, mean (95% CI) | 5.83 (5.17–6.50) | 4.58 (4.14–5.02) | p = 0.002 b |
| Objective signs, n | 41 (98%) | 101 (99%) | 0.99 (0.94–1.04) |
| Pallor | 28 (67%) | 71 (70%) | 0.96 (0.75–1.23) |
| Lymphadenopathy | 28 (67%) | 62 (61%) | 1.10 (0.84–1.43) |
| Skin/mucosal bleeding | 16 (38%) | 53 (52%) | 0.73 (0.48–1.13) |
| Hepatomegaly | 13 (31%) | 30 (29%) | 1.05 (0.61–1.81) |
| Splenomegaly | 10 (24%) | 27 (27%) | 0.90 (0.48–1.69) |
| Arthritis | 3 (7%) | 0 (0%) | — |
| Gingival hyperplasia | 2 (5%) | 6 (6%) | 0.81 (0.17–3.87) |
| Other | 5 (12%) | 17 (17%) | 0.71 (0.28–1.82) |
| None | 1 (2%) | 1 (1%) | 2.43 (0.15–38.30) |
- Note: Significant prevalance ratios are marked with bold. Children could present with more than one symptom and objective sign.
- Abbreviations: CI, confidential interval; CNS, central nervous system; n, number of observations; ‘—’, not possible to calculate prevalence ratio.
- a Includes paresis, impaired sensation and cranial nerve palsy.
- b p Value calculated by unpaired t test.
3.3 Laboratory Values
The laboratory values of the children with and without musculoskeletal symptoms prior to the diagnosis of AML are compared in Table S1. In the total cohort, thrombocytopenia was most prevalent, followed by anaemia, elevated ferritin and lactate dehydrogenase (LDH), both at the time closest to the first symptoms and at the time of diagnosis. For the laboratory values closest to the first symptom, elevated LDH (90% vs. 74%, PR = 1.22, 95% CI: 1.01–1.47) and ferritin (100% vs. 70%, PR = 1.43, 95% CI: 1.14–1.80) were more frequent in the group with musculoskeletal symptoms. No differences were found in the remaining laboratory values. Complete blood counts were available in 32 of 42 children with prior musculoskeletal. Of these, 100% had at least one, 81% had at least two and 59% had three cell lines affected. Complete blood counts were available in 91 of 102 children without prior musculoskeletal symptoms. Of these, 99% had at least one, 89% had at least two and 57% had three cell lines affected. The laboratory values closest to diagnosis did not differ between the children with and without musculoskeletal symptoms.
3.4 Diagnostic Time Intervals
The diagnostic time intervals of children with and without prior musculoskeletal symptoms are illustrated in Table 4 and Figure S2. For this analysis, six children were excluded as their diagnosis of AML was discovered during routine ambulatory controls for ITP, Down syndrome or conditions with increased cancer risk. The results revealed a tendency towards longer parental and primary care intervals among the children with musculoskeletal symptoms. When comparing the total interval, the difference was even more pronounced with a median time of 53 days (IQR 29; 67) for the children with musculoskeletal symptoms compared to 32 days (IQR 17; 63) for children without (p = 0.07). The diagnostic intervals at the hospital were quite short in both groups, all with 75 percentiles below 1 week, with outliers in both groups. When comparing the treatment interval, a significant but clinically insignificant difference was revealed as the children without musculoskeletal symptoms had a longer treatment interval (p = 0.03). However, the median time was 1 day with a maximum range of 1 week in both groups.
| Time interval in days | Musculoskeletal symptoms (n = 42) | No musculoskeletal symptoms (n = 102) | p a | ||
|---|---|---|---|---|---|
| Median (IQR), range | N | Median (IQR), range | N | ||
| Parental interval | 21 (2; 55), 0–365 | 24 | 14 (5; 28), 0–180 | 63 | 0.34 |
| Primary care interval | 0 (0; 1), 0–23 | 26 | 1 (0; 6), 0–59 | 56 | 0.12 |
| Parental and primary care interval | 30 (7; 60), 0–365 | 39 | 22 (10; 42), 0–190 | 93 | 0.33 |
| Secondary care interval | 6 (4; 22), 1–236 | 41 | 5 (3; 9), 1–236 | 93 | 0.15 |
| First hospital doctor interval | 1 (0; 4), 0–163 | 40 | 1 (0; 1), 0–225 | 95 | 0.22 |
| Specialist interval | 3 (1; 6), 0–120 | 40 | 2 (1; 5), 0–78 | 95 | 0.14 |
| Treatment interval | 1 (0; 1), 0–6 | 41 | 1 (0; 2), 0–7 | 94 | 0.03 |
| Total diagnostic interval | 7 (4; 27), 1–236 | 25 | 8 (3; 18), 1–98 | 56 | 0.24 |
| Total interval | 53 (29; 67), 6–366 | 38 | 32 (17; 63), 5–281 | 90 | 0.07 |
- Note: Significant p values are marked with bold.
- Abbreviations: IQR, interquartile range; N, number of patients this variable was known for.
- a p values calculated by t test with a bootstrapping approach.
In the total cohort, a general paediatrician served as the first hospital doctor in 76% (109/144) of the cases. For children with prior musculoskeletal symptoms, the first hospital doctor was an orthopaedist or non-specialist in the emergency department in 12% (5/42) of the cases compared to only one case among children without musculoskeletal symptoms (PR = 12.14, 95% CI: 1.45–101.58). One child initially consulted a paediatric rheumatologist due to recurrent fever episodes.
3.5 Survival
The survival of children with and without musculoskeletal symptoms is illustrated in a Kaplan–Meier curve with 1- and 5-year overall survival in Figure S3. For all survivors, the median follow-up time was 14.8 years (IQR 9.4–19.2). For the total cohort, 26% (37/144) died due to cancer or treatment-related side effects. The overall survival did not differ between the groups (log rank, p = 0.5). The group with musculoskeletal symptoms had a 5-year survival of 71% (95% CI: 55.21–82.65) compared to 76% (95% CI: 66.68–83.39) for the children without musculoskeletal symptoms.
3.6 The Characteristics of Children With Musculoskeletal Misdiagnoses
Finally, we describe the subgroup with a musculoskeletal misdiagnosis to identify clinically important patterns or red flags. The 11 children with a prior musculoskeletal misdiagnosis were evaluated, and the main characteristics, laboratory values, diagnostic imaging, mistreatment and diagnostic time intervals are listed in Table 5. The mean age at diagnosis was 6.8 years and six were males (6/11). The main AML subtype was acute myeloblastic leukaemia (M1–M2) (5/11). Death due to the cancer or side effects of treatment occurred in three children.
| Patient (sex) | Case 1 (M) | Case 2 (F) | Case 3 (F) | Case 4 (M) | Case 5 (M) | Case 6 (F) | Case 7 (F) | Case 8 (M) | Case 9 (F) | Case 10 (M) | Case 11 (M) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | |||||||||||
| Age at diagnosis (years) | 6.3 | 3.6 | 14.2 | 2.3 | 2.4 | 7.9 | 9.8 | 13.8 | 0.4 | 1.9 | 12.4 |
| Presenting symptoms |
Pain (leg, abdominal) Fever Bleeding |
Arthritis (hip) | Arthralgia (ankle) | Arthritis (hip) |
Leg pain Fever |
Leg pain Fever |
Fever Fatigue Abdominal pain Haematuria |
Back pain | Bleeding |
Arthritis (knee, hip) Fever |
Arthralgia (knee) |
| Musculoskeletal symptoms up to diagnosis | Leg pain |
Arthritis Night-time pain |
Arthralgia Pain (leg, back) Limp a Medullary compression b |
Arthritis | Leg pain |
Pain (leg, back, night-time) Limp a |
Leg pain Arthralgia Impaired sensation (knees) |
Pain (leg, back, night-time) Limp a Medullary compression b |
Arm pain | Arthritis | Arthralgia |
| Musculoskeletal misdiagnosis | Growing pains | Coxitis simplex | Injury in sports | Coxitis simplex | Suspected injury | Growing pains | SLE | Guillain–Barré syndrome | Battered child syndrome | Reactive arthritis | Growing pains |
| Mistreatment | None | Analgesic | None | None | None | Analgesic | None | Analgesic IVIG c | None | Analgesic antibiotic | None |
| AML subtype | (M5) | (M1/2) | (M4) | (M4) | (M1/2) | (M5) | (M1/2) | (M1/2) | (M4) | (M7) | (M1/2) |
| Cytogenetic abnormalities (risk) d | Unfav. | Interm. | Interm. | Unfav. | Interm. | Interm. | Interm. | Interm. | Interm. | Interm. | Interm. |
| Number of symptoms at time of diagnosis | 4 | 7 | 7 | 2 | 2 | 10 | 9 | 4 | 4 | 5 | 8 |
| Death e | No | No | Yes | No | Yes | No | No | No | Yes | No | No |
| Diagnostic time intervals (days) | |||||||||||
| Parental interval | 7 | — | 0 | 60 | 1 | 2 | 2 | — | — | — | — |
| Primary care interval | 0 | — | 0 | 0 | 0 | 2 | 0 | — | — | — | — |
| Parental and primary interval | 7 | 60 | 0 | 0 | 1 | 4 | 2 | 30 | 21 | 30 | 90 |
| Secondary care interval | 5 | 44 | 38 | 236 | 62 | 3 | 164 | 36 | 12 | 29 | 4 |
| First hospital doctor interval | 4 | 36 | 35 | 141 | 56 | 1 | 163 | 32 | 10 | 12 | 0 |
| First hospital doctor (specialisation) f | Ped. | Ortho/ED | Ortho/ED | Ortho/ED | Ortho/ED | Ped. | Ped. | Ortho/ED | Ortho/ED | Ped. | Ped. |
| Specialist interval | 1 | 6 | 2 | 95 | 1 | 2 | 1 | 4 | 2 | 19 | 2 |
| Treatment interval | 0 | 2 | 1 | 0 | 5 | 0 | 0 | 0 | 0 | 1 | 2 |
| Total diagnostic interval | 5 | — | 37 | 236 | 57 | 5 | 164 | — | — | — | — |
| Total interval | 12 | 104 | 38 | 296 | 63 | 7 | 166 | 66 | 33 | 59 | 94 |
| Laboratory values closest to first symptom | |||||||||||
| Haemoglobin, | 5.8 | 6.3 | 6.8 | — | — | 5.7 | 7.3 | — | — | 4.7 | 4.1 |
| Platelets, | 129 | 237 | 220 | — | — | 151 | 390 | — | — | 150 | 220 |
| Leukocytes, | 232 | 6.1 | 15.7 | 17 | 4.4 | 246 | 13.3 | — | — | 2.2 | 41.5 |
| Neutrophils | 2.32 | 0.9 | — | — | 1.6 | — | 8.93 | — | — | — | 0.21 |
| LDH, | 2780 | 257 | 658 | — | — | 3300 | — | — | — | 706 | 1192 |
| Uric acid, | 0.40 | 0.16 | 0.21 | — | — | 0.32 | — | — | — | — | 0.35 |
| CRP, | 109 | 14 | 3 | 24 | — | 15 | 202 | — | — | 53 | 11 |
| ESR, | 3 | 54 | — | — | — | — | 70 | — | — | 72 | 120 |
| Ferritin, | 544 | 177 | — | — | — | — | — | — | — | — | — |
| Blasts in blood smear | Yes | Yes | Yes | — | No | Yes | — | — | — | No | Yes |
| Diagnostic imaging | |||||||||||
| X-ray | — | NAD | — | Periosteal covering in femur | Periosteal covering in femur | NAD | — | NAD | NAD | NAD | — |
| US | — | Increased synovial fluid | — | Increased synovial fluid | — | NAD | — | — | — | NAD | — |
| CT | — | — | NAD | — | — | — | — | — | — | — | — |
| MR | — | — | Epidural tumour g | Bone tumour | — | — | — | Retroperitoneal tumour h | — | Unspecific | — |
| BS | — | — | — | Pathological uptake | — | — | — | — | — | Pathological uptake | — |
- Abbreviations: , elevated values, adjusted for age; , decreased values, adjusted for age; BS, bone scintigraphy; CRP, C-reactive protein; CT, computed tomography; ER, emergency department; ESR, erythrocyte sedimentation rate; F, female; GP, general practitioner; LDH, lactate dehydrogenase; M, male; MR, magnetic resonance imaging; NAD: nothing abnormal detected; SLE, systemic lupus erythematosus. , billions per litre; , micrograms per litre; , millimetre per hour; , millimoles per litre; , units per litre; , micrograms per litre; US, ultrasonography; ‘—’, missing data or test not conducted.
- a Earlier referred to as gait abnormalities.
- b Symptoms related to medullary compression.
- c Intravenous immunoglobulins.
- d Fav., favourable risk; Interm., intermediate risk; Unfav., unfavourable risk.
- e Death related to cancer or side effects to treatment.
- f Ped.: general paediatrician; Ortho/ED., orthopaedist or non-specialist doctor in the emergency department; Ped., general paediatrician.
- g Large epidural tumour masses from vertebra level T4–T10.
- h Retroperitoneal tumour blocking medulla spinalis. Total destruction of vertebral corpus L2.
The most frequent presenting symptoms were fever (5/11), leg pain (3/11) and arthritis (3/11). Only two cases did not initially present with musculoskeletal symptoms but developed them prior the diagnosis of AML. The most frequent musculoskeletal symptom was leg pain (6/11) followed by gait abnormalities, arthritis, night-time pain and back pain each occurring in three cases. Moreover, arthralgia and signs of medullary compression occurred in two cases each.
Diagnostic imaging was performed in eight cases, revealing positive findings in seven of them. In four cases, ultrasound was performed revealing increased synovial fluid in two cases. Magnetic resonance imaging (MRI) was performed in four cases: three revealed tumour masses, two in relation to the medulla spinalis and one in the femoral bone with subsequent biopsies confirming myeloid sarcomas as part of the AML. No other children with misdiagnosis had myeloid sarcomas.
For the diagnostic time intervals, the total interval ranged from 7 to 296 days with an interval above 1 month in 82% (9/11) of the cases. This delay was primarily due to a diagnostic delay in the secondary care intervals. However, secondary care intervals also showed considerable variations, ranging from 3 to 236 days.
The laboratory values closest to first symptom revealed pancytopenia in four cases. In four cases, only one cell line was affected, most frequently as leucocytosis. Other common abnormal laboratory values included elevated CRP (7/8), LDH (5/6), ferritin (2/2) and ESR (4/5). Peripheral blood smear showed blasts in five cases (5/7). However, the amount of missing data was notable.
4 Discussion
This is the first study to evaluate the prevalence of musculoskeletal symptoms and misdiagnoses in a national, non-selected cohort of children with AML. The study revealed a high prevalence of musculoskeletal symptoms (29%) and a subgroup with musculoskeletal misdiagnoses (8%), mainly non-specific and pain-related including growing pains and injuries in half of the cases. The remaining included reactive arthritis, SLE and Guillain–Barré syndrome. The children with and without musculoskeletal symptoms did not differ markedly up to the diagnosis of AML and blood counts were equally affected in both groups. However, the children with prior musculoskeletal symptoms were more likely to have elevated levels of LDH and ferritin. Furthermore, they revealed a tendency towards a longer total interval, but the overall survival did not differ.
The prevalence of musculoskeletal symptoms prior to the diagnosis of AML ranges from 3% to 19%, which is lower compared to ALL [11, 12, 18-21]. We report a notably higher prevalence comparable to observations in ALL. This may be attributed to methodological differences in definitions of musculoskeletal symptoms and whether symptoms have been collected prior to or at the time of diagnosis. While some studies only included cases of bone and joint pain at diagnosis, our assessment of subjective symptoms 6 months prior to diagnosis encompassed a wider spectrum [18, 20]. Nevertheless, studies incorporating subjective complaints tend to report a higher estimate [18, 19, 29].
In the present study, the most frequently reported musculoskeletal symptoms were leg pain (19%), gait abnormalities (6%), arthralgia (5%) and unspecified pain (5%). This is consistent with the findings of Robazzi et al., revealing leg pain in 26%, gait abnormalities in 10% and arthralgia in 5% prior to diagnosis [19]. Robazzi et al. evaluated a mixed cohort of children with AML and ALL from a single centre in Brazil, and it is the largest study to report isolated results for children diagnosed with AML (n = 93) to date. However, they did not evaluate survival or the presence of misdiagnoses [19]. In the literature, arthritis has been found in up to 10% of children with AML [11, 19, 23]. We revealed arthritis in three children (2%). These findings may indicate tendencies towards a rheumatic pattern as observed in children with ALL, however with a lower prevalence [16, 19, 23, 30].
A recent Italian multi-centre study has investigated the prevalence of musculoskeletal symptoms in childhood cancer including 84 children with AML [11]. They stated that 71% of children with cancer and musculoskeletal complaints had at least one non-specific general symptom at presentation [11]. This aligns with our findings as 96% reported non-specific general symptoms, most frequently fever, in accordance with additional literature [11, 19, 21, 31, 32]. Doctors evaluating children with several non-specific complaints, including musculoskeletal, must be aware of the possibility of an underlying malignancy. However, identification of certain red flags was complicated by the non-specific presentation and subjective complaints.
In previous studies of acute childhood leukaemia, children with prior musculoskeletal symptoms tend to exhibit less affected blood counts and absence of peripheral blasts at initial presentation compared to children without musculoskeletal symptoms [11, 14, 16, 18, 20, 33]. We did not observe a similar pattern in children with AML as all children demonstrated markedly affected laboratory values at presentation. Most prevalent was thrombocytopenia, followed by anaemia, elevated ferritin and LDH. Previous studies also reported anaemia, thrombocytopenia and elevated LDH as initial prominent parameters in acute childhood leukaemia [21, 34]. Notable, among children with prior musculoskeletal symptoms, we identified a higher prevalence of elevated LDH and ferritin. LDH is a marker of high cell turnover while ferritin serves as a non-specific marker of inflammation [35, 36]. Elevated levels of LDH have previously been reported in children with cancer and prior musculoskeletal symptoms, suggesting it as a predictive factor for malignancy [6, 14, 16, 37]. Early elevation of LDH may be explained by an increased presence of leukaemic infiltrates in children with musculoskeletal symptoms, causing inflammation and higher cell turnover. This suggests that inflammation contributes to musculoskeletal pain. Therefore, elevation of LDH and ferritin might be indicators of AML in children with musculoskeletal symptoms, when observed concomitantly with haematological abnormalities. However, several other theories exist regarding the occurrence of musculoskeletal symptoms, including malignant cell infiltration of the joints, bones, bone marrow or muscles, presence of intra- or peri-articular bleeding as well as paraneoplastic effects [6, 11, 16].
The presence of musculoskeletal symptoms has previously been reported to cause a diagnostic delay in childhood cancer [12, 32]. The present study is the first to compare diagnostic time intervals using a standardised definition in a cohort of children with AML [38]. The time from first symptom until diagnosis has previously been reported as 1 month in children with AML which is comparable to the group without musculoskeletal symptoms in the present study [32, 39]. Notably, we found a tendency towards a longer total interval in children with AML and musculoskeletal symptoms of 53 days which corresponds to the findings by Robazzi et al., revealing a total interval of 65 days for children with AML and initial musculoskeletal complaints [19]. In mixed cohorts of AML and ALL, the total interval ranges from 30 to 52 days among children with prior musculoskeletal symptoms [18, 21]. Thus, comparison is difficult as the literature mainly contains ALL, which has been reported to have a shorter median time to diagnosis [9].
The present study found no significant differences in survival between children with and without prior musculoskeletal symptoms. This is in alignment with literature investigating the influence of musculoskeletal symptoms on survival in children with cancer [12, 18, 29]. Nevertheless, these outcomes are predominantly influenced by cases of ALL and various other types of cancer.
Children with Down syndrome were only present in the group without musculoskeletal symptoms. AML associated with Down syndrome has a favourable prognosis, despite an increased risk of treatment-related toxicity [40]. This may have improved the overall survival of this group as 14% had Down syndrome. The absence of musculoskeletal symptoms among children with Down syndrome may be attributed to their distinct AML pathogenesis that often initially mimics myelodysplastic syndrome [40]. Other contributing factors could be congenital and communicative limitations.
The survival of children with AML has increased during the last decades. The global 5-year survival ranges from 33% to 78% [41-43]. We revealed a 5-year survival of 69% and 74% in children with and without musculoskeletal symptoms, respectively. This might reflect the collaboration of the Nordic Society of Paediatric Haematology and Oncology (NOPHO) that facilitates AML treatment after international protocols [44].
Several studies indicate that prior musculoskeletal symptoms may lead to a misdiagnosis in 3–13% of cases before the diagnosis of acute childhood leukaemia [11, 18, 19, 31]. This study observed musculoskeletal misdiagnoses in 8% supporting the presence in children with AML. However, comparison poses a challenge as existing literature is based on mixed cohorts with an unequal representation of ALL and AML. Juvenile idiopathic arthritis (JIA), arthralgia and reactive arthritis are frequently reported misdiagnoses in children with ALL [30]. Similarly, case reports have previously demonstrated JIA, arthritis, acute osteomyelitis, discitis and septic arthritis as misdiagnoses in AML [14, 22-25]. Among these, we only identified few cases of reactive arthritis and arthralgia. Most misdiagnoses were non-specific and pain-related, including accidental lesions with contact to the emergency department, as reported in the literature [24, 31].
Significant strengths of this study include its population-based nationwide design and the utilization of a non-selected cohort identified through the DCCR over a 23-year period [26]. Another strength is the free and equal access to tax-supported healthcare in Denmark, thereby minimizing the potential impact of socioeconomic status [45].
The following limitations are important for interpreting the results. Firstly, AML is a rare disease among children leading to few cases and small subgroups, which may limit the statistical precision. Secondly, retrospective evaluation of the clinical presentation based on doctors' notes from medical charts may introduce recall bias, particularly for time intervals and symptoms. Furthermore, the validity of the study is also limited by the 23-year span. Nevertheless, the diagnostic criteria mainly remained unchanged during the period, although refinements in diagnostic genetics occurred. Therapy has improved survival close to 80% [46], but children with musculoskeletal symptoms were equally distributed throughout the 23 years. Lastly, information from primary care is lacking, presumably underestimating the number of prior musculoskeletal diagnoses.
5 Conclusion
In a national non-selected cohort of children with AML, musculoskeletal symptoms occurred in 29% resulting in musculoskeletal misdiagnoses in 8% of the total cohort. This prevalence is higher than reported earlier for AML. The subgroup with musculoskeletal symptoms had a non-specific clinical presentation, markedly affected blood counts and a tending delayed time to the diagnosis of AML, though not affecting overall survival. In conclusion, AML should be considered as an underlying cause in children with unexplained musculoskeletal symptoms and abnormal blood counts. Concomitant elevation of LDH and ferritin should strengthen the suspicion of AML.
Author Contributions
Data collection and analysis were performed by Anne Birthe Helweg Jensen, Sarah Thorius Jensen and Helene Riis Pontoppidan Andersen. Kasper Amund Henriksen assisted in data collection from Rigshospitalet, Copenhagen. The article was written with an equal contribution by Anne Birthe Helweg Jensen, Sarah Thorius Jensen and Helene Riis Pontoppidan Andersen. Supervision of the process was done by Ninna Brix and Christina Friis Jensen. All authors contributed substantially to the manuscript and critically reviewed and approved the final version.
Acknowledgements
The authors would like to thank Eigil Kjeldsen, Professor of Cancer Cytogenetics at Aarhus University Hospital, for his expertise and assistance in the interpretation of cytogenetics.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




