Research progress on the hematopoietic microenvironment in aplastic anemia
Dijiong Wu and Baodong Ye contributed as senior authors.
Abstract
Aplastic anemia (AA) is a disease of bone marrow hematopoietic failure, and the main clinical manifestation is pancytopenia. Its pathogenesis is still unclear. In recent years, more research has been done on its immune abnormalities to explain its pathogenesis and less on the hematopoietic microenvironment, but there are still some advances. This article summarizes the research on the hematopoietic microenvironment of AA in recent years to provide new ideas for the clinical treatment of AA.
Novelty statements
What is the new aspect of your work?
We review the latest literature on the pathogenesis of the abnormal bone marrow hematopoietic microenvironment in aplastic anemia (AA) and the corresponding therapies.
What is the central finding of your work?
There are significant abnormalities in the haematopoietic microenvironment in AA. Treatment of the abnormal haematopoietic microenvironment has therapeutic potential.
What is (or could be) the specific clinical relevance of your work?
Treatment of the abnormal haematopoietic microenvironment in AA offers clinicians new options in addition to the current first-line treatments.
1 INTRODUCTION
Aplastic anemia (AA) is an acquired bone marrow failure syndrome characterized by pancytopenia and bone marrow hypoplasia.1, 2 It is now widely acknowledged that deficiencies in hematopoietic stem and progenitor cells (HSPCs), immune dysregulation, abnormal bone marrow hematopoietic microenvironment, and genetic background abnormalities are the main factors in the pathology of AA.3 The incidence of AA is approximately 2.0–7.4 per million population, with the incidence in Asia being higher than that in Western countries.4-7
The first-line treatment of AA includes immunosuppressive therapy (IST) and allogeneic hematopoietic stem cell transplantation (HSCT), which is mainly based on the pathogenesis of immune dysregulation and HSPC deficiency.8 However, the severe side effects, treatment-related mortality and severe complications of HSCT, as well as the poor response and high relapse rate of IST have always been limitations and challenges of these treatment options. Therefore, it is urgent to start with the pathogenesis of AA and seek a novel approach for treating AA to meet clinical demands. In this review, we discuss recent advances related to the possible pathogenesis of AA hematopoietic microenvironment abnormalities and summarize the relevant literature published in recent years.
2 BONE MARROW HEMATOPOIETIC MICROENVIRONMENT
2.1 Composition of the bone marrow hematopoietic microenvironment
The hematopoietic microenvironment, the area in which hematopoietic stem cells (HSCs) reside, also called the “hematopoietic stem cell niche,” usually includes the endosteal niche and the perivascular niche.9 It is a multidimensional and complex system involving biochemical factors (stromal cells, microvasculature and biomolecules, etc.) and physicochemical factors (O2 concentration and extracellular matrix [ECM], etc.) that regulate HSC maintenance, self-renewal, proliferation, and differentiation.9-12 Hematopoietic stromal cells include perivascular cells, endothelial cells, osteoblasts, adipocytes, and macrophages.13, 14 Endothelial cells constitute the microvasculature, including sinusoids and arterioles.15, 16 Perivascular cells are the cells located near the microvasculature, also named mesenchymal stromal cells,15 and mainly include bone marrow mesenchymal stem cells (BMSCs), also named nestin (+) cells,17 CXC chemokine ligand 12 (CXCL12)-abundant reticular (CAR) cells,18 leptin receptor (LEPR)(+) cells,19 mast cells,20 and chondroitin sulfate proteoglycan-4 (Cspg4) (+) pericytes.16 Biomolecules include cell growth factors and cytokines, such as stem cell growth factors (SCF), thrombopoietin (TPO), angiopoietin-1 (Ang-1), FMS-like tyrosine kinase 3 ligand (Flt3-L), stromal cell-derived factor (SDF-1), granulocyte colony stimulating factor (G-CSF), interleukin-3 (IL-3), interleukin-6 (IL-6), and vascular endothelial growth factor (VEGF).21-23 The ECM mainly includes polysaccharides, proteoglycans, insoluble proteins, and other macromolecules, such as collagen, adhesin, elastin, and fibronectin.11
2.2 The main role of the bone marrow hematopoietic microenvironment
2.2.1 The role of the endosseous niche
The endosteal niche, also known as the intraosseous niche, is densely distributed with osteoblasts and osteoclasts, is important for the homing and long-term maintenance of HSCs and serves as a quiescent HSC reservoir.9 In this area, the HSCs are very close to the osteoblasts. Osteoblasts participate in HSC homing by secreting CXCL-12, G-CSF, and Ang-1 and activate the angiopoietin signaling pathway to maintain HSC survival, quiescence, and adhesion in the hematopoietic microenvironment.24 CXCL-12 secreted by osteoblasts also plays a role in the generation of early lymphoprogenitor cells.25 Osteoclasts mobilize quiescent HSCs in this inflammatory and hypoxic environment by means of CXC chemokine receptor-4 and matrix metalloproteinase-9.26 Endosteal macrophages can secrete G-CSF to promote the activation of HSCs from a quiescent state.27 Normal endosseous niche is summarized in Figure 1.
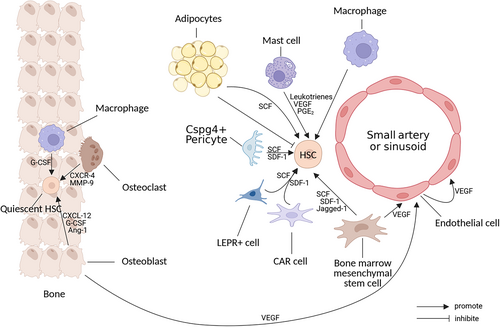
2.2.2 The role of the perivascular niche
In contrast to the endosteal niche, the perivascular niche supports the expansion and differentiation of short-term HSPCs.28 Quiescent HSCs move from the endosteal niche to the perivascular niche after activation. Perivascular cells such as BMSCs and CAR cells promote the expansion, differentiation, and recycling of activated HSCs by producing SDF-1 and SCF.29 BMSCs are the only bone marrow stromal cells that have been shown to self-renew and have the ability to differentiate into adipocytes, osteoblasts, and chondroblasts.30 BMSCs can secrete TPO, Flt3-L, SDF-1, SCF, IL-6, and other factors through paracrine mechanisms to support HSC proliferation and differentiation21, 31 and they secrete VEGF to promote microvascular system generation.32 In addition, BMSCs express several adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular cell adhesion molecule-1, and lymphocyte function-associated antigen-3, which provide immunomodulatory signals33 and participate in immune regulation by inhibiting the proliferation and activation of T lymphocytes and B lymphocytes and regulating helper T-cell (Th) differentiation.31 SDF-1 secreted by BMSCs can activate and induce the migration of HSPCs, endothelial cells, and most leukocytes.34 CAR cells, LEPR(+) cells and Cspg4(+) pericytes are a group of stromal cells that express high levels of SDF-115 and release SCF, binding to the tyrosine kinase receptor (c-kit) on the surface of HSCs prevents HSC apoptosis and promotes HSC expansion through phosphorylation activation of several signaling pathways, including Ras/Raf/Erk, PI3K, and JAK/STAT.35 Cspg4+ pericytes promote HSC quiescence and are essential for HSC population maintenance in the bone marrow.16 Mast cells can secrete angiogenic factors such as VEGF to promote mobilization of hematopoietic and/or endothelial progenitor cells and form new blood vessels in bone marrow.36 It has been reported that mast cells in the bone marrow microenvironment are able to maintain hematopoietic homeostasis and promote hematopoietic stem cell migration and homing through the release of leukotrienes and prostaglandin E2.37 Normal perivascular niche is summarized in Figure 1.
2.2.3 The role of the bone marrow microvascular system
The bone marrow microvascular system consists of endothelial cells, which form a vascular network divided into two main types: small arteries and sinusoids. Blood enters the bone marrow through the arteries and they gradually branch to form thinner arteries. The small arteries near the endosteal region connect to the downstream sinus, which returns blood into the central sinus and out of the bone marrow.38 In addition to the mechanical barrier and blood cell transport functions of the microvascular system, endothelial cells regulate HSC development, hematopoiesis, and osteogenesis by interacting with them38 and they are essential for angiogenesis of the bone marrow microvascular system.39 Osteoblasts, BMSCs, and endothelial cells activate endothelial cells by secreting VEGF and stimulating angiogenesis,40-42 as well as secreting cytokines such as SCF, SDF-1, and Jagged-1 to regulate HSC expansion and bone marrow hematopoietic function.19, 43 Conditional deletion of the gp130 cytokine receptor in endothelial cells leads to myeloid cytopenia and a decrease in the number of HSCs.44 The adhesion molecule E-selectin is expressed only by endothelial cells in the bone marrow, and it can promote HSC proliferation.45
2.2.4 The role of adipocytes
Bone marrow adipocytes (BMAs) are differentiated from BMSCs, and their proportion gradually increases with age. In long-term clinical observations, it is generally accepted that BMA is a negative regulator of hematopoiesis,46 yet there is increasing evidence that BMA promotes hematopoiesis. Studies have shown that after radiation and chemotherapy, BMA provides a source of SCF to support HSC expansion and maintenance and that adipocyte-specific deletion of SCF inhibits hematopoietic recovery.47 This result suggests that adipogenesis may be a rapid emergency response to cytopenia to promote short-term hematopoietic recovery. In addition, with the development of single-cell sequencing, studies have revealed the existence of a population of bone marrow adipogenic lineage precursors (MALPs), which maintain the bone marrow microvasculature and inhibit osteogenesis by secreting factors such as VEGF and angiopoietin 4.48 In conclusion, BMA may secrete multiple factors that affect the function of HSC and stromal cells, which potentially suggest new targets for AA therapy.
2.2.5 The role of the ECM
The ECM provides physical and structural support for almost all cell types and influences their growth, survival, and differentiation,49, 50 serving as a structural basis for water retention, mechanical cushioning, and physical scaffolding of cells,9, 51 with roles in promoting HSC expansion and homing, regulating HSC differentiation, and promoting endothelial cell development.52, 53 The physical properties of the ECM, such as its stiffness, are also involved in regulating the self-renewal and differentiation of HSCs. The adhesion and motility of HSPCs are significantly higher on high-stiffness surfaces than on low-stiffness surfaces.54 A matrix with endosteal stiffness supports HSC differentiation into primitive bone marrow progenitor cells, while a softer matrix may promote differentiation toward the erythroid lineage spectrum.49
Bone marrow is a relatively hypoxic microenvironment, and oxygen levels in the hematopoietic microenvironment are negatively correlated with the self-renewal and differentiation ability of HSCs.55 HSCs have better maintenance of quiescence and expansion potential at a 1% oxygen concentration than at a 20% oxygen concentration, which may be related to hypoxia-inducible factor-1α activation.56, 57
3 ABNORMALITIES IN THE HEMATOPOIETIC MICROENVIRONMENT OF AA
3.1 Abnormalities in bone marrow stromal cells
Bone marrow pathology and immunohistochemistry show that patients with AA have significantly reduced numbers of bone marrow stromal cells, including osteoblasts, vascular endothelial cells, BMSCs, and bone marrow central macrophages,40, 58 while the proportion of adipocytes and mast cells are significantly increased compared to healthy individuals.59 These cell reductions lead to decreased production of cytokines such as VEGF, SCF, and SDF-1 and ultimately to a decrease in circulating lymphocytes, erythroid progenitor cells, HSCs, and microvascular system.18, 32, 40
Compared to healthy individuals, BMSCs from AA patients exhibit abnormal morphology, diminished proliferative potential, increased apoptosis, abnormal differentiation potential, altered gene expression, and a reduced ability to support HSCs in vitro.32, 60-64 In addition to reduced expression of VEGF, Notch-1, Jagged-1 transcription factor GATA-2,65 basic fibroblast growth factor-2 (FGF-2),66 and VCAM-167 are all downregulated. The expression of peroxisome proliferator-activated receptor-γ (PPAR-γ),65 adiponectin Q (AdipoQ),68 and miR-144-3p69 are upregulated. AdipoQ and PPAR-γ are major regulators of adipocyte differentiation, and they play an important role in their self-renewal.70, 71 The decrease in GATA2 expression accelerates the differentiation of BMSCs into adipocytes.72 In vitro studies confirmed that BMSCs isolated from AA patients had a stronger tendency toward lipogenic differentiation and a weaker capacity for osteogenic and chondrogenic differentiation.68, 69 Downregulation of FGF-2 affects HSC stability and diminishes the pro-angiogenic effect.73, 74 Reduced VCAM-1 leads to decreased and delayed endothelial cell differentiation and bone marrow microvasculature formation.67 Transcriptome sequencing of BMSCs from AA patients also showed downregulation of many genes, including those involved in the cell cycle, cell division, proliferation, chemotaxis, mediators of hematopoietic cell interactions, adipogenesis, and immune response, and genes involved in apoptosis were upregulated.61, 68 In vitro cultured BMSCs from AA patients also showed increased apoptosis, and coculture with peripheral blood mononuclear cells (PBMCs) inhibited PBMC proliferation, which may be associated with higher concentration of IL-6, gamma-interferon (IFN-γ), tumor necrosis factor-α, and IL-1β cytokines.64 In vitro and in vivo studies have demonstrated that IFN-γ negatively affects the maintenance of BMSCs and induces senescence-like features in BMSCs.75, 76
Mast cells, SCF-dependent cells,77 increased in the BM of AA patients.78 Indeed, decreased concentration of SCF has been confirmed in SAA, which indicates the increased mast cells are a relative increasing due to the destruction of other bone marrow cells by immune factors.79 However, further studies are needed to confirm whether there is a direct correlation between the increase in mast cells and the development of AA. Abnormalities in bone marrow stromal cells of AA are summarized in Figure 2.

3.2 Abnormalities in the bone marrow microvascular system
Bone marrow microvascular density (MVD) is currently used to evaluate the abundance of the microvascular system in the bone marrow hematopoietic microenvironment.80 Several studies have shown that bone marrow MVD is significantly lower in patients with AA than in healthy humans and correlates with the severity of AA.40, 80-82 As the severity of AA increases, angiogenesis in the bone marrow decreases, possibly due to reduced VEGF expression in the bone marrow of patients with SAA and VSAA83 and reduced or abnormal function of osteoblasts, BMSCs, and endothelial cells, resulting in reduced secretion of VEGF and failure to stimulate angiogenesis.40, 42
3.3 Abnormalities in the ECM
In a chemotherapy-mediated mouse model of AA, it was found that the production of reactive oxygen species negatively affects the hematopoietic ecotone, which is regulated through the Notch-1 signaling pathway and alters the epigenetic status of HSCs, leading to HSC damage and bone marrow cell destruction.83 In turn, there are bone marrow immunohistochemical results showing a markedly lower proportion of osteonectin+ cells in specimens from AA patients than in healthy subjects,84 which suggests that ECM abnormalities may also be involved in the pathogenesis of AA.
4 THE ROLE OF THE BONE MARROW HEMATOPOIETIC MICROENVIRONMENT IN THE TREATMENT OF AA
4.1 Application of hematopoietic stromal cells
There are two sources of HSCs used for HSCT: bone marrow collection and peripheral blood collection. Current clinical studies have found a significant survival advantage and lower incidence of graft-versus-host disease after receiving HSCT with untreated bone marrow-derived HSCs compared to receiving peripheral blood-derived HSCs.85 The reason may be that untreated bone marrow provides more components of the cotransplanted hematopoietic microenvironment, such as BMSCs, endothelial cells, and osteoblasts,86 which are able to secrete more hematopoietic growth factors to stimulate HSC expansion, differentiation, and maintenance, chemokines to promote HSC homing, and VEGF to stimulate angiogenesis, and these all facilitate the restoration of the HSC niche and hematopoietic reconstitution.58, 87
4.2 Application of BMSCs
BMSCs are of clinical importance in AA. Similar to HSCT, BMSCs promotes hematopoietic improvement through very similar mechanisms (e.g., immunomodulation, release of growth factors, and osteogenic support).31 Moreover, BMSCs have immunomodulatory properties, and intravenous donor-derived BMSCs can modulate the regulatory T-cell (Treg)/helper T lymphocyte-17 balance via the Notch/RBP-J/FOXP3/RORγt pathway,88, 89 exert additional immunosuppressive effects on T lymphocytes, natural killer cells, dendritic cells and B lymphocytes, and provide a better hematopoietic microenvironment for hematopoietic recovery.90-92
BMSC transplantation can be a potential complementary option for refractory SAA, especially for AA that is nonresponsive to IST and ineligible for HSCT. A clinical trial from the National Institutes of Health (NIH) of 18 patients with IST treatment-naive AA who received donor-derived BMSCs observed an increased proportion of Treg cells, and 6 (33.3%) achieved a complete or partial response to BMSCs therapy.89 The results of another multicentre Phase II clinical trial from the NIH showed that 74 AA patients who failed to respond to IST treatment had an overall response rate of 28.4% (95% CI, 19%–40%), a complete response rate of 6.8%, and a partial response rate of 21.6% with four consecutive infusions of ex vivo expanded allogeneic bone marrow-derived BMSCs.93 Few clinical studies have used BMSCs alone to treat AA, only in severe cases that have failed to respond to HSCT or IST. These studies suggest that transplantation of BMSCs alone is safe and promising but may not be sufficient to restore bone marrow hematopoietic function in AA.
4.3 Application of biomimetics
Current studies have demonstrated the presence of a variety of biomolecules in the microenvironment to support the proliferation of HSCs that affect hematopoiesis. In previous studies, attempts to restore hematopoietic microenvironment function in AA patients using hematopoietic growth factors produced by hematopoietic stromal cells such as G-CSF, SCF, and interleukins, with the exception of TPO, have been unsuccessful.94 Several preclinical studies have confirmed the ability of TPO to stimulate HSC expansion.95-97 TPO mimetics, such as eltrombopag, hetrombopag, avatrombopag, and romiplostim, which were approved to treat immune thrombocytopenia, are used as agonists of C-MPL (TPO endogenous receptor) to treat AA.98-101 Several ongoing studies (Table 1) are evaluating the efficacy and safety of these drugs individually or in combination with standard first-line treatments.
| Drug | ClinicalTrials.gov identifier | Title | Conditions | Sponsors/collaborators | Phase | N |
|---|---|---|---|---|---|---|
| Romiplostim | NCT05323617 | Efficacy of romiplostim in treatment of SAA in adults previously untreated with or refractory to immunosuppressive therapy | Severe aplastic anemia | Amgen | 2 | 40 |
| NCT04478227 | TPO-mimetic use in children for hematopoietic failure | Aplastic anemia | Anjali Sharathkumar University of Iowa Amgen | 1 | 25 | |
| Eltrombopag | NCT03025698 | A Phase II dose-escalation study characterizing the PK of eltrombopag in pediatric patients with previously untreated or relapsed severe aplastic anemia or recurrent aplastic anemia | Aplastic anemia | Novartis Pharmaceuticals | 1/2 | 207 |
| NCT03988608 | Study to assess the safety and efficacy of eltrombopag in Chinese refractory or relapsed severe aplastic anemia (SAA) aubjects | Aplastic Anemia | Novartis Pharmaceuticals | 2 | 20 | |
| Hetrombopag | NCT05018936 | Efficacy and safety of hetrombopag in non-severe aplastic anemia | Aplastic anemia | Peking Union Medical College Hospital | 2/3 | 40 |
| NCT04961710 | Extension study of hetrombopag in severe aplastic anemia | Treatment-naive severe aplastic anemia | Jiangsu HengRui Medicine Co., Ltd. | 3 | 180 | |
| NCT05660785 | Herombopag added to cyclosporine in non severe aplastic anemia | Non severe aplastic anemia untreated | Institute of Hematology & Blood Diseases Hospital Jiangsu Hengrui Pharmaceuticals Co., Ltd | 2 | 54 | |
| Avatrombopag | NCT05720234 | Avatrombopag combined with IST as first-line treatment for SAA | Severe aplastic anemia | Institute of Hematology & Blood Diseases Hospital | 2 | 53 |
| Luspatercept | NCT05399732 | Efficacy and safety in transfusion independent non-severe aplastic anemia | Aplastic anemia | Bing Han Peking Union Medical College Hospital | 2 | 90 |
- Abbreviation: SAA, severe aplastic anemia.
- Source: Data retrieved from ClinicalTrials.gov on 23 April 2023.
4.4 Application of biologics with potential to modulate the hematopoietic microenvironment in AA
Ruxolitinib, an oral JAK1/JAK2 inhibitor, has been demonstrated to suppress the proliferation and activation of effector T cells in the bone marrow, which are the main producers of IFN-γ in AA.102 Besides, it can block JAK/STAT pathway to protect bone marrow stromal cells and HSC from impairment by IFN-γ.103
Luspatercept, a recombinant fusion protein, has been demonstrated to improve hemoglobin or transfusion burden in thalassaemia and ineffective erythropoiesis in myelodysplastic syndromes by blocking the TGF-β signalling pathway.104, 105 One study reported that Luspatercept could inhibit SDAM2/3 phosphorylation in BMSCs to reverse SDF-1 secretion, which could increase the subsequent homing of co-cultured HSPCs.106 Therefore, Han sponsored a clinical study (Table 1) to evaluate whether Luspatercept can alleviate hematopoietic failure in none sever aplastic anemia.
5 CONCLUSION AND OUTLOOK
The hematopoietic microenvironment and aplastic anemia mutually interact and influence each other. The explanation for the reduction and abnormal function of bone marrow stromal cells in AA patients is not yet clear, but related studies such as transcriptome sequencing suggest that it may be related to the genetic background, and additional studies are required to clarify it. Bone marrow stromal cells have been used clinically for the treatment of AA, but only BMSCs have been purified, expanded and identified for treatment, and there are no relevant specialized studies on other stromal cells, such as endothelial cells, osteoblasts, and reticulocytes. Moreover, the current studies on the combined transplantation of BMSCs and HSCs are mostly single-arm historical controlled trials, and there is a need to evaluate the efficacy of BMSCs combined with HSC transplantation in large multicentre two-arm studies. In addition, except TPO mimetics, there are only observational studies on the use of other biomolecules produced by the hematopoietic microenvironment, such as CXCL-12, FGF-2, VEGF, and Flt3-L, for AA treatment, without dedicated clinical studies or even in vitro cellular and animal studies. Therefore, further exploration of the pathogenesis of the abnormal bone marrow hematopoietic microenvironment of AA is expected to break the bottleneck of current therapy methods and create new hope for the clinical treatment of AA.
AUTHOR CONTRIBUTIONS
All authors contributed to the manuscript and were involved in revisions and proofreading. All authors approved the submitted version, helped in study concept, and drafting of manuscript. All authors critically revised the manuscript. Dijiong Wu and Baodong Ye supervised the study.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (NO. 82274273, 82174138), Research project Zhejiang Chinese Medical University (NO. 2022FSYYZQ06), Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (NO. 2021KY822), Zhejiang Scientific Research Fund of Traditional Chinese Medicine (NO. 2020ZB085, NO. 2022ZA059) and Health technology Plan of Zhejiang Province (NO. 2022RC216).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data sets generated for this study are included in the article.