Prognostic impact of translocation t(11;14) and of other cytogenetic abnormalities in patients with AL amyloidosis in the era of contemporary therapies
Abstract
Objectives
Translocation t(11;14) is the most common cytogenetic abnormality in patients with systemic AL amyloidosis with prognostic and therapeutic relevance, which has not been clearly defined in the most recent therapeutic era.
Methods
We assessed its prognostic role in 146 newly-diagnosed patients who received novel agent-based treatment combinations. Event-free survival (EFS), a composite endpoint defined by hematological progression, start of a new treatment-line or death, and overall survival (OS) were the primary endpoints.
Results
Half of the patients had at least one FISH abnormality; 40% had t(11;14) which was inversely associated with other cytogenetic abnormalities. At 1, 3, and 6-month landmarks, hematologic response rates were numerically but not statistically higher in the non-t(11;14) group. Patients with t(11;14) were more frequently switched to second-line treatment within 12 months (p = .015). At median follow-up of 31.4 months, t(11;14) was associated with shorter EFS [17.1 (95% CI 3.2–10.6) vs. 27.2 months (95% CI 13.8–40.6), p = .021] and retained its prognostic significance in the multivariate model (HR:1.66, p = .029). The effect on OS was neutral, possibly due to the use of effective salvage therapies.
Conclusions
Our data support the use of targeted therapies for patients with t(11;14) to avoid delays in the achievement of deep hematologic responses.
Novelty statements
What is the NEW aspect of your work?
We have assessed the prognostic role of translocation t(11;14) in a non-transplant cohort of patients with systemic AL amyloidosis who received novel agent-based treatment combinations.
What is the CENTRAL finding of your work?
Translocation (11;14) has a negative prognostic effect on event-free survival in AL amyloidosis but the effect on overall survival is neutral possible due to the use of effective salvage therapies for patients that do not achieve satisfactory responses to first-line therapy.
What is (or could be) the SPECIFIC clinical relevance of your work?
Our data support the use of targeted therapies in patients with AL amyloidosis who harbor t(11;14) since delays in the achievement of a deep hematologic response may have detrimental effects, despite the use of effective salvage therapies.
1 INTRODUCTION
Light chain amyloidosis (AL) is usually characterized by a clonal plasma cell disorder of low proliferative level, which produces immunoglobulins that form amyloid fibrils that are deposited extracellularly in vital organs, and a devastating phenotype that results from progressive organ failure. “Amyloidogenic” free light chains are skewed toward λ Ig light chain and specific Ig light chain genotypes are linked to organ tropism.1 Cardiac involvement is seen most commonly (65%–75%), followed by renal (60%–70%) and then lung, gastrointestinal, liver, soft tissue, and autonomous and peripheral nervous system involvement2-5 In multiple myeloma (MM) the chromosomal abnormalities of the plasma cell clone is an area of research due to their prognostic importance and are incorporated in the staging and risk stratification. Although the plasma cell clones in MM and AL amyloidosis share the same aberrations, their frequency is different and also have different prognostic implications. Translocation t(11;14), which brings in proximity the immunoglobulin heavy chain locus (IgH) with the oncogene cyclin D1, is the most commonly observed abnormality by iFISH in patients with AL amyloidosis,6-9 found in 40%–60% of patients, compared to a much lower frequency in myeloma (about 15%). This translocation is associated with Cyclin D1, B-cell lymphoma 1 and 2 (bcl-1 and bcl-2) overexpression, apoptosis inhibition, and increased BCL-2 to MCL-1 ratio.10 In patients with AL amyloidosis, the presence of t(11;14) has been associated with lower response rates to standard therapies based on bortezomib or IMiDs10 and with poorer prognosis9, 11 compared to patients who lack this translocation. Inferior progression and event free survival (PFS, EFS) and overall survival (OS) have been linked to a lower sensitivity to bortezomib.11 The importance of evaluating cytogenetic abnormalities and t(11;14) in particular, is clinically relevant as there is an increased understanding of differing sensitivities based on mutations status and as new therapeutic options become available. Thus, it has been proposed that patients with t(11;14) treated with regimens containing melphalan (either low dose or high-dose with autologous stem cell transplantation) may overcome the detrimental effect of the cytogenetics.9, 12 Daratumumab (an anti-CD38 monoclonal antibody) seems to be agnostic of t(11;14) when added to VCd, in a subgroup analysis of a randomized study, providing additional options.13 On the other hand, venetoclax (a bcl-2 targeting drug) may offer new options for these patients, since it is preferentially active in patients with t(11;14).14
We, therefore, aimed to assess the prognostic relevance of t(11;14), as assessed by iFISH, in a cohort of newly diagnosed patients with systemic AL amyloidosis treated without ASCT but in the more recent era, in which daratumumab was also available as primary or salvage treatment option.
2 MATERIALS AND METHODS
Per standard clinical practice, we prospectively assess cytogenetics using iFISH in all patients with AL amyloidosis. The study included 146 consecutive newly-diagnosed patients with systemic AL amyloidosis using fluorescence in situ hybridization, which were diagnosed and treated in the Department of Clinical Therapeutics, in Athens, from 2016 to 2021. The study was approved by the “Alexandra” Hospital institutional review board and followed the principles of the Declaration of Helsinki.
2.1 FISH studies
CD138-enriched chromosome-specific FISH panels for MM obtained from bone marrow aspirate samples were studied, all samples were collected at our institution. A minimum of 100 interphase nuclei per probe was required for evaluation and the threshold was set at 10% for gains, losses, and translocations.15 We report data for the following chromosomes with respective probe sets: 17 (17p13.1 and 17q21), 1 (both 1q21 CKS1B/1q23 PBX1 and 1p CHD5) and translocations involving the immunoglobulin heavy chain (IgH) and the following partners, 11q13 (CCND1), 4p16.3 (FGFR3), and 16q23 (MAF). In analogy to MM t(4;14), t(14;16) and deletion 17p13 were subsumed as high-risk (HR) aberrations. Patients were divided into subgroups based on the above FISH data, including a binary assessment of abnormal (presence of any abnormality) versus normal FISH samples.
2.2 Data collection
All laboratory values were collected at the time of diagnosis and recorded in the patients' file. Kappa (k) and lambda (λ) light chain restriction were recorded, κ/λ ratio and difference between involved and uninvolved light chains (dFLC). Organ involvement was recorded and patients were categorized as having cardiac, renal, hepatic, nervous system (autonomic and peripheral) involvement and soft tissue involvement. The diagnosis was based on tissue biopsies that demonstrated apple-green birefringence under polarized light and amyloid deposits were mostly typed with immunofluorescence or immunohistochemistry (fewer with mass spectrometry or immunoelectron microscopy). The diagnosis of organ involvement was made based on the criteria by the International Society of Amyloidosis (ISA).3, 16 Cardiac staging was based on the European modification of the Mayo 2004 staging system17, 18 cardiac response on modified ISA criteria.19 Renal staging and response were also assessed using established criteria.20 Hematological responses were recording according to the 2012 ISA criteria19 and the 2021 clarification.21
2.3 Statistical analysis
Patients' characteristics were summarized using median and range for continuous variables and frequency and percentage for categorical variables. Pairwise comparisons of clinical and hematological factors with respect to chromosomal aberrations were conducted using Wilcoxon rank-sum (Mann–Whitney) test for continuous variables and Fisher's exact test for categorical variables. Due to the limitations in the definition of progression free survival for patients with AL amyloidosis, the primary endpoints were EFS and OS. EFS was defined as the time from date of start of treatment for AL amyloidosis to any of the following (A) hematological progression (B) start of a new line of therapy due to a suboptimal hematological response (C) death. All analyses were performed on an intent-to-treat basis. OS was defined as the time from date of AL diagnosis to death from any cause, censoring those who were still alive at the date of last follow-up. Survival distributions of OS and EFS were estimated using Kaplan–Meier survival function and log-rank tests were used to evaluate the survivor functions between different groups of patients. Median follow-up time was estimated using the reverse Kaplan–Meier method. Cox proportional hazard model was used to evaluate the association between patient characteristics and EFS/OS. The statistical analysis was performed using SPSS statistics V28.0.1.
3 RESULTS
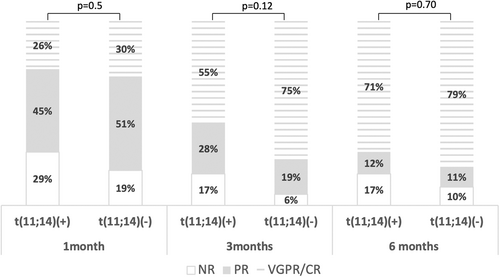
The clinical characteristics of the cohort (n = 146) are shown in Table 1. Briefly, the median age of the enrolled patients was 65 years (38–95) and 58% were males. The most frequently involved organ at diagnosis was the heart (84%), followed by kidneys (59%), nervous system (28%), soft tissue (29%), and liver (19%). In 95% of patients, first-line therapy was bortezomib-based and first-line therapy included daratumumab in 30% of patients. Half of the patients in the cohort had at least one FISH abnormality; 40% were positive for the presence of t(11;14), followed by 13q in 36% and amp/add1q21 in 28%. The rates of standard HR cytogenetic abnormalities seen in MM were considerably lower, at 2% for t(4;14), 5% for t(14; 16), and 7% for del17p. HR aberrations [t(4;14), t(14;16), del17p or + 1q21] were present in 31% of patients (n = 130). In 24% of patients, t(11;14) was the only cytogenetic aberration and 32% of patients had cytogenetic abnormalities other than t(11;14). Two or more cytogenetic abnormalities were present in 32% of patients and the median number of cytogenetic abnormalities was 1 (range 1–4). The baseline clinical profile, organ involvement patterns, and treatment regimens were comparable between patients with and without t(11;14) (Table 1); however, t(11;14) was associated with higher incidence of κ-LC amyloidosis (35% vs. 18%, p = .016; Table 1). Importantly the presence of t(11;14) was inversely associated with del13q (0.044), +1q21 (p < .001) and the presence of other HR cytogenetics (p < .001). The median percentage of positive cells for t(11;14) was 55% (range 10%–99%). There was a correlation of percentage positivity with the BM infiltration (p = .011) but there was no prognostic significance of t(11;14) percentage with any of the outcomes.
| All (n = 146) | t(11;14) (+) n = 59 | t(11:14) (−) n = 87 | p-valuea | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age (median) years | 65 (38–95) | 66 | 65 | .76 |
| Gender, male (%) | 58% | 61% | 55% | .56 |
| PS | p = .93 | |||
| 0–1 | 70% | 73% | 68% | |
| 2–4 | 30% | 27% | 32% | |
| dFLC (mg/L) | 254 | 330 | 208 | .63 |
| eGFR_MDRD (ml/min/1.73 m2 | 75.1 | 73.6 | 76.3 | .41 |
| BMPC infiltration (%) | 20% | 20% | 15% | .73 |
| Diagnosis: κLC/λLC | 26%/74% | 35%/64% | 18%/82% | .016 |
| Urine total protein, mg/24 h | 1818 | 1800 | 2583 | .59 |
| NT-proBNP, ng/L, median (range) | 2651 | 2558 | 3778 | .98 |
| Organ involvement | ||||
| Renal | 59% | 58% | 60% | All p > .05 |
| Heart | 84% | 90% | 79% | |
| Liver | 19% | 22% | 16% | |
| NS | 28% | 21% | 31% | |
| Soft tissue | 29% | 22% | 33% | |
| Median number of organs involved | 2 | 2 | 2 | .98 |
| Mayo stage 1/2/3A/3B | 13%/36%/27%/24% | 10%/39%/30%/21% | 15%/35%/24%/26% | .7 |
| Renal stage I/II/III | 48%/45%/7% | 46%/46%/8% | 50%/44%/6% | .92 |
| Treatment type (first line) | ||||
| Bortezomib-based | 95% | 93% | 95% | .47 |
| Daratumumab-containing | 30% | 27% | 32% | .28 |
| Dara @ any Tx line | 53% | 47% | 57% | .22 |
| Cardiac response | ||||
| 6 months | 47% | 52% | 44% | .55 |
| Renal response | .37 | |||
| 6 months | 44% | 40% | 54% | |
| Hematologic response | NR/PR/≥VGPR | NR/PR/≥VGPR | NR/PR/≥VGPR | |
| 1 month | 23%/29%/48% | 29%/45%/26% | 19%/51%/30% | .5 |
| 3 months | 10%/22%/68% | 17%/28%/55% | 6%/19%/75% | .12 |
| 6 months | 13%/11%/76% | 17%/12%/71% | 10%/11%/79% | .7 |
- Abbreviations: BMPC, bone marrow plasma cell; Dara@ any Tx line, treatment has included daratumumab at any treatment line; dFLC, difference between involved and uninvolved free light chain; eGFR, estimated glomerular filtration rate using MDRD formula; NS, peripheral and autonomic nervous system; NT-proBNP, N-terminal pro-B-type natriuretic peptide; PS, performance status by ECOG.
- a p values refer to the comparison between patients with present and absent t(11.14).
At 1, 3, and at 6-month landmarks hematologic response rates were numerically but not statistically higher in the non-t(11;14) group (Figure 1). However, patients with t(11;14) were more frequently switched to second-line treatment within 12 months since diagnosis for inadequate hematologic response (p = .015). In addition, at 12 months, hematologic responses (independently of line of therapy) were significantly better in the non-t(11;14) cohort (VGPR or better in 88% versus 62% in the t(11;14) positive group, p = .04). Per our clinical practice, we evaluate the presence of minimal residual disease (MRD) in patients who achieve at least VGPR with low dFLC response (i.e., dFLC <10 mg/L) after first line therapy, using next-generation flow cytometry (sensitivity at 2 × 10−6). Among the 146 patients, 74 had available data for assessment of MRD, however, only 2 out of 74 patients assessed for MRD had t(11;14). The rate of MRD negativity was 32% (23 of 72 without and 1 of those with t(11;14)).
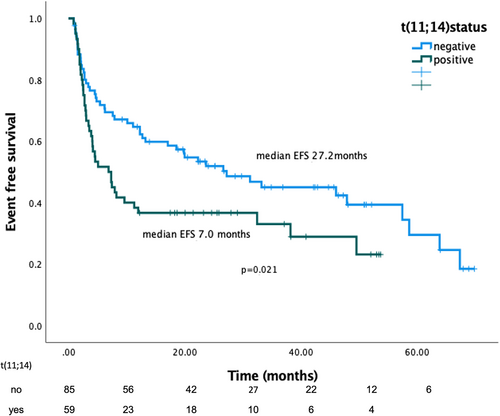
The median follow-up for the cohort was 31.4 months. At the time of data cutoff the median EFS was 17.1 months (95% CI 4.6–29.6) and was significantly shorter for patients with t(11;14) (7.0 months, 95% CI 3.2–10.6, vs. 27.2 months, 95% CI 13.8–40.6 for non-t(11;14) patients, p = .021) (Figure 2). In Table SS1, the type of events in each group are shown. Looking at the treatment type at first-line, among patients that received bortezomib-based (non-daratumumab, alkylating agent or IMiD containing regimen) (n = 77), median EFS was significantly shorter for patients with t(11;14) compared to patients without the translocation (4.6 months, 95% CI 1.1–8.1 vs. 18.6 months, 95% CI 9.1–28.0 respectively, p = .022). However, among patients who received treatment that was daratumumab, IMiD, and alkylating agent-based (+/− bortezomib) (n = 69) EFS was not significantly different based on the t(11;14) status (38.2 months, 95% CI 2.8–73.5 and 46.0 months, 95% CI 22.3–69.7 respectively, p = .92). In our cohort, 69 patients (43%) received daratumumab-based salvage treatment. Treatment was daratumumab-based at first line in 43 patients, and only 14% required salvage and change of therapy. Use of daratumumab in the first line was also associated with lower probability for and longer time to second line therapy (p < .001). In multivariate analysis, the adverse prognostic effect of t(11;14) on EFS was independent of Mayo stage, dFLC (> or ≤50 mg/L) at baseline, BMPC infiltration (≥ or <10%) and of daratumumab containing 1st-line treatment (HR:1.66, p = .029) (see Table 2). We subsequently evaluated the prognostic impact of classical “high risk”’ cytogenetics, as defined in MM patients. EFS was not statistically different for patients with or without HR cytogenetics (p = .333).
| HR | CI 95% | p | |
|---|---|---|---|
| dFLC at baseline ≤ versus >50 mg/L | 0.930 | 0.438–1.977 | .851 |
| BMPC <10 versus ≥10% | 0.795 | 0.408–1.549 | .500 |
| Dara in 1st line | 0.420 | 0.239–0.736 | .002 |
| Mayo stage | <.001 | ||
| II versus I | 1.549 | 0.678–3.538 | .300 |
| IIIa versus I | 2.586 | 1.080–6.194 | .033 |
| IIIb versus I | 5.169 | 2.092–12.768 | <.001 |
| t(11;14) (presence vs. absence) | 1.663 | 1.053–2.628 | .029 |
- Abbreviations: BMPC, bone marrow plasma cells; CI, confidence interval; Dara, daratumumab; dFLC, difference between involved and uninvolved light chain; HR, Hazard ratio.
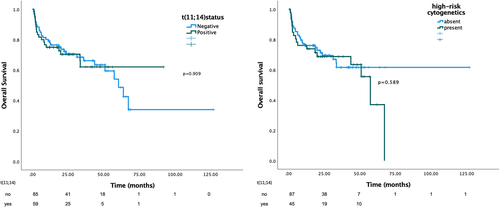
Median OS for the whole cohort was 60.4 months (95% CI 47.6–73.3 months) and the 24-month OS rate was 46%. Early mortality, defined as survival <3 months was 17% for patients harboring t(11;14) versus 16% for non-t(11;14) (p = .84). Median OS was not statistically different between patients with and without t(11;14) (p = .909) with a 24 months OS of 25% versus 49% respectively (p = .909) (see Figure 3). It remained similar between the two groups (HR:1.33, p = .70) even after adjustment for Mayo stage, dFLC (> or ≤50 mg/L) at baseline, BMPC infiltration (≥ or <10%) and of daratumumab containing 1st-line treatment (Table 3). The presence of HR cytogenetics (either +1q21, t(4;14), t(14;16), del17p) was not associated with OS (p = .589, median OS; Figure 3) and when adjusting for t(11;14) there was still no prognostic effect for OS.
| HR | CI 95% | p | |
|---|---|---|---|
| dFLC at baseline > versus ≤50 mg/L | 1.331 | 0.383–4.630 | .653 |
| BMPC <10 versus ≥10% | 0.390 | 0.170–0.893 | .026 |
| Dara in 1st line | 0.514 | 0.215–1.229 | .134 |
| Mayo stagea | <.001 | ||
| IIIa versus I and II | 2.103 | 0.905–4.887 | .084 |
| IIIb versus I and II | 13.959 | 3.713–18.144 | <.001 |
| t(11;14) (presence versus absence) | 1.133 | 0.599–2.141 | .702 |
- Abbreviations: BMPC, bone marrow plasma cells; CI, confidence interval; Dara, daratumumab; dFLC, difference between involved and uninvolved light chain; HR, Hazard ratio.
- a Mayo stages I and II were grouped together as there were no events (deaths) for Mayo stage I patients in our cohort.
4 DISCUSSION
In this study, we confirm that t(11;14) is the most common cytogenetic abnormality in patients with AL amyloidosis, seen in about 40% of patients. Furthermore, its presence was associated with a shorter EFS, especially when bortezomib-based therapy, without daratumumab, was used, however, it was not prognostic for OS. These data do not contradict previously published series,9, 22 however, they expand our current knowledge, since this a non-transplant cohort that also includes many patients who received daratumumab either at first line or as salvage (Table S2).
Previous studies have shown that t(11;14) may be associated with inferior outcomes, at least among patients treated with bortezomib-based regimens.9, 11, 23 The addition of melphalan to bortezomib or the use of HDM may have abrogated this effect, while the use of bortezomib induction followed by high-dose melphalan consolidation may have blunted the adverse prognostic effect of t(11;14) in other series.11 In our study, t(11;14) was associated with numerically lower overall hematologic responses which were also of lower quality (i.e., less than hemVGPR). This is also reflected in the lower number of patients with t(11;14) that reached a depth of response to be assessed for MRD: Per our clinical practice, we evaluate the presence of MRD in patients who achieve at least VGPR with low dFLC response (i.e., dFLC <10 mg/L) after 1st line therapy, but among 74 assessed patients only 2 had t(11;14). Although these numbers should be interpreted with caution they are indicative of the lower quality for responses in t(11;14) patients. Thus, the contemporary approach to treatment that dictates deep hematologic responses within the first few months as the main goal of therapy, has resulted in the modification of therapy in more patients with t(11;14) than without, in the first 12 months at least, as reflected by the difference on the EFS. Importantly, however, the OS was not different indicating these patients could be salvaged very effectively with 2nd lines therapy. In our cohort, 95% of patients received bortezomib-based first line therapy and 30% received daratumumab as part of first line and also many as salvage therapy (53% received daratumumab in any line). The adverse prognostic impact of t(11;14) on EFS, was independent of the use of daratumumab at first line in the MVA model (or daratumumab in any line, data not shown). In our study, salvage therapy with daratumumab was also effective, however, the detrimental effect of delays in the achievement of deep hematologic responses cannot be evaluated in this cohort. In the recent subanalysis from the ANDROMEDA study, the addition of daratumumab to VCd seemed to be able to overcome the adverse effect of t(11;14), hematologic complete response rates, and EFS favored D-VCd over VCd across all cytogenetic subgroups.13, 24 The D-VCd combination overcomes the decreased sensitivity of amyloid clones that harbor t(11;14) to bortezomib-based combinations. No biological mechanism has been proposed to suggest increased sensitivity to anti-CD38 treatment compared to clones that lack the translocation.
The biology of t(11;14) plasma cells in AL amyloidosis is intriguing. We did not identify an association of major baseline clinical and laboratory parameter with the presence of t(11;14), although there was an association with κ LC disease; this finding should be validated also in other series. The presence of t(11;14) was associated with a low-frequency of concurrent aberrations in del13q and 1q21 and of the other “classical” HR primary translocation (t(4;14), t(14;16)) and of del17p. It is known that the presence of t(11;14) is associated with increased expression of cyclin D1, B-cell lymphoma 1 and 2 (bcl-1 and bcl-2), apoptosis inhibition and increased BCL-2: MCL-1 ratio and “B-cell like” phenotype. The increased frequency of t(11;14) in AL amyloidosis may be related to the ability of these cells to sustain and survive the increased proteotoxic stress associated with the production of amyloidogenic light chains or other factors, perhaps explaining their relative “resistance” to proteasome inhibition. Plasma cells harboring t(11;14) are also very sensitive to BCL-2 inhibition, and such drugs have emerged as a promising treatment option. Venetoclax, an oral small molecular bcl-2 inhibitor leading to bcl-2 sequestration and an increased rate of apoptosis25 in plasma cells, has shown excellent activity in patients with AL amyloidosis, particularly those harboring t(11;14) and/or those who overexpress bcl-2.14, 26-29 As a result, ongoing clinical trials are assessing the efficacy of venetoclax alone (NCT05451771) or in combination regimens (NCT05486481) and other, newer, bcl-2 inhibitors are tested in patients with relapsed or refractory AL amyloidosis (NCT05199337).
This is a retrospective analysis and is subject to bias, however, patients were consecutively enrolled and treated in a single center so that treatment strategies regarding the use of salvage therapy for “inadequate’ response have remained largely unchanged. It is also notable that no patient received ASCT as part of 1st line therapy or as standard consolidation strategy, providing a more homogeneous treatment approach, with 95% receiving bortezomib-based therapy while very few had received venetoclax. Although data from a randomized study24 indicated that addition of daratumumab to VCd could overcome the effect of t(11;14) in terms of CR rates, our study provides a more unselected, real world patient population (in which 24% were at HR, i.e., stage 3B).
In conclusion, despite the negative effect on EFS, the effect of t(11;14) on OS in patients with AL amyloidosis is neutral, possibly due to the use of effective salvage therapies for patients that do not achieve satisfactory responses to first-line therapy. Nevertheless, our data support the use of more targeted therapies to patients with t(11;14) since delays in the achievement of a deep hematologic response may have detrimental effects, despite the use of effective salvage therapies.
ACKNOWLEDGMENTS
Despina Fotiou and Efstathios Kastritis designed the study, performed the analysis and wrote the manuscript. Despina Fotiou, Foteini Theodorakakou, Efstathios Kastritis, Panagiotis Malandrakis, Evangelos Eleutherakis Papaiakovou, Nikolaos Kanellias, Magdalini Migkou, Ioannis Ntanasis-Stathopoulos performed data collection and revised the manuscript. Evangelos Terpos, Meletios Athanasios Dimopoulos, Efstathios Kastritis, Charikleia Gakiopoulou, and Asimina Papanikolaou performed senior manuscript revision.
FUNDING INFORMATION
No support or funding was received for this work.
CONFLICT OF INTEREST STATEMENT
Efstathios Kastritis: Consultancy: Janssen, Pfizer, Amgen Honoraria: Janssen, Pfizer, Takeda, Genesis Pharma, Amgen Maria Gavriatopoulou; Honoraria: Janssen, Genesis Pharma, Amgen, Karyopharm, Sanofi, GSK, Takeda, Evangelos Terpos; Consultancy: Janssen, Amgen, Genesis Pharma, Celgene, Takeda, Sanofi; Honoraria: Novartis, Janssen, Takeda, Genesis Pharma, Amgen, Celgene, Sanofi, BMS, GSK; Meletios Athanasios Dimopoulos: Honoraria: Takeda, BMS, Amgen, Beigene, Janssen. Despina Fotiou; Honoraria: Janssen. Foteini Theodorakakou, Magdalini Migkou, Panagiotis Malandrakis, Nikolaos Kanellias, Ioannis Ntanasis-Stathopoulos, Evangelos Eleutherakis Papaiakovou, Asimina Papanikolaou, and Charikleia Gakiopoulou declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The full dataset is available upon request.