Correlated response to selection for increased body weight on fecundity in Hermetia illucens
Abstract
Genetic improvement through artificial selection holds potential for improving production of the black soldier fly, Hermetia illucens L. (Diptera: Stratiomyidae). A long-term artificial selection for increased larval body weight is in place for the black soldier fly. To investigate the impact of body weight selection on egg production in this species, four tests were conducted, assessing the phenotypic relationship between pupal body weight, egg clutch weight, number of eggs, and egg size. To measure fecundity, egg clutches were collected from individual females. The egg clutches from the body weight (BW) line, selected for 14, 21, and 32 generations, were compared with those of the base population (BP) line to evaluate the effect of long-term selection for body weight on the reproductive output of the black soldier fly. The maternal pupae weight showed a strong positive correlation (0.73) with egg clutch weight and a moderate positive correlation (0.47) with the number of eggs. The egg clutch weight showed a strong positive correlation (0.79) with the number of eggs and a moderate positive correlation (0.51) with the length of an egg. The BW line showed significantly higher performance over the BP line, with about an 18%–49% increase in egg clutch weight per female, a 24%–30% increase in the number of eggs per clutch, and a 3%–4% increase in the length of an egg. The linear mixed model showed that the selection had significantly increased egg clutch weight over the generations of selection in the BW line. Assessment of phenotypic relationships showed no evidence of reproductive trade-offs with higher body weight in female black soldier flies in this study. This research provides the first empirical evidence of increased fecundity in response to artificial selection for increased larval body weight in the black soldier fly.
INTRODUCTION
Insects exhibit a wide range of variations in body weight–fecundity relationships (Leather, 1988). In several arthropods, including insects, maternal size or weight is positively correlated with fecundity under constant environmental conditions because of the allometric increase in ovariole number in bigger individuals (Honěk, 1993). However, this is not universal for all insect species (Head, 1995; Legaspi & Legaspi Jr., 2005; Prenter et al., 1999) and body weight–fecundity relationships are hard to establish under varying environmental conditions prevailing in nature (Leather, 1988). Several factors may determine the body weight–fecundity relationship, like resource acquisition for reproductive investment (Tammaru & Haukioja, 1996), extent of parental care (Gilbert & Manica, 2010), foraging behavior, and several other environmental and genetic factors (Hilker et al., 2023). Trade-offs in the number of offspring and offspring size are central to life history theory (Roff, 1992). According to this, under fixed resources, an individual can direct the resources allocated for reproduction either in producing many small offspring or few(er) big offspring. The clutch size–egg size trade-off is particularly evident in arthropods that are semelparous, use larval energy reserve for reproduction, and provide no parental care (Fox & Czesak, 2000). Black soldier fly (BSF), Hermetia illucens L. (Diptera: Stratiomyidae), is such a species that fits into this profile and is particularly interesting because it is widely used in industrial insect breeding. Moreover, both the larval body weight and fecundity are economically important traits in the mass production of this species.
The mass rearing of insects has developed over the past decade as a lucrative industry for sustainable animal feed. Insects are valued for their ability to valorize organic side-streams into utilitarian products, including, among others, insect-derived proteins and fats that can be used in animal feed. The insect farming sector is projected to generate a market turnover of € 2 billion per year by the next decade (IPIFF, 2021). Within the insect feed sector, the BSF is trailblazing commercial insect farming, dominating the current global insect feed market (Chauhan & Deshmukh, 2022).
Increasing production efficiency is key to meeting the growing demands for insect-based products in a cost-effective manner. Black soldier fly proteins are currently priced higher compared with cheaper but less sustainable alternatives like soy and fish meals, due to the high cost of BSF production. A typical BSF factory consists of two distinct production domains: (1) larvae production for input into the primary products and (2) egg production, which supplies the starter neonates (seedstock) for the larvae production. The second part involves BSF breeding, which is a complex process demanding specific knowledge on appropriate growth environments and operational processes fitting the biological lifecycle. Additionally, breeding requires dedicated infrastructure, equipment, technology, and skilled labor. These can add to significant costs of production. For companies that outsource their seedstock, the cost price of starter larvae is more expensive than the costs of producing their own eggs (Groeneveld et al., 2021). Improving egg production could cut down the total costs of BSF larvae. A black soldier fly female typically lays around 500–1500 eggs (Booth & Sheppard, 1984). Despite being a prolific breeder, there are many opportunities to improve BSF egg production in an industrial production system. Thus far, optimizing BSF egg production has focused on fine-tuning various extrinsic factors like temperature, humidity, nutrition (Bertinetti et al., 2019; Macavei et al., 2020), ovipositing structure/substrate (Booth & Sheppard, 1984), light intensity (Macavei et al., 2020), stocking density (Hoc et al., 2019; Park et al., 2016), sex ratio, and artificial photoperiod (Hoc et al., 2019).
Whereas extrinsic factors are important, enhancing intrinsic factors like colony genetics remains a less explored area, albeit with immense potential. Genetic improvement through selective breeding for economically relevant traits in insects for feed has previously focused on increasing larval body weight in BSF (Facchini et al., 2022) and pupal size in the yellow mealworm (Morales-Ramos et al., 2019). Genetic improvement with novel approaches like gene editing has also been explored in BSF (Zhan et al., 2020). Protix, a commercial BSF producer based in the Netherlands, has a long-term artificial selection program for BSF since 2019 for commercially important traits. The artificial selection for increased larval body weight in BSF has already proven to be successful on an industrial scale. Under automated production settings, an average increase of 39% in larval weight and 34% in wet yield per crate was achieved in the selected body weight (BW) line compared with the base population (BP) line (Facchini et al., 2022).
There is a knowledge gap in how single-trait selection in BSF affects other traits of importance, like egg production. Phenotypic relationships between different traits of interest need to be understood better to guide the selection program. Reproductive success under selection pressure is crucial to understand, as it determines the genetic integrity of the population. Artificial selection can be constrained by genetic correlations that can lead to unfavorable correlated responses in other traits (Mrode, 2014). General concerns on the trade-off of single-trait selection are therefore inevitable, especially due to patterns seen in selection in the livestock industry, where animals bred for growth tend to manifest trade-offs, including but not limited to diminished reproductive performance (Nestor & Noble, 1995; Rauw et al., 1998; Robinson et al., 1993). Reproductive trade-offs arising from unfavorable genetic correlations are crucial to understand, both because of economic implications on the costs of egg production and because reproduction is a crucial component in life history trait evolution.
This study aimed to investigate the phenotypic relationship between maternal body weight and fecundity and the impact of artificial selection for higher larval body weight on fecundity in BSF. Fecundity is defined as the physiological reproductive potential often measured as a quantitative parameter, that is, number of offspring per female or, in other words, clutch size. Commonly, past studies have derived estimates of fecundity in BSF via cage yields, but this does not reveal the individual body weight–fecundity relationship. In this study, measurements were conducted at an individual level, measuring the fecundity of BSF in relation to maternal body weight. Besides the number of eggs (clutch size), we further measured other qualitative indices of fecundity, like egg clutch weight, single egg weight, and egg length, to see the effect of selection for increased body weight over time. Specifically, comparisons were made between the BW and the BP lines. We present the results of tests done at Protix, both in controlled laboratory conditions and in a large-scale production environment over the span of 4 years from 2021 to 2024 with different generations of the selected BW line. The three main research objectives of the tests were to evaluate: (1) whether the maternal body weight is phenotypically correlated with egg clutch weight and clutch size in the BW line; (2) whether a heavy egg clutch contains more, bigger, or heavier eggs; and (3) whether the selected BW line has different fecundity compared with that of the BP line across selected generations.
MATERIALS AND METHODS
The BSF populations used in this study were sourced from the two genetic lines available at Protix locations: the Genetics Lab and the Protix Production Factory, both located in Bergen op Zoom, the Netherlands. The BW line was continually selected for increased larval body weight in the Genetics Lab starting in 2019 (Facchini et al., 2022), whereas the BP line is the original source population at the Protix Production Factory that did not undergo any intentional selection.
The study comprised four tests, each with different sets of measurements that varied in genetic line, selected generation, and test location (Table 1). The first test (T1) was carried out in the Genetics laboratory to establish correlation between the pupal weight of female BSF with egg clutch weight and the number of eggs. This was carried out on the BW line, which had undergone 15 generations of selection for higher larval body weight.
| Test ID | Study period | Treatmenta | Line | Generations selected | Location | Variables measured | |||
|---|---|---|---|---|---|---|---|---|---|
| Pupal body weight | Egg clutch weight | No. of eggs in a clutch | Length of an egg | ||||||
| T1 | Apr 2021 | 1BW-S15-G | BW | 15 | Genetics laboratory | ✓ | ✓ | ✓ | |
| T2 | Feb 2022 | 2BP-S0-P | BP | - | Protix Production | ✓ | ✓ | ||
| Feb 2022 | 2BW-S14-P | BW | 14 | Protix Production | ✓ | ✓ | |||
| T3 | Apr 2023 | 3BP-S0-P | BP | - | Protix Production | ✓ | ✓ | ||
| Apr 2023 | 3BW-S21-P | BW | 21 | Protix Production | ✓ | ✓ | |||
| T4 | Mar 2024 | 4BP-S0-G | BP | - | Genetics laboratory | ✓ | ✓ | ✓ | |
| Apr 2024 | 4BW-S32-G | BW | 32 | Genetics laboratory | ✓ | ✓ | ✓ | ||
- a Treatment indicates test ID (T1–T4), genetic line (selected Body Weight [BW] or Base Population [BP]), generation selected for body weight (S0–S32), and location of rearing history (Genetics Laboratory [G] or Production facility [P]).
Three additional tests were carried out to compare fecundity indices between the two genetic lines with a common garden approach. Two tests (T2 and T3) were conducted to compare the fecundity of the BW line with the BP line at the Protix Production Factory. The BW lines used in T2 and T3 had undergone 14 and 21 generations of larval body weight selection, respectively, in the Genetics laboratory. It was then introduced into the production factory under relaxed selection for about 1 year to allow adaptation to production conditions prior to the tests T2 and T3. These were compared with the BP line available in production within the same week or month as the BW line, as the two lines did not run in parallel.
The fecundity comparison was repeated at the Genetics laboratory in another test (T4). The BP line was introduced into the Genetics laboratory and maintained in the same environmental conditions as the BW line but without selection for about 4 months before the test T4 was conducted. This BP line was compared with the in-house BW line, which had been selected for 32 generations in the Genetics laboratory. Depending on the research questions, different variables were measured in the four tests and are listed in Table 1. A visual illustration of the methodology in the four tests is shown in Figure S1.
Adult flies used in this study were sourced by randomly sampling pupae (at least 1000) from the running batches of the BW and the BP line available at Protix location in each test (Table 1). The experimental setup consisted of three or four major steps: (1) weighing pupae and tagging of adult females (only in T1); (2) female isolation, egg clutch collection, and weighing; (3) egg clutch separation and counting to estimate the number of eggs; and (4) egg length measurement. As the tests were done in two locations and over the span of 4 years, there were slight differences in test setup and methodology due to logistic reasons and optimization of the setup over the years (details in Table S1). However, within a test, the methods stayed the same and were conducted parallel in time. Therefore, performance comparisons between the two genetic lines were only made between treatments within the same test.
Pupae body weight and tracking
In the first test (T1), pupae of the BW line were weighed individually and placed in 35-mL clear plastic Solo cups (Dart Container Corporation, Twin Falls, USA) covered with a cardboard lid until the adult flies emerged. The flies were visually sexed by examining their genitalia. Only the females were tagged with opalistic number labels developed for labeling queen bees (Leuchtplättchen mit nr.1–99; Werner Seip Biozentrum GmbH & Co. KG, Butzbach, Germany). The tag was glued onto the thorax of each female before she was released into a breeding cage. Individual tagging enabled us to trace each female and link its egg clutch data with the pupal weight data.
Female isolation and egg clutch collection
Once >95% of the pupae had eclosed, adult flies were moved into a breeding cage (Ikea, Breda, the Netherlands; plastic cages modified with mesh lid, details in Table S1). Fly eclosion rate was only quantified on a batch level, thus the data on the age of individual flies were unavailable. The ages of the flies ranged from 1 to 5 days old when introduced into the breeding cage. A plastic cup with wet cotton was placed inside the breeding cage as a water source. One breeding cage was set up per treatment in each test with a 1:1 sex ratio. The number of flies in the breeding cage in each test was different due to practical implications on workload (see Table S1). The breeding cage was placed in a climate room underneath artificial LED lights (L12:D12 photoperiod) at 30 ± 0.5°C, 76 ± 3% r.h. when in the Genetics Laboratory and 32 ± 0.5°C, 57 ± 3% r.h. when in the Protix Production facility, to induce mating. After approximately 2.5 days (63–69 h), female flies were randomly handpicked and placed individually into containers of known weight and ID for egg clutch collection. The sample size of collected female flies in all treatments is provided in Table S2. Either a small 10-mL transparent plastic cover cup with lid (Grip bags, Ede, the Netherlands) or 1.5- to 2-mL Eppendorf tubes (Eppendorf, Hamburg, Germany) were used as containers for females to lay eggs in (Table S1). The plastic cup had a small hole drilled in the lid whereas the Eppendorf tube was covered by Parafilm (Heathrow Scientific, Vernon Hills, USA), to permit respiration. When using an Eppendorf tube, the fly was placed with its ovipositor pointing downward.
After female flies were collected, the cups were placed in a tray, whereas the Eppendorf tubes were placed in an Eppendorf tube rack and left inside the climate room at the same breeding conditions available at the respective location for about 6 h during the day (usually between 10:00 and 16:00 h) to allow oviposition. No additional substrate, support, or chemical cue was provided for egg laying, as the combination of high temperature and humidity in the narrow space inside a cup or Eppendorf tube was enough to induce oviposition. The collected female flies were checked regularly over the 6-h test period to spot a complete egg clutch. The egg clutch was considered complete when the fly ceased oviposition behavior (i.e., female stopped probing with the ovipositor and laid still with its ovipositor completely retracted). To assess whether this was a reliable method of collecting complete egg clutches, all female flies used in T1 were immediately euthanized after the test the next day by freezing at −20°C. From this, a sample of eight female flies (four that had laid eggs in during 6 h and four that did not lay eggs at all) were dissected. The dissection showed that the abdomen of female flies that laid eggs during the test was completely empty, whereas the flies that did not lay eggs during the test still had eggs or fat bodies in their abdomen (Figure S2). This confirmed that female flies that lay eggs are likely to lay complete egg clutches in the container provided.
Once a complete egg clutch was present, the fly was removed, and the container was weighed on an analytical weighing scale (Nimbus NBL 4201e; Adam Equipment, Kingston, UK) to determine the fresh weight of the egg clutch in it. To assess whether a female could lay multiple egg clutches within the test period, the fly was again transferred to a new pre-weighed container with a new ID to collect a second egg clutch. The process was repeated if the female laid another egg clutch, continuing until the end of the day. The total number of collected females, the number of ovipositing females, the percentage of ovipositing females inside and outside the test period, and the percentage of females laying more than one egg clutch in each treatment are provided in Table S2. Of the flies that laid eggs within the test period, none or only a few (<11%) BSF females laid more than one egg clutch during the 6-h test period. After the test period, all collected female flies were left in the breeding room overnight to confirm whether additional egg clutches were laid after the test period. For the analysis, only females that laid egg clutches exclusively during the test period of 6 h were included; females that laid additional clutches after this period were excluded. The reason for excluding females that laid additional eggs overnight from the analysis was that eggs lose weight over time due to dehydration, and the extent of weight loss can be strongly influenced by the initial egg clutch weight (Figure S3). Consequently, the weight of the eggs laid overnight does not accurately reflect the original fresh weight for analysis. Furthermore, any egg clutches that appeared visibly damaged by the female or contained external debris or a body part from the female were excluded from the analysis. Only fully intact and clean egg clutches collected during the test period were considered for this study. This ensured that we captured fecundity as accurately as possible. The percentage of total egg clutches successfully collected in each test is provided in Table S2.
Additionally, to check for any bias introduced by time elapsed between egg laying and weighing during the 6-h test period, the egg clutches in test T4 were weighed both immediately after laying and then every hour over the 6-h test period. The results showed that egg clutches lost, on average, 2% of their fresh weight, with heavier egg clutches showing more consistent weights over time (Figure S3). We considered this to be an acceptable loss in weight, given the high amount of work involved in checking multiple replicates simultaneously and weighing them immediately. Thus, egg clutch weights measured in this study refer to the weights taken within 6 h after laying. All egg clutches collected in the test period were frozen immediately at −20°C for the next step.
Egg clutch separation and counting
Egg clutches were randomly sampled from the freezer for counting. To estimate the number of eggs in an egg clutch, individual eggs need to be separated and counted. The BSF eggs are held together very firmly in an egg clutch, which makes it challenging to effectively separate them for counting. Thus, only a few fully intact egg clutches per treatment were counted (sample size mentioned in Table S4). An in-house method of separating eggs with acetone (99+%; Interchema, Vollenhove, the Netherlands) as a solvent was developed at the Protix Genetics Laboratory. Acetone is highly volatile; thus, all steps with acetone were carried out in a fume hood. Acetone was added to the egg clutch with a 1000 μL pipette. When using an Eppendorf tube, the tube was closed and shaken to detach the egg clutch from the container. The eggs were then moved into a 50-mL glass beaker and gently pipetted up and down several times until the eggs were completely separated from each other. With the same pipette, the acetone and eggs were transferred onto a glass Petri dish (4 cm in diameter; Anumbra, Šumperk, Czech Republic). Both the egg container and the beaker were carefully examined to verify that all eggs were transferred successfully. If some eggs were left in the container, the container was flushed again with acetone and the process was repeated until all the eggs were transferred. The eggs were then spread evenly by carefully moving the Petri dish in a circular motion. As eggs sank to the bottom, any excess acetone was pipetted out carefully, and the Petri dish was kept aside to wait for the acetone to evaporate completely. Once this step was complete, the pipette and containers were examined again to make sure all eggs had been transferred into the Petri dish. Any egg clutches in which eggs were missed or spilled were excluded from analysis.
Once dry, a picture of the Petri dish was taken against a dark background and uploaded to an open application program ImageJ (Schneider et al., 2012). The counting tool in ImageJ was used to manually count the eggs in the egg clutch. Some images showed that some eggs remained grouped together even after separation, leading to minor inaccuracies in the counting method. However, relative to the total counts, the counting error was minimal and thus accepted as an artifact of the sample processing method and human error.
Estimation of single egg weight
Egg length measurement
The egg clutches in Eppendorf tubes from tests T3 and T4, stored in the freezer at −20°C, were used to measure individual egg lengths. After a few minutes of thawing, a small portion of the egg clutch was randomly sampled by carefully transferring eggs to a glass slide with a small paint brush. The eggs were spread evenly on a glass slide and observed through a binocular (NexiusZoom; Euromex, Duiven, the Netherlands) supplemented with a measuring lens and calibrated in the appropriate setting (WF10×/22 eyepiece, Zoom 1:6.7 objective with 4.5×). The measurements were done on six egg clutches per treatment, which were randomly selected.
From each egg clutch, 10 eggs were selected randomly for measuring length. Only the longest dimension of each egg was measured, as width is variable. The thawed eggs appeared intact, with no visible signs of dehydration or damage. To check the effect of freezing on egg length, the lengths of some fresh eggs were also measured (data not shown). The fresh eggs were found to be of similar lengths. Furthermore, lengths measured were comparable to data found in the literature (Bogdan et al., 2021). Thus, the accuracy of size measurements using frozen egg clutches was deemed to be acceptable.
Statistical analysis
Statistical analysis was conducted using the open-source software R (R v.2024.04.2; R Core Team, 2024). Spearman's rank correlation test was used to estimate the phenotypic correlations between female pupal weight and egg clutch weight, female pupal weight and the number of eggs, egg clutch weight and the number of eggs, as well as egg clutch weight and egg length. A Wilcoxon rank sum test was used to compare egg clutch weight, the number of eggs, estimated single egg weight, and egg length between the BW and BP lines.
RESULTS
Correlation between female body weight, egg clutch weight, and clutch size
The female pupae weight showed a highly significant, strong positive correlation with egg clutch weight (Spearman's correlation: r = 0.73, S = 626.64, p < 0.001; n = 24, y = −3.92 + 0.15 × x; Figure 1A). The correlation between the female pupae weight and the number of eggs per female showed a moderate positive correlation (Spearman correlation: r = 0.47, S = 1222.8, p = 0.02; n = 24, y = 435.91 + 2.91 × x; Figure 1B).
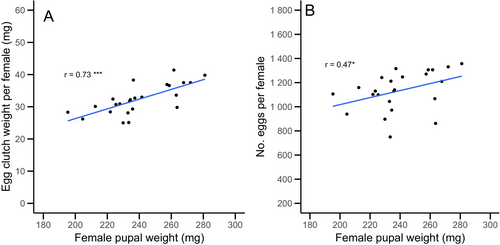
Correlation between egg clutch weight, clutch size, and egg length
The egg clutch weight showed a highly significant, strong positive correlation with the number of eggs per clutch (Spearman's correlation: r = 0.79, S = 10 144, p < 0.001; n = 66, y = 388.47 + 21.89 × x). Except for the BP line in T2 (Spearman's correlation: r = 0.40, S = 12, p = 0.52; n = 5), all treatments showed a significant positive correlation between egg clutch weight and the number of eggs per clutch (Figure 2A; Table S3). The egg clutch weight and the length of an egg also showed a significant, positive correlation (Spearman's correlation: r = 0.51, S = 1 117 865, p < 0.001; n = 240, y = 831.42 + 3.19 × x). The egg clutch weight and egg length were positively correlated in the BW line in both tests T3 (Spearman's correlation: r = 0.68, S = 11 695, p < 0.001; n = 60) and T4 (Spearman's correlation: r = 0.89, S = 3846, p < 0.001; n = 60). On the contrary, in the BP line egg clutch weight and egg length showed a weak positive correlation in T3 (Spearman's correlation: r = 0.38, S = 22 303, p = 0.003; n = 60) and a weak negative correlation in T4 (Spearman's correlation: r = −0.31, S = 47 305, p = 0.014; n = 60; Figure 2B).
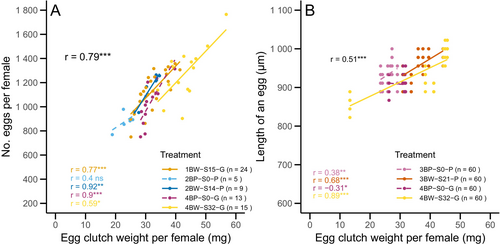
Fecundity in BW line compared with BP line
The variables measured in egg clutches from the BW line were compared with those from the parallel BP line in tests T2, T3, and T4 (Figure 3). The BW line females produced significantly heavier egg clutches compared with the BP line in all tests irrespective of location, and with progressive increase in selected generations (Figure 3A–C). The egg clutches of the BW line also had a significantly higher number of eggs in a clutch (Figure 3D,E). In terms of egg lengths, the BW line eggs were also significantly longer than the BP line eggs (Figure 3F,G). However, no significant difference was found in estimated single egg weights between the two lines (Figure 3H,I).
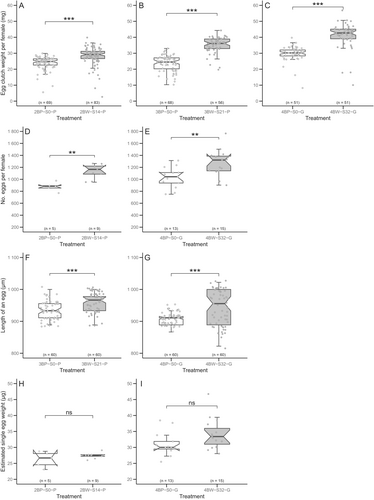
The mean values of all variables measured on egg clutches, along with the performance difference between the BW line and the parallel BP line for each test and treatment, are provided in Table S4. A positive performance difference of 18%–49% in egg clutch weight, 24%–30% in the number of eggs per clutch, 5%–10% in single egg weight, and 3%–4% in egg length was found in the BW line compared with the parallel BP line.
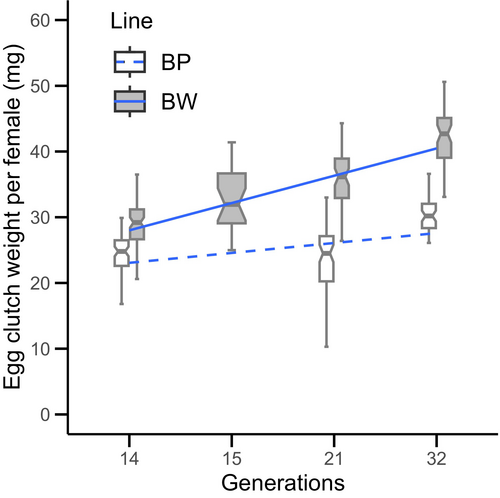
The estimates of the LMM on egg clutch weight are shown in Table 2. The linear regression of egg clutch weight is plotted against generation in Figure 4, where the increase in the egg clutch weight over generations was steeper in the BW line compared with the BP line. The variance of random effect of “location” was found to be 1.59, whereas the residual variance was 30.88. The ANOVA revealed significant main effects of “generation” (LMM: F1,41 = 58.01, p = 0.002) and “line*generation” interaction (LMM: F1,396 = 23.24, p < 0.001). The main effect of “line” itself was non-significant (LMM: F1,387 = 0.17, p = 0.68). These results not only show that generation had a significant impact on egg clutch weight, but also that this impact varied significantly between lines (the interaction effect). Although the line factor itself was not significant in the model, likely due to large variances around the means, the differences between the lines are reflected in the interaction effect.
| Variables | Estimate | SE | d.f. | t | p value |
|---|---|---|---|---|---|
| Intercept | 20.51 | 1.97 | 7.45 | 10.40 | <0.001 |
| Line (L) | 0.72 | 1.75 | 386.61 | 0.41 | 0.67 |
| Generation (G) | 0.24 | 0.07 | 66.74 | 3.30 | 0.002 |
| L × G | 0.38 | 0.08 | 396.38 | 4.82 | <0.001 |
- Note: Line = selected body weight (BW) and base population (BP), generation = no. of generation selected for increased body weight.
DISCUSSION
The maternal pupae weight of BSF was positively correlated with both egg clutch weight as well as clutch size. The egg clutch weight was shown to be a good proxy for clutch size, showing a strong positive correlation with the number of eggs in it. The egg clutch weight was also positively correlated with egg size. The comparison between the two lines showed that the BW line females had higher fecundity compared with the BP line for all fecundity indices measured (except for estimated single egg weight), including egg clutch weight, clutch size, and egg size in all tests.
Our result on maternal pupal weight–clutch size relationship is consistent with other capital breeding insect species, where pupal weight was seen to be a good proxy of potential fecundity (Fitt, 1990; Honěk, 1993; Klemola et al., 2004; Miller, 2005; Tammaru & Haukioja, 1996). This is not surprising, as capital breeding insects like BSF primarily rely on metabolic resources gathered during the larval stage and females do not need to feed as adults to lay eggs. In this study, adult BSF females were only provided with water to extend longevity, which is also a common practice in captive cultures around the world (Barrett et al., 2023). Females under such constant conditions usually require minimal effort in search for a mate and a suitable oviposition site, compared with when they are in the wild. Therefore, pupal weight typically reflects adult reproductive potential and can serve as a good indicator of fecundity in controlled environments. In the wild however, environmental conditions experienced by adults could play a more important role, consequently fecundity can be harder to predict based on body weight (Leather, 1988).
Individual-level variation in clutch size for pupae with similar weight was observed in this study. Several environmental and genetic factors could play a role in individual variation (Evenden et al., 2006; Heisswolf et al., 2009; Proshold et al., 1982). We compared individual pupal body weights to predict fecundity, which though highly correlated with the adult body weight, might not entirely reflect the environmental variation encountered as an adult fly and thus its reproduction potential. Notably, we did not control for maternal age in our tests. Our study consisted of random populations of both females and males, all aged 4–8 days on egg laying day. Maternal age is known to negatively affect clutch size and egg size in the house fly (McIntyre & Gooding, 2000). If this is true for BSF, it could be that older females diverted some energy stored toward somatic maintenance, at the expense of reproduction (Kirkwood, 1977). This could explain individual variation in clutch size. A methodological aspect could have also caused some variation. In a study by Dickerson et al. (2024), older BSF females were found to exhibit egg dumping behavior, with most of those eggs being infertile. In this study, we spotted few egg clutches laid in the cage before collecting females to harvest egg clutches. It was not possible to prevent females from exhibiting this behavior, nor to distinguish which females engaged in such a behavior in our setup. This would require continuous observation over the breeding cycle which lasts multiple days. Therefore, we decided to accept this methodological artifact. The amounts deposited in the cage were relatively small and might explain some outliers. The previous test done to assess the methodology showed a relatively low percentage of females laying incomplete egg clutches or laying twice (Table S2). Therefore, we consider this to have had a minimal influence on the overall correlation estimates given our sample sizes. Nonetheless, this factor may have contributed to individual variation in egg clutch weights and clutch size from pupae of similar weights. Additionally, it is not known whether other factors, such as individual differences in maternal mating frequency and size or quality of nuptial gift obtained from mated males, impact realized fecundity in BSF.
Egg clutch weight was found to be a strong indicator of clutch size. However, there was a difference in regression slopes between different tests and lines, indicating that this could be specific across populations as well as different environments. Egg clutch weight, or the grams of eggs harvested from a cage, is commonly used as an industry standard to estimate the number of eggs/neonates, as they are relatively low effort to measure. We think egg clutch weight will continue to serve as a useful proxy for fecundity, given the high positive correlation and ease of measurement. Nonetheless, we recommend caution when estimating the number of eggs by generalizing per-unit egg weight, which can be highly variable across studies and lines, as demonstrated in this study.
No evidence of a trade-off between clutch size and egg size in BSF was found in this study. The clutch size–egg size trade-off under limited resources is a well-known concept (Smith & Fretwell, 1974). Fox and Czesak (2000) reviewed egg number–size trade-off across several insect species and suggested it is common in semelparous, capital breeding insects like BSF, as they have fixed resources as an adult. In contrast, our results indicate that the heavy egg clutches had both more eggs and larger eggs. A key condition for the clutch size–egg size trade-off is that the resources available should be constant. In our setup, the larval densities and feeding are optimized to fit specific needs of each line (Facchini et al., 2022). Thus, it is not surprising that we did not observe a clutch size–egg size trade-off in the absence of feeding constraints. It remains unknown whether this relationship holds true under resources limitations, such as competition for feed.
Interestingly, the clutch size and egg lengths of the BW line were significantly higher than that of the BP line in both test locations. This suggests that, despite the environment playing a strong role in determining fecundity, there may be a shared genetic basis linking body weight and fecundity. However, we only evaluated phenotypic relationships in this study; therefore, no conclusions can be drawn about the underlying genetic relationships between body weight, egg clutch weight, and egg size. Additional investigation into genetic correlations would be necessary. The review by Fox and Czesak (2000) also suggested that maternal size was positively associated with egg size in other insect orders, including Diptera. We observed similar results, as BW line females are bigger and laid bigger eggs. However, in our study we did not obtain parallel data on maternal weight and egg length. We derived the maternal weight–egg length relationship based on the egg clutch weight–egg length relationship. A direct comparison of the clutch size and egg size, along with maternal weight, would be necessary to conclude whether a maternal weight–egg size or clutch size–egg size trade-off is truly absent in this species, and to identify other factors that determine egg size.
Notably, we observed a high degree of variation in egg size within the same egg clutch. Bogdan et al. (2021) found egg lengths within ranges comparable to ours and reported variation in egg size within the same clutch. Variation in size between eggs from the same clutch is quite common in insects, with maternal age often playing a role (Fox & Czesak, 2000). Diversifying egg size within a clutch is seen as an adaptive response and a bet-hedging strategy to increase fitness under different environmental conditions (Philippi & Seger, 1989). Within-clutch egg size variation could also explain some of the variation in the number of eggs estimated for clutches of similar weight. This adds an additional layer of complexity when quantifying clutch size or egg size based on egg clutch weight in BSF. What is interesting though, is the ability of BSF females to produce both more and larger eggs simultaneously after 32 generations of body weight selection. This suggests that constraints due to physical restrictions (Beck & Beck, 2005), such as abdomen space and structure of ovipositor, have not been reached yet. These findings suggest that there might be an allometric increase in abdomen and ovary size with body size, as commonly seen in other insects (Honěk, 1993). Therefore, further improvement in fecundity through selection should be possible.
In this study, being a larger female came with no costs on the quantity or quality of eggs in BSF. A similar trend was observed in Orgyia spp., where large females benefitted from higher fecundity (Tammaru et al., 2002). Moreover, the concept of large females enjoying a “fecundity advantage” leading to the evolution of females toward larger size is widely accepted (Andersson, 1994; Darwin, 1874; Shine, 1988). The only trade-off of large size observed so far when disseminating the BW line at the factory, is longer development time from pupae to adult stage (approximately +2 days, data from the factory). This trade-off in development time is very common across other insect species; however, of little economic concern in BSF, as it can be mitigated by accelerating development through temperature regulation at a comparatively low cost (internal perspective). From an evolutionary perspective, being larger is not constrained by increased predation or lower chance of finding a mate in captive breeding, where predators are absent, and sex ratio is regulated. However, body size can impact mating success in BSF, where although a large female is more fecund, hatch rates are ultimately determined by the paternal traits (Jones & Tomberlin, 2021).
Our findings show that selection for higher larval body weight has a positive effect on fecundity. Similar results were reported by Morales-Ramos et al. (2019), where selection for higher body weight resulted in higher fecundity. A progressive increase was observed in egg clutch weight in the BW line over selected generations in this study. This is in line with the positive correlation found with pupal bodyweight in test T1, as the BW line had higher mean pupal weights that increased with each generation of selection (data not shown). The intercepts for egg clutch weights of both the BW line and BP line were similar, which is expected as they originate from the same population. However, slope for egg clutch weight per generation was steeper in the BW line (0.69) compared with the BP line (0.33), indicating that body weight selection had a positive impact on egg clutch weights. The significant interaction between line and generation in LMM confirmed this.
The egg clutch weight in the BP line also showed a slight positive increase. The increase for BP performance was seen primarily in test T4, where the BP line was reared in the Genetics Laboratory for 4 months. The rearing conditions and feed type in the Genetics Laboratory differ from that of production, and the population of animals is housed in considerably lower volumes, which we believe might have in some way benefited the BP line in test T4. This study only compared fecundity differences on a population level, where improvement in fecundity was observed in the batches from the selected line with higher body weight. To evaluate whether selection in the BW line has improved productivity per unit of body weight, further investigation is needed, in which fecundities can be directly compared with individual body weight/size across the two populations. Moreover, investigations of genetic correlations between these traits would shed light on if these observed phenotypic relationships have a shared genetic basis. The BW line in tests T2 and T3, had spent at least 1 year in the factory to adapt to the production environment prior to testing, with no selection carried out during that time. Even after almost a year in the production environment, the BW line continued to exhibit higher fecundity. This demonstrates that the benefits of increased fecundity, resulting from artificial selection for body weight, can be effectively realized and maintained in a real production environment.
The mean egg clutch weights per female (23.7–41.2 mg) observed in this study for both lines are higher than values reported in other published studies. Studies published earlier reported 29.1 mg (Booth & Sheppard, 1984), 15.2 mg (Tomberlin et al., 2002), 17.4–22 mg (Hoc et al., 2019), 10.8–13.1 mg (Macavei et al., 2020), and 4.3–6.4 mg (Cai et al., 2022), under various conditions. Similarly, other studies also reported lower fecundity estimates (few 100–1000) compared with this study. Only one other study (Bogdan et al., 2021), reported egg clutch weight and fecundity estimates comparable to or higher than those in this study (8.6–44.7 mg egg clutch weight, corresponding to 378–1659 eggs per clutch). One possible reason for disparity across studies could be that the earlier studies collected eggs from corrugated cardboard, or wooden frames placed in cages with a group of females. Although this method reflects the reality of production conditions, it has pitfalls in terms of accurately measuring egg clutch weight or fecundity per female. Firstly, it is impossible to ensure that an egg clutch is laid by only one female in such a setting where multiple females can lay next to each other. Conversely, a female can also lay at two different locations. Egg clutch weights could thus be overestimated and underestimated. Secondly, both cardboard and wood absorb moisture from the eggs, which could lead to underestimations of egg clutch weight in aforementioned studies. These two critiques have also been mentioned by Hoc et al. (2019). Thirdly, none of the studies measured fresh egg clutch weight. They were weighed after a day, except for Bogdan et al. (2021), where the clutches were weighed within a similar time frame (6 h) to this study, making it the most directly comparable result. Therefore, the method presented here offers a more accurate approach for estimating true fecundity. The only limitation of this method is that it does not account for competition between females for egg-laying sites. Yet, the ranges of egg clutch weights observed in this study are also realized at an industrial scale, where the BW line consistently outperforms the BP line in reproductive performance under the comparable conditions and methods. Hence, we are confident that the values in this study closely reflect real cage conditions and demonstrate a tangible improvement.
To fully capture and translate potential fecundity to realized fecundity, male fertility under a selection program and paternal effect on egg production in this species will be crucial to our understanding. In this study, we could not discern if the eggs were fertile, because estimating fecundity with acetone is a destructive process. Other methods of estimating fecundity, such as using 70% ethanol and 50% glycerin, also effectively separate eggs from a clutch for counting (Bogdan et al., 2021). However, these methods are also destructive. A non-destructive way of separating eggs would be useful in the future, as it would allow distinguishing the fertile eggs from non-fertile ones. This would shed light on fertility contribution from males, and consequently, realized fecundity. Munsch-Masset et al. (2023) state that sperm counts in BSF male range between 9700 and 39 600, which is well above the threshold required to fully fertilize the eggs from a female(s). Further investigation is needed to assess whether male fertility would be a limiting factor in future body weight selection program. Based on our study and observations in the production facility, we have not yet observed reduced fertility in the BW line eggs. The fecundity improvements observed translated to tangible gains in large-scale production.
CONCLUSION
In conclusion, maternal pupal weight in BSF exhibited a positive phenotypic relationship with both the egg clutch weight and the number of eggs per clutch. No evidence of a trade-off was found between egg clutch weight and individual egg weight or egg size. This study presents the first empirical evidence of improved egg production in response to artificial selection for increased larval body weight in BSF. Future studies investigating additional maternal traits, such as age, adult body weight, as well as the influence of paternal traits on egg production, will provide further insights into the opportunities and challenges associated with the artificial selection of this economically interesting species.
AUTHOR CONTRIBUTIONS
Kriti Shrestha: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; visualization; writing – original draft; writing – review and editing. Petra Junes: Conceptualization; data curation; formal analysis; investigation; methodology; supervision; validation; writing – original draft; writing – review and editing. Estelle van den Boer: Project administration; resources; writing – review and editing. Ilse Christianen: Methodology; writing – review and editing. Roland Jacobse: Methodology; writing – review and editing. Eric Schmitt: Formal analysis; funding acquisition; project administration; validation; writing – review and editing.
ACKNOWLEDGMENTS
This work was supported by the Provincie Noord-Brabant with Economie, Kennis en Talentontwikkeling (EKT) subsidy. We thank Ljubinka Francuski, Lotte Joosten, and Bregje Wertheim for critically reviewing the manuscript. We also thank Elena Facchini for support in the initial phase of conceptualization of research ideas.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




