Impact of newly diagnosed extramedullary myeloma on outcome after first autograft followed by maintenance: A CMWP-EBMT study
Abstract
Background
No adequate data exist on the impact of multiple myeloma (MM) with extramedullary disease (EMD) after autograft and maintenance therapy.
Methods
We identified 808 patients with newly diagnosed MM who received first autograft, of whom 107 had EMD (83 paraskeletal and 24 organ involvement), and who had been reported to the EBMT registry December 2018. Distribution according to type of involvement was similar between the treatment groups (p = .69). For EMD, 46 (40%) received thalidomide, 59 (51%) lenalidomide, and 11 (10%) bortezomib.
Results
The median follow-up from maintenance start was 44 months. Three-year progression-free survival (PFS) was 52% (48%–57%) for no EMD, 56% (44%–69%) for paraskeletal involvement, and 45% (22%–68%) for organ involvement (p = .146). Early PFS (within first year) appeared to be significantly worse for organ involvement (hazard ratio, 3.40), while no significant influence was found after first year from maintenance start. Three-year overall survival (OS) was 81% (77%–84%), 88% (80%–96%), and 68% (47%–89%; p = .064), respectively. With thalidomide as reference, lenalidomide was significantly associated with better PFS and OS, whereas bortezomib appeared to improve outcome specifically in EMD.
Conclusion
Lenalidomide maintenance is standard of care for MM without EMD, whereas extramedullary organ involvement remains a significant risk factor for worse outcome, especially for early events after maintenance start.
NOVELTY STATEMENTS
What is the new aspect of your work?
First comprehensive real-world analysis of impact of EMD on maintenance efficacy after upfront autologous transplant.
What is the central finding of your work?
Lenalidomide maintenance is standard of care for MM without EMD, bortezomib appeared to improve outcome in EMD.
What is (or could be) the specific clinical relevance of your work?
Extramedullary organ involvement is a cohort of unmet clinical event and studies early in disease course including upfront strategies to improve outcomes (e.g., Bortezomib-containing maintenance) are urgently needed.
1 INTRODUCTION
Multiple myeloma (MM) represents a genetically and clinically heterogeneous hematologic neoplasm with survival ranging from several months to decades.1, 2 High-dose chemotherapy with melphalan and autologous stem cell transplantation (ASCT) was established in the late 1980s and is a standard of care in newly diagnosed patients eligible for high-dose therapy.3-5 Several strategies using novel agents have been evaluated to improve response and outcome after ASCT by incorporating consolidation and maintenance therapy.
Although consolidation therapy directly after transplantation has shown deeper responses and improved long-term outcome,6 this strategy is still lacking approval. In contrast, maintenance therapy is recommended for all patients.7 Lenalidomide is considered treatment of choice while bortezomib may be considered in high-risk patients.7 Despite the improvements in survival over the past two decades, only ~15% of patients achieve expected survival when matched to the general population.8 Therefore, patient selection becomes crucial from diagnosis, to identify those who may benefit the most from specific treatments.
This may be particularly important for real-world settings where treatment scenarios may significantly differ from randomized trials well as for patients for whom evidence of current treatment options remains limited. One such subgroup is MM with extramedullary disease (EMD), defined as paraskeletal or organ involvement.9 Patients with EMD, especially those with organ manifestation, showed consistently worse outcome in comparison to patients without EMD, even in the era of novel agents.10, 11 With respect to posttransplant therapy, randomized trials only included a small proportion of EMD patients and did not report subgroup analysis.12 As a result, no adequate data exist to date on maintenance in this cohort.13
Here, we aimed to evaluate the characteristics and outcomes of newly diagnosed MM patients with or without EMD after autologous transplant and different maintenance therapies.
2 PATIENTS AND METHODS
2.1 Study design and data collection
We included adult patients with MM who had available data on extramedullary involvement at time of diagnosis who received first ASCT and who had been reported to the EBMT registry from 2009 to 2018. Patients were considered eligible for analysis if they received documented maintenance therapy (defined as single-agent therapy within 6 months after first ASCT without experience of relapse), and if there were full data available on extramedullary involvement (yes or no) at time of diagnosis, its location, and the number of sites. Single-agent maintenance was restricted to lenalidomide, thalidomide, and bortezomib to reflect current recommendation. This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Chronic Malignancies Working Party of the EBMT.
The EBMT is a nonprofit, scientific society representing more than 600 transplant centers, mainly in Europe. Data are entered, managed, and maintained in a central database with internet access. Audits are routinely performed to determine the accuracy of the data. Data on extramedullary involvement were extracted from the database using Med-B forms. Patients whose transplant data are reported provided informed consent to use the information for research purposes and data are anonymized.
2.2 End points and statistical analysis
The primary end points were 3-year progression-free survival and overall survival. Progression-free survival was defined as the time from maintenance start to disease progression or death from any cause. Overall survival was defined as the time from maintenance start to death from any cause or last follow up. Survival probabilities were estimated by the Kaplan–Meier product limit estimation method, and the Log-Rank test was used for univariate subgroup comparisons. Median follow up was determined according to the reverse Kaplan–Meier method. Non-relapse mortality was defined as death without evidence of relapse or progression, with relapse or progression as competing events. Remission, progression and relapse were defined according to standard EBMT criteria.14 Remission status was categorized as complete remission (CR), very good partial remission (VGPR), and partial remission (PR). Relapse and non-relapse mortality were analyzed together in a competing risks framework. Subgroup differences in cumulative incidences were assessed using Gray's test.
Multivariable Cox regression was applied to investigate the simultaneous impact of multiple covariates on OS and PFS, when a sufficient number of patients and subsequent events were available. Both models use the same covariate structure: involvement (paraskeletal, organ vs. no), treatment (Lenalidomide, Bortezomib vs. Thalidomide), ISS (II, III vs. I), Karnofsky score (<90 vs. ≥90), age at transplant and disease stage at transplant (PR, <PR vs. CR/VGPR). The proportional hazards assumption was verified using graphical methods. Scaled Schoenfeld residuals and graphical checks proposed by Klein and Moeschberger were performed to find evidence of violations. Violations in categorical risk factors are resolved by stratifying the corresponding hazard ratio's according to patient follow-up. Significance of individual hazard ratio's was determined by means of the Wald test. Categorical variables were compared by means of the χ2 test. Continuous variables were analyzed using the Kruskal-Wallis test. All p-values were two-sided and p < .05 was considered significant. Where necessary, to adjust for multiple comparisons, the false discovery rate was controlled by the Benjamini-Hochberg method. Statistical analyses were performed in R version 3.6.0 (R Development Core Team), using packages “survival,” “prodlim” and “cmprsk.”
3 RESULTS
3.1 Characteristics according to disease
We identified 808 patients with newly diagnosed MM who received first ASCT of whom 107 had EMD (83 paraskeletal and 24 organ involvement). Median age at time of ASCT of the total cohort was 59.4 years (95% CI, 52.5–64.4 years), with no significant difference according to type of involvement (p = .14). At time of ASCT, most patients (42%) had PR. Less patients with organ involvement had myeloma IgA (13%) in comparison to no EMD and paraskeletal involvement (21%, respectively), while more patients had light chain disease (29% vs. 18% and 24%, respectively). More patients with organ involvement had International Staging System stage III (50% vs. 24% and 15%, respectively). The remaining characteristics according to type of involvement are shown in Table 1.
| Total | No involvements | Paraskeletal | Organ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Missing | N (%) | Missing | N (%) | Missing | N (%) | Missing | N (%) | p | |
| Total | 808 (100.0%) | 692 (100.0%) | 83 (100.0%) | 24 (100.0%) | ||||||
| Treatment | Thalidomide | 287 (35.5%) | 241 (34.8%) | 33 (39.8%) | 8 (33.3%) | .694 | ||||
| Lenalidomide | 446 (55.2%) | 387 (55.9%) | 41 (49.4%) | 15 (62.5%) | ||||||
| Bortezomib | 75 (9.3%) | 64 (9.2%) | 9 (10.8%) | 1 (4.2%) | ||||||
| MM class | IgG | 9 | 462 (57.8%) | 8 | 401 (58.6%) | 1 | 41 (50.0%) | 13 (54.2%) | .04 | |
| IgA | 162 (20.3%) | 141 (20.6%) | 17 (20.7%) | 3 (12.5%) | ||||||
| light chain | 151 (18.9%) | 124 (18.1%) | 20 (24.4%) | 7 (29.2%) | ||||||
| other Ig | 12 (1.5%) | 12 (1.8%) | 0 | 0 | ||||||
| non-secretory | 12 (1.5%) | 6 (0.9%) | 4 (4.9%) | 1 (4.2%) | ||||||
| Patient sex | Male | 492 (60.9%) | 426 (61.6%) | 44 (53.0%) | 14 (58.3%) | .313 | ||||
| Female | 316 (39.1%) | 266 (38.4%) | 39 (47.0%) | 10 (41.7%) | ||||||
| Stage | CR | 15 | 143 (18.0%) | 15 | 122 (18.0%) | 16 (19.3%) | 3 (12.5%) | .97 | ||
| VGPR | 265 (33.4%) | 225 (33.2%) | 29 (34.9%) | 10 (41.7%) | ||||||
| PR | 334 (42.1%) | 286 (42.2%) | 33 (39.8%) | 10 (41.7%) | ||||||
| >PR | 51 (6.4%) | 44 (6.5%) | 5 (6.0%) | 1 (4.2%) | ||||||
| ISS | I | 155 | 261 (40.0%) | 131 | 222 (39.6%) | 17 | 28 (42.4%) | 2 | 8 (36.4%) | .015 |
| II | 235 (36.0%) | 203 (36.2%) | 28 (42.4%) | 3 (13.6%) | ||||||
| III | 157 (24.0%) | 136 (24.2%) | 10 (15.2%) | 11 (50.0%) | ||||||
| Karnofsky | ≥90 | 64 | 542 (72.8%) | 56 | 460 (72.3%) | 4 | 55 (69.6%) | 3 | 20 (95.2%) | .055 |
| <90 | 202 (27.2%) | 176 (27.7%) | 24 (30.4%) | 1 (4.8%) | ||||||
| HCT-CI | Low | 330 | 308 (64.4%) | 285 | 261 (64.1%) | 32 | 35 (68.6%) | 9 | 9 (60.0%) | .431 |
| Intermediate | 105 (22.0%) | 91 (22.4%) | 7 (13.7%) | 5 (33.3%) | ||||||
| High | 65 (13.6%) | 55 (13.5%) | 9 (17.6%) | 1 (6.7%) | ||||||
| ASCT year | Median (IQR) | 2012 (2009–2017) | 2012 (2009–2017) | 2012 (2009–2016) | 2011 (2009–2016) | .961 | ||||
| Age at ASCT | Median (IQR) | 59 (52–64) | 59 (52–64) | 56 (51–63) | 59 (54–64) | .14 | ||||
| Velcade pre | no | 16 | 209 (26.4%) | 13 | 172 (25.3%) | 1 | 29 (35.4%) | 1 | 7 (30.4%) | .138 |
| yes | 583 (73.6%) | 507 (74.7%) | 53 (64.6%) | 16 (69.6%) | ||||||
- Abbreviations: ASCT, autologous transplantation; CI, confidence interval; CR, complete remission; HCT-CI, comorbidity index; ISS, International Staging System; OS, overall survival; PFS, progression-free survival; VGPR, very good partial remission.
3.2 Characteristics according to treatment
Distribution according to type of involvement was similar between the three treatment groups (p = .69). For EMD patients, 46 (40%) received thalidomide, 59 (51%) lenalidomide, and 11 (10%) bortezomib. For no EMD, 241 (35%) received thalidomide, 387 (56%) lenalidomide, and 64 (9%) bortezomib. Median age was significantly different between treatment groups (p = .005), being 60 years for lenalidomide, 58 for thalidomide, and 57 for bortezomib. The thalidomide group received ASCT in median year of 2009, while both the lenalidomide and bortezomib group received ASCT in a median of 2015 (p < .001). Frequencies of remission status at transplant in the treatment groups (thalidomide/lenalidomide/bortezomib) was 21/16/21% for CR, 25/40/28% for VGPR, 46/39/42% for PR, and 8/5/9% for <PR (p = .003). More patients in the lenalidomide group (86%) and bortezomib group (73%) received bortezomib pretreatment before ASCT than the thalidomide group (54%; p < .001). The remaining characteristics according to maintenance therapy are shown in Table 2.
| Total | Thalidomide | Lenalidomide | Bortezomib | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Missing | N (%) | Missing | N (%) | Missing | N (%) | Missing | N (%) | p | |
| Total | 808 (100%) | 287 (100%) | 446 (100%) | 75 (100%) | ||||||
| MM class | IgG | 9 | 462 (57.8%) | 3 | 174 (61.3%) | 5 | 248 (56.2%) | 1 | 40 (54.1%) | .115 |
| IgA | 162 (20.3%) | 53 (18.7%) | 92 (20.9%) | 17 (23.0%) | ||||||
| light chain | 151 (18.9%) | 45 (15.8%) | 90 (20.4%) | 16 (21.6%) | ||||||
| other Ig | 12 (1.5%) | 3 (1.1%) | 8 (1.8%) | 1 (1.4%) | ||||||
| non-secretory | 12 (1.5%) | 9 (3.2%) | 3 (0.7%) | 0 (0.0%) | ||||||
| Involvement | No | 9 | 692 (86.6%) | 5 | 241 (85.5%) | 3 | 387 (87.4%) | 1 | 64 (86.5%) | .694 |
| Paraskeletal | 83 (10.4%) | 33 (11.7%) | 41 (9.3%) | 9 (12.2%) | ||||||
| Organ | 24 (3.0%) | 8 (2.8%) | 15 (3.4%) | 1 (1.4%) | ||||||
| Patient sex | Male | 492 (60.9%) | 180 (62.7%) | 267 (59.9%) | 45 (60.0%) | .732 | ||||
| Female | 316 (39.1%) | 107 (37.3%) | 179 (40.1%) | 30 (40.0%) | ||||||
| Stage | CR | 15 | 143 (18.0%) | 2 | 59 (20.7%) | 9 | 69 (15.8%) | 4 | 15 (21.1%) | .003 |
| VGPR | 265 (33.4%) | 71 (24.9%) | 174 (39.8%) | 20 (28.2%) | ||||||
| PR | 334 (42.1%) | 132 (46.3%) | 172 (39.4%) | 30 (42.3%) | ||||||
| >PR | 51 (6.4%) | 23 (8.1%) | 22 (5.0%) | 6 (8.5%) | ||||||
| ISS | I | 155 | 261 (40%) | 63 | 95 (42.4%) | 73 | 145 (38.9%) | 19 | 21 (37.5%) | .17 |
| II | 235 (36%) | 82 (36.6%) | 127 (34.0%) | 26 (46.4%) | ||||||
| III | 157 (24%) | 47 (21.0%) | 101 (27.1%) | 9 (16.1%) | ||||||
| Karnofsky | ≥90 | 64 | 542 (72.8%) | 15 | 206 (75.7%) | 47 | 279 (69.9%) | 2 | 57 (78.1%) | .144 |
| <90 | 202 (27.2%) | 66 (24.3%) | 120 (30.1%) | 16 (21.9%) | ||||||
| HCT-CI | Low | 330 | 308 (64.4%) | 160 | 77 (60.6%) | 145 | 196 (65.1%) | 25 | 35 (70.0%) | .79 |
| Intermediate | 105 (22.0%) | 32 (25.2%) | 64 (21.3%) | 9 (18.0%) | ||||||
| High | 65 (13.6%) | 18 (14.2%) | 41 (13.6%) | 6 (12.0%) | ||||||
| ASCT year | Median (IQR) | 2012 (2009–2017) | 2009 (2008–2011) | 2015 (2011–2018) | 2015 (2010–2017) | <.001 | ||||
| Age at ASCT | Median (IQR) | 59 (52–64) | 58 (51–62) | 60 (53–64) | 57 (52–66) | .005 | ||||
Velcade pre |
no | 16 | 209 (26.4%) | 7 | 128 (45.7%) | 9 | 61 (14.0%) | 20 (26.7%) | <.001 | |
| yes | 583 (73.6%) | 152 (54.3%) | 376 (86.0%) | 55 (73.3%) | ||||||
- Abbreviations: ASCT, autologous transplantation; CI, confidence interval; CR, complete remission; HCT-CI, comorbidity index; ISS, International Staging System; OS, overall survival; PFS, progression-free survival; VGPR, very good partial remission.
3.3 Outcomes according to disease
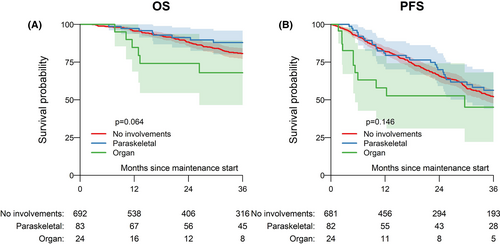
The median follow-up time from maintenance start was 44 months (95% CI, 40–48 months). The estimated 3-year progression-free survival was 52% (48%–57%) for no EMD, 56% (44%–69%) for paraskeletal involvement, and 45% (22%–68%) for organ involvement (p = .146). Of note, early outcome after maintenance start appeared to be significantly worse for organ involvement, with 1-year progression-free survival of 58% in comparison with 81% for paraskeletal involvement and 82% for no EMD (p = .002). The estimated 3-year overall survival was 81% (77%–84%) for no EMD, 88% (80–96%) for paraskeletal involvement, and 68% (47%–89%) for organ involvement (p = .064). Survival curves are depicted in Figure 1. The estimated 3-year cumulative incidence of relapse and non-relapse mortality were 45% (41%–50%) and 2% (1%–4%) for no EMD, 44% (31%–56%) and 0% for paraskeletal involvement, and 55% (32%–78%) and 0% for organ involvement (p = .13).
3.3.1 Outcomes according to treatment
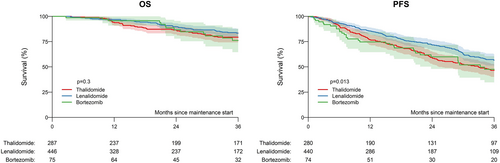
The median interval between autologous transplant and maintenance start was 2.8 months (95% CI, 2.3–4.2 months) for thalidomide, 3.6 months (95% CI, 2.7–4.6 months) for lenalidomide, and 3.2 months (95% CI, 2.2–4.1 months) for bortezomib. In the total cohort, the estimated 3-year progression-free survival according to type of single-agent maintenance after ASCT was 47% (40%–53%) for thalidomide, 57% (51%–63%) for lenalidomide, and 48% (35%–61%) for bortezomib (p = .013; Figure 2). The 3-year estimated overall survival was 79% (74%–85%) for thalidomide, 83% (79%–88%) for lenalidomide, and 76% (65%–88%) for bortezomib (p = .30). Survival curves according to treatment are depicted in Figure 1B. The estimated 3-year cumulative incidence of relapse was 52% (45%–58%) for thalidomide, 41% (35%–47%) for lenalidomide, and 50% (38%–63%) for bortezomib (p = .006). The estimated 3-year non-relapse mortality was 2% (0–3% for thalidomide, 2% (1%–4%) for lenalidomide, and 2% (0–6%) for bortezomib (p = .80). Best response rates after start of maintenance were significantly different between the three groups, with more patients receiving lenalidomide and bortezomib showing complete response (Table 3).
| Thalidomide | Lenalidomide | Bortezomib | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Missing | N (%) | Missing | N (%) | Missing | N (%) | p | |
| Total | 97 (100%) | 237 (100%) | 35 (100%) | |||||
| Best response | sCR/CR | 0 (0.0%) | 29 (29.9%) | 2 (0.8%) | 99 (42.1%) | 1 (2.9%) | 14 (41.2%) | .006* |
| VGPR | 26 (26.8%) | 80 (34.0%) | 7 (20.6%) | |||||
| PR | 42 (43.3%) | 56 (23.8%) | 13 (38.2%) | |||||
| Time to response | Median (IQR) | 1.1 (0.4–2.1) | 1.4 (0.6–2.5) | 1.2 (0.4–3.2) | .074 | |||
- * Conditional on having responded and best response occurred after maintenance treatment.
- Abbreviations: CR, complete remission; VGPR, very good partial remission.
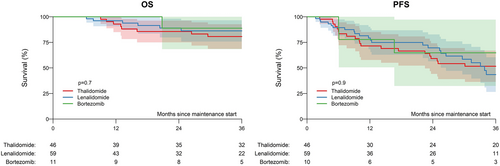
In patients with EMD, estimated 3-year progression-free survival was 52% (36%–67%) for thalidomide, 43% (27%–60%) for lenalidomide, and 65% (32%–97%) for bortezomib (p = .90). In terms of overall survival, estimated 3-year rates were 81% (69%–93%) for thalidomide, 86% (76%–97%) for lenalidomide, and 89% (68%–100%) for bortezomib (p = .70; Figure 3).
3.3.2 Other factors on outcome
In terms of progression-free survival, International Staging System and ASCT year showed impact on outcome. Estimated 3-year rates were 61% (54%–68%) for stage I, 43% (35%–50%) for stage II, and 51% (41%–61%) for stage III (p = .008), and 50% (45%–55%) for ASCT 2008–2011, 49% (40%–58%) for 2012–2015, and 59% (49%–70%) for 2016–2018 (p = .08). In terms of overall survival, the interval between diagnosis and first ASCT showed estimated 3-year overall survival rates of 85% (81%–90%) for 0–6 months, 78% (72%–83%) for 6–12 months, and 79% (71%–87%) for more than 12 months (p = .09). The complete univariate analysis is shown in Table S1.
3.3.3 Multivariable analysis
We then developed a multivariable model using Cox regression to adjust for confounding effects of other factors on outcome and clinically significant variables (including maintenance, type of involvement, ISS, performance status, age, and remission status at time of autograft).
For progression-free survival, the effect of type of involvement was estimated separately within the period 0–1 year after maintenance start and after 1 year after maintenance start. As a result, the hazard ratio (compared to no EMD) before 1 year was 1.26 (0.69–2.33; p = .50) for paraskeletal involvement and 3.40 (1.55–7.47; p = .002) for organ involvement. No difference for both groups in comparison to no EMD was seen after 1 year (Table S2). Comparing groups with EMD, organ involvement showed significantly increased risk for relapse/progression or death compared with paraskeletal involvement (p = .03) but similar risk after 1 year from maintenance start. Overall survival appeared to be comparable between both EMD groups (p = .56). In terms of treatment, compared with thalidomide, the hazard ratio was 0.71 (0.55–0.91); p = .003) for lenalidomide and 0.87 (0.59–1.28; p = .50) for bortezomib. Another risk factor for worse progression-free survival was stage II according to ISS compared with stage I with a hazard ratio of 1.38 (1.06–1.79; p = .015) and PR compared to CR/VGPR showing a hazard ratio of 1.28 (1.01–1.63; p = .043).
In terms of overall survival, the hazard ratio (with no EMD as reference) was 0.93 (0.56–1.54; p = .8) for paraskeletal involvement and 1.66 (0.76–3.62; p = .17) for organ involvement. For the comparison of maintenance treatment, the hazard ratio (with thalidomide as reference) was 0.73 (0.52–1.02; p = .06) for lenalidomide and 0.54 (0.29–0.99; p = .045) for bortezomib. No other independent predictors for worse overall survival were identified. The full multivariable analysis is shown in Table S2.
4 DISCUSSION
This registry study on posttransplant maintenance for newly diagnosed MM with or without EMD showed significantly worse early progression-free survival for patients with organ involvement, while different maintenance treatment did not seem to affect outcome in EMD. For patients without EMD, lenalidomide showed significantly higher progression-free survival compared with thalidomide. In multivariable analysis including disease and maintenance, lenalidomide was independently associated with better outcomes in comparison with thalidomide, whereas bortezomib appeared to be associated with improved overall survival.
This is the first study to evaluate the outcome in patients with EMD receiving posttransplant maintenance. A large meta-analysis on 1208 patients found median progression-free survival of 52.8 months for the lenalidomide group and 23.5 months for the placebo or observation group.12 The cumulative incidence rate of a second primary malignancy before disease progression was higher with lenalidomide maintenance versus placebo or observation, whereas the cumulative incidence rates of progression, death, or death as a result of myeloma were all higher with placebo or observation versus lenalidomide maintenance. Although 188 patients had reported EMD, no subgroup analysis are available to date. Another meta-analysis on 5073 participants showing lenalidomide-based regimens (lenalidomide-prednisone, lenalidomide alone) as the most effective options, in transplant and non-transplant settings.15 However, this meta-analysis did also not report on results of patients specifically with EMD.
Here, we showed significantly worse progression-free survival, especially early after maintenance start for patients with organ involvement, while patients with paraskeletal involvement appeared to be associated with similar outcomes in comparison with patients without EMD. This is in line with previous studies without the available data on maintenance treatment.10, 11, 16 Other EMD-specific factors for worse outcome were previously identified, including number of manifestations11 and size of involvements.16, 17 In the present analysis, due to small sample size, we were not able to further stratify outcome in patients with EMD.
In terms of different maintenance strategies, our results on better outcomes for lenalidomide are in line with previous analysis and underline current recommendations for patients without EMD.7 The impact of lenalidomide maintenance in patients with high risk MM, however, still is unclear. In a meta-analysis, no significant overall survival benefit was seen in these subsets of high risk patients.12 However, in a more recent trial that was not part of the meta-analysis, benefit was seen in high risk patients.18 Bortezomib administered every other week has been shown to improve overall survival, particularly in patients with del(17p).19 Bortezomib-based maintenance is therefore considered preferable for high-risk patients.20 This can either consist of bortezomib alone given every other week, or low intensity triplet combination with lenalidomide and dexamethasone.21 In patients unable to access or tolerate bortezomib, ixazomib has become a reasonable alternative that has shown benefit in a placebo controlled randomized trial.22 However, all these trials defined high risk according to cytogenetics or disease status and did not specifically include EMD patients. We found no difference in subgroup analysis in EMD for either treatment, with slightly higher survival rates for bortezomib, but these results should be interpreted with caution due to small sample size of only 11 patients who received bortezomib.
Since intensification seems to be the key to EMD control,16, 23 it is possible to speculate that new drugs may offer a higher level of treatment intensity than conventional drugs. In the pre-new drug era, this goal was obtained only with ASCT. In order to evaluate whether the high efficacy of new drugs results in a more aggressive relapse, a meta-analysis assessed progression-free survival 2 and observed that EMD patients benefited from a similar disease control when compared to patients without EMD (42 vs. 46 months, respectively). This suggests that patients retain the benefit beyond the first line. In contrast, EMD patients consistently showed poor responses to novel drugs, including monoclonal antibodies and novel immunomodulatory drugs and proteasome inhibitors.24, 25 In order to minimize selection bias, we focused our analysis on patients receiving lenalidomide, thalidomide, or bortezomib, and excluded patients who received daratumumab or other single agent maintenance.
This analysis was conducted with the use of retrospective data and is therefore subject to the several shortcomings. Data on how the EMD diagnosis was assessed are not routinely documented in the EBMT registry while no harmonization in assessing extramedullary involvement exists so far; and information on certain risk stratifications (according to R-ISS or different cytogenetic profile and the technology used for cytogenetic results) are not generally captured in the registry.23 Another limitation of the study is the lack of information on the duration of maintenance (especially bortezomib maintenance, usually consisting of a fixed duration strategy) and subsequent therapy. Furthermore, the incorporation of new strategies such as quadruplet induction and consolidation, monoclonal antibody or doublet maintenance may influence outcome, whereas no reports exist with respect to patients with EMD.26-28 Another risk of bias might be introduced by the fact that patients in the thalidomide group were probably exposed to different induction regimens (old therapies) compared with patients exposed to lenalidomide or bortezomib induction. Treatment at relapse after maintenance might also be different, due to the fact that patients were treated during different time periods. This reporting and selection bias might limit generalizability of our results. However, because prospective trials specific in EMD are unlikely, we used regression modeling as a means of controlling for differences between cohorts in the most possible manner, but such adjustment cannot account for all discrepancies in clinical and diagnostic characteristics between patients. Thus, our results need to be interpreted in the context of the limitations of the study.
In conclusion, organ involvement was associated with worse early progression-free survival, despite maintenance treatment. Different maintenance treatment did not seem to affect outcome in EMD. For patients without EMD, lenalidomide showed significantly better outcomes when compared with thalidomide.
AUTHOR CONTRIBUTIONS
Nico Gagelmann designed the study, collected data, interpreted analyses and wrote the first draft of the manuscript; Dirk-Jan Eikema collected and analyzed data and interpreted analyses. Linda Koster collected data. Tanja Netelenbos, Andrew McDonald, Anne-Marie Stoppa, Roland Fenk, Achilles Anagnostopoulos, Gwendolyn van Gorkom, Eric Deconinck, Claude-Eric Bulabois, Michel Delforge, Donald Bunjes, William Arcese, Péter Reményi, Maija Itälä-Remes, Lorenz Thurner, Ali Zahit Bolaman, Yafour Nabil, Hélène Labussière-Wallet, Patrick J. Hayden, Meral Beksac, Stefan Schönland and Ibrahim Yakoub-Agha wrote and reviewed the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank all data managers and all patients.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.