Artificial light at night has species-specific effects on oviposition behavior of mosquitoes
Abstract
Artificial light at night (ALAN) is a pervasive and growing issue worldwide. ALAN disrupts the physiology and natural crepuscular and nocturnal behavior of organisms, with widely observed effects on insects. Mosquitoes (Diptera: Culicidae) are disease vectors that evaluate and select freshwater habitats as oviposition sites. This behavior has both ecological and epidemiological implications. However, it is unknown how ALAN affects mosquito oviposition. We compared oviposition rates in outdoor mesocosms exposed to light-emitting diodes (LED, 3000 K, ca. 13 lux) with dark controls. We assayed the oviposition behavior of natural populations of mosquitoes by quantifying mosquito eggs (Culex restuans Theobald and Ochlerotatus japonicus Theobald) deposited in the experimental mesocosms over 7 days. Mosquitoes had species-specific responses to ALAN. Mean cumulative Cx. restuans egg raft deposition was greater in control pools than in ALAN pools (21 vs. 10 eggs). We observed no response of Oc. japonicus, potentially reflecting the risk associated with the alternative oviposition strategies of the two species (eggs rafts vs. skip oviposition). Our results show that ALAN has species-specific effects on organisms, thereby complicating our understanding of the behavioral and potential ecological and epidemiological effects of this novel anthropogenic stressor.
INTRODUCTION
Artificial light at night (ALAN) is a pervasive worldwide stressor that has increased steadily over the last several decades due to increased human population, development, and urbanization. Indeed, ALAN affects 23% of the land surface between 75°N and 60°S (i.e., most continental land besides Antarctica; Falchi et al., 2016). The effects of ALAN are wide-ranging across multiple levels of biological organization (Zapata et al., 2019), but there is substantial evidence for effects on hormone levels, behavior, and cognition of organisms (Sanders et al., 2021). Insects, in particular, are strongly affected by ALAN because it disorients their navigation abilities by adding new light sources to the environment, which interfere with natural celestial cues (reviewed in Owens & Lewis, 2018). For example, Perkin et al. (2014) captured 27× as many insects at light-exposed intercept traps near an oxbow lake compared to the same traps with no lights, and most of these insects were aquatic, primarily Diptera, Ephemeroptera, and Trichoptera. It is estimated that 30–40% of insects that are attracted to and disoriented by street lights die (Eisenbeis, 2006) owing to collision damage, ALAN-assisted predation, overheating, or dehydration (Yoon et al., 2010; Minnaar et al., 2015). Thus, artificial light often acts as an ecological trap for insects (Szaz et al., 2015), with some orders such as Diptera, Coleoptera, and Lepidoptera more vulnerable than others (Perkin et al., 2014; van Grunsven et al., 2014).
Artificial light at night potentially disrupts the demographic habitat selection of organisms, which signifies long-term habitat use, such as to complete a life cycle stage, e.g., den-, nest-, and oviposition-site selection. Due to relatively longer temporal spans, demographic habitat selection has greater importance to community structure than transient habitat selection, which is typical of foraging behavior (Resetarits et al., 2021). Oviposition site selection is a form of demographic habitat selection that is crucial to the reproductive success of an organism, and if disrupted, can result in severe fitness consequences (Honnen et al., 2016). For example, oviposition site selection is the only form of parental care for many organisms. Thus, there is strong selection pressure to ensure the selection of safe and suitable oviposition sites that maximize growth and success of their typically less-mobile offspring. Aspects of risk (e.g., predation-risk effects) typically exert the strongest influence over these reproductive decisions (Resetarits, 1996). As many organisms oviposit nocturnally, ALAN may compromise the safety of an otherwise suitable oviposition site by increasing predation risk. For example, there is evidence that ALAN is disruptive to natural oviposition rhythms of the moth Bombyx mori (L.) (Yamaoka & Hirao, 1981). Thus, ALAN has potential to affect the distribution and fitness of insects and may function as a potent environmental cue for unsuitable oviposition sites.
Mosquitoes have complex, multi-stage life cycles and use a variety of environmental cues to inform their oviposition site selection (Day, 2016). Whereas most aquatic insects utilize positive polarotaxis for navigation to aquatic habitats (Horváth & Csabai, 2014), current evidence suggests that mosquitoes primarily use chemosensory (olfactory) cues (Meltser et al., 2008; Lerner, 2014). This exception was demonstrated in a series of experiments by Bernáth et al. (2008, 2012) that revealed female Aedes aegypti (L.) are insensitive to polarized light when choosing an oviposition site. However, Ae. aegypti are polarotaxic when they are deprived of chemical cues. Additionally, atmospheric polarized light can increase the prevalence of long, roving flight behavior in both Aedes and Culex spp., which ceases when polarization is disrupted (e.g., by clouds). However, it is unknown whether this behavior translates to oviposition site selection (Wellington, 1974). Thus, mosquitoes can detect polarized light, but they do not utilize polarized light as their primary cue during navigation to oviposition sites, making mosquitoes relatively unique among aquatic insects.
Mosquito genera also differ in their oviposition strategies, which may modulate their response to ALAN. For example, mosquitoes in genera such as Culiseta and Culex land on the water surface and deposit all eggs as one floating raft, thereby investing all reproductive output into one habitat. Thus, Culex spp. are generally more selective and responsive to spatial environmental heterogeneity when ovipositing (Eveland et al., 2016; Resetarits & Silberbush, 2016; Bohenek et al., 2017). Artificial light at night may increase the contrast of their body and egg rafts resulting in increased predation-risk. Mosquitoes in other genera such as Aedes and Ochlerotatus employ a skip oviposition strategy, which serves as a form of spatial bet-hedging that trades careful oviposition site selection for multi-site selection (Hopper, 1999). These genera oviposit on structures just above the water surface (e.g., plant vegetation, container walls). Their eggs are resistant to desiccation and can hatch weeks or months after oviposition (Andreadis et al., 2001). Thus, their oviposition strategy also functions as a form of temporal bet-hedging because current environmental cues of small aquatic habitats are not generally reliable of future conditions (Day, 2016). Because skip-ovipositor eggs are deposited terrestrially, ALAN may have limited effects on body and egg contrast and, thus, not increase predation risk from aquatic predators.
The impacts of ALAN on insects with aquatic larval stages and terrestrial adult stages (i.e., emergent aquatic insects) may have ecological and epidemiological effects. For instance, Meyer & Sullivan (2013) found decreases in family richness and mean body size of emergent aquatic insect communities under experimental light additions in streams. Owing to the nutritional reliance of riparian consumers on emergent aquatic insects (Henschel et al., 2001; Baxter et al., 2005; Kautza & Sullivan, 2016), ALAN-induced changes in the flux of emergent aquatic insects might be expected to alter riparian food webs. Additionally, as mosquitoes have few predator defenses and ALAN facilitates predation by reducing the refuge of darkness (Sanders et al., 2021), ALAN potentially results in greater predation risk to adult mosquitoes and their offspring. Artificial lighting at night may also affect mosquitoes in other ways such as modulating blood feeding and diapause activity (reviewed in Coetzee et al., 2022). Owing to mosquito-borne diseases such as Zika virus, West Nile Virus, dengue, and malaria, ALAN effects on mosquitoes may have important implications for public health (Kernbach et al., 2021; Coetzee et al., 2022).
To determine the effects of ALAN on mosquito oviposition behavior, we constructed an outdoor mesocosm experiment with freely-colonizable aquatic mesocosms that were either lit or unlit with ALAN. We assayed oviposition preferences of natural populations of mosquitoes for 7 days. We hypothesized that the female mosquitoes would avoid ovipositing in aquatic mesocosms exposed to ALAN.
METHODS
Study site
The study was conducted at The Wilma H. Schiermeier Olentangy River Wetland Research Park (ORWRP) in Columbus, OH, USA (40°01′15.0”N, 83°01′10.3”W) in the Level III Ecoregion Eastern Corn Belt Plains of the United States Plains. The ORWRP is surrounded by urban development and the Olentangy River, but it is sufficiently large to contain multiple habitats: mixed riparian forest, multiple wetlands, tallgrass prairie, small fields, and artificial ponds. The study took place on a 10 × 30 m gravel pad in a fenced, experimental area in the northwest corner of the ORWRP.
Experimental design
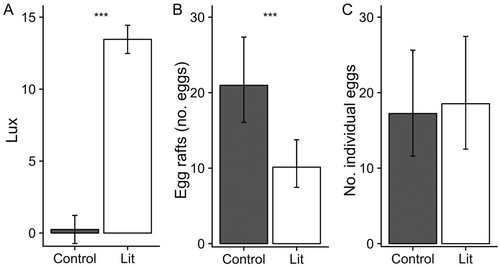
The layout of the experiment consisted of eight blocks, each with two mesocosms and a light source (Figure 1). Blocks were arranged into one large array that consisted of two rows of four blocks. Each block was 3 m from its nearest neighboring block. Within each block, the three components were laid out in a 2 m equilateral triangle such that the lights were positioned interiorly and 2 m from each mesocosm, which, in turn, were positioned 2 m from each other. Mesocosms were either controls or exposed to a 3000 K LED lights (750 lumens; Energetic Lighting, Chino, CA, USA). At 2 m from the light, each lit mesocosm received 13.46 ± 0.65 lux (mean ± SE), whereas dark mesocosms received light levels typical of night skies (0.24 ± 0.01 lux) (Figure 2A). Lux levels were significantly greater in lit mesocosms, which confirmed treatment establishment (F1,6.96 = 417.86, P < 0.001; Figure 2A). These levels of lux represent ecologically meaningful intensities that have been observed in aquatic habitats in Columbus, OH (Meyer & Sullivan, 2013; Sullivan et al., 2019). The lights were shielded with opaque black plastic material to direct a beam of light only to the treatment mesocosm in each block. Each dark control mesocosm was measured to ensure that no light trespass occurred from any direction. The lights were on during the entire experiment.
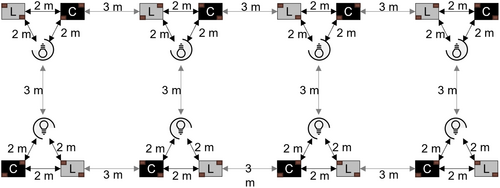
The mesocosms were small, rectangular, plastic bins of 71 × 51 × 15 cm and they held 37.9 L of water. To serve as a nutrient base and to replicate natural depressional pools that serve as breeding sites for mosquitoes, a supply of leaf litter was collected from a nearby riparian forest. Each mesocosm was randomly assigned 100 g of hand-homogenized leaf litter. Two filter papers were haphazardly placed in each mesocosm such that they were partially submerged and located in diagonally opposite corners. Filter paper served as additional oviposition substrate for mosquitoes with skip oviposition strategies (i.e., distribute their eggs from the same batch among several different sites), such as Aedes and Ochlerotatus spp. Diagonal placement was used to minimize potential variation between surfaces oriented toward or away from lights. Mesocosms were filled with water on 1 June 2021. Egg rafts and filter papers were collected daily each morning for 7 days and transferred to a laboratory at ORWRP. Filter papers were replaced daily upon collection. Individual eggs were quantified under a microscope, left to dry for 2 days, and then added to individual hatching and rearing containers until they reached fourth instar for identification. Egg rafts were immediately placed into hatching containers where they were likewise raised to fourth instar for identification. Species identification was based on Darsie & Ward (2005).
Statistical analysis
The two main units of analyses were cumulative egg rafts deposited on the water surface (Culex sp.) per mesocosm and cumulative eggs deposited on filter papers (Ochlerotatus sp.) per mesocosm. We compared control and treatment mesocosms using a generalized linear mixed model with a Poisson distribution and log-link function where treatment (lit and control) was a fixed effect and block was a random effect. Significance was tested using a likelihood ratio test comparing the treatment model to a null model.
We used R v.4.0.2 (R Core Team, 2022) for all analyses (α = 0.05), lme4 v.1.1–26 (Bates et al., 2015) for mixed effects models, tidyverse v.1.3.1 (Wickham et al., 2019) for graphics and data manipulation, ggsci v.2.9 (Xiao, 2018) for plot color schemes, cowplot v.1.1.1 (Wilke, 2020) for plot themes, and emmeans v.1.5.3 (Lenth, 2023) for marginal mean estimates.
RESULTS
Over a 7-day period, there was a cumulative total of 261 egg rafts deposited onto the water surface and a cumulative total 197 individual eggs deposited on filter papers in our experiment. A subset of 228 egg rafts (87%) were raised to fourth instar for species identification and 100% were identified as Culex restuans Theobald. Hatch rates for individual eggs were poor and only a subset of 11 individual eggs (6%) from filter paper were reared to the adult stage for identification. However, they represented at least five out of 12 oviposition events from skip ovipositors (42%). Of the individual eggs, 100% were identified as Ochlerotatus japonicus Theobald.
Mosquito oviposition patterns across treatments were species-specific. The mean total of Cx. restuans egg rafts deposited on the water surface was greater in dark controls compared to lit mesocosms (χ2 = 32.40, d.f. = 1, P < 0.001; Figure 2B). The mean total of Oc. japonicus eggs deposited on filter paper showed no difference between treatments (χ2 = 0.25, d.f. = 1, P = 0.61; Figure 2C).
DISCUSSION
The effects of ALAN are myriad, with even closely related taxa exhibiting a range of behaviors from positive to negative phototaxis or no response at all (Sanders et al., 2021). Our results provide further evidence for species-specific avoidance of ALAN. Though our experiment cannot resolve all questions, the fundamental differences between oviposition strategies (rafts vs. skip oviposition) may be the reason for differential sensitivity to environmental cues among taxa (Vonesh & Blaustein, 2010). Culex spp. (and other raft depositing genera) should be more susceptible to predation under ALAN given visual contrast as they stand vulnerably on the water surface during oviposition, including an increased risk to their offspring. Ochlerotatus spp. (and other skip ovipositors), on the other hand, utilize water-adjacent terrestrial surfaces that may preclude them from strong contrast effects of ALAN and less susceptibility to aquatic predators.
Mosquitoes are important vectors of human disease. Culex restuans is a vector of St. Louis encephalitis (Chamberlain et al., 1959), whereas Oc. japonicus, a fairly recent invader in the USA (Peyton et al., 1999), is a vector of Japanese encephalitis, and both species are a vector of West Nile virus (Kaufman & Fonseca, 2014). Our results here show that localized ALAN can deter mosquitoes from oviposition sites. It is unknown how this effect may scale with respect to the large amount of ALAN output from urban centers, but it is possible that ALAN may function as a ‘push’ factor on the landscape, at least for Culex spp. during oviposition. Mosquito demography and assemblage composition may therefore be sensitive to widespread ALAN from urban centers. For example, Culex populations may become more sustained and concentrated in natural dark habitats, whereby Ochlerotatus/Aedes populations may proliferate in urban areas due to reduced larval competition with Culex, which opt for darker oviposition sites. However, there is evidence that Culex are most abundant under moderate ALAN (Kernbach et al., 2018). As Ochlerotatus spp. (Molaei et al., 2009) and Aedes spp. (Ponlawat & Harrington, 2005) are highly competent vectors that prefer human hosts (relative to Culex; Farajollahi et al., 2011), this pattern potentially leads to increased disease transmission.
However, using ALAN as a mitigation tool for these disease vectors is unlikely to be effective for several reasons. Primary among these reasons is that the effects of ALAN are often species-specific and may vary across levels of biological organization. For example, Fyie et al. (2021) found that female Culex pipiens L. in highly lit urban areas may actively reproduce and bite later in the season, potentially extending the period of disease risk for human populations. Likewise, Kernbach et al. (2019) also found that ALAN increases West Nile virus exposure risk by extending the infectious period (vector competence) of house sparrows, Passer domesticus (L.), a known reservoir for the disease. In a separate study, Kernbach et al. (2021) found that West Nile virus transmission via Culex spp. was greatest in peri-urban areas with low levels of ALAN, suggesting that disease transmission may be greatest at urban edges where dark oviposition sites are abundant but ALAN is still present to increase vector competence (Kernbach et al., 2019). In terms of feeding, Rund et al. (2020) found that ALAN exposure increases nighttime blood-feeding of Ae. aegypti. Lastly, ALAN is an ecological trap for a diverse array of insects (Owens & Lewis, 2018), thus, the costs to ecosystems at large likely outweigh any potential benefits that ALAN can provide for vector management.
ALAN can also alter community-to-ecosystem properties of aquatic ecosystems (e.g., Sullivan et al. 2019), with potential cascading consequences for other organisms (Sanders et al., 2021). In particular, the aquatic-to-terrestrial flux (and vice versa) of emergent aquatic insects such as mosquitoes link aquatic ecosystems and their adjacent terrestrial zones (reviewed in Baxter et al., 2005). Alterations in the composition timing and biomass of emergent aquatic insects can strongly affect the distribution, abundance, and behavior of a suite of riparian consumers (e.g., arthropods, birds, mammals, and herpetiles) and alter food-web dynamics (Murakami & Nakano, 2002; Kautza & Sullivan, 2016). Emergent aquatic insects can also be quantitatively important vectors of contaminants, such as mercury, from aquatic to terrestrial ecosystems (Sullivan & Rodewald, 2012). Our findings on mosquito oviposition implicate one of many potential mechanisms by which ALAN may alter emergent aquatic insect communities and thus, nutritional and contaminant transfers to terrestrial ecosystems.
Results from this study contribute to a growing body of literature on species-to-ecosystem effects of ALAN (Owens & Lewis, 2018; Sanders et al., 2021). However, it remains unknown how the behavioral effects of ALAN mechanistically contribute to impacts on demography, community composition, and ecosystem functions such as aquatic-terrestrial fluxes of energy, contaminants, and disease vectors. Our experiment utilized 3000 K LED lights at 13.5 lux, which matches the currently used specifications of the Ohio Department of Transportation and many other local and state agencies. However, organisms have been shown to respond to variation in light technology, spectral composition, and illuminance (Meyer & Sullivan, 2013; Alaasam et al., 2018). Additional research examining different intensities of ALAN and different spectral compositions can provide further insight into the effects of ALAN on mosquito oviposition patterns and the potential ecological and epidemiological consequences. The effects of ALAN are difficult to study in situ because they co-occur with urbanization and are confounded with other urban impacts. As shown here, the careful use of experimentation can help illuminate the ecological consequences of ALAN.
AUTHOR CONTRIBUTIONS
Sabrina M Daufel: Data curation (lead); investigation (lead); methodology (equal); writing – original draft (equal). Jason Bohenek: Conceptualization (lead); data curation (equal); formal analysis (lead); funding acquisition (supporting); investigation (equal); methodology (lead); project administration (supporting); resources (supporting); software (lead); supervision (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). S. Mazeika Patricio Sulliván: Funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – original draft (equal); writing – review and editing (equal).
ACKNOWLEDGMENTS
Funding support was provided by the Schiermeier Olentangy River Wetland Research Park (ORWRP), The Ohio State University, and McIntyre-Stennis funds. We also thank personnel in the Stream and River Ecology (STRIVE) Laboratory and ORWRP for their assistance. We would like to specifically thank R. Bardsley, J. Patel, M. Jones, and K. Peña for emotional support, and K. Volkers and P. Daufel for logistical support.
Open Research
DATA AVAILABILITY STATEMENT
Data available on figshare. https://figshare.com/articles/dataset/Data_for_Artificial_light_at_night_has_species-specific_effects_on_oviposition_behavior_of_mosquitoes_/19230045.




