How hindgut microbiota may shape sympatric speciation in an invasive phytophagous scarab
Abstract
Following the introduction of new host plants, rapid evolutionary changes in invasive phytophagous insects can sometimes result in sympatric speciation. The underlying processes and facilitation factors are still to be investigated in detail. The role of hindgut microbiota is one of these factors. In this paper, we examined the differences in the gut microbiota of two species of Costelytra scarabs (Coleoptera: Scarabaeidae, Melolonthinae), one non-invasive [Costelytra brunneum (Broun)] and one invasive (Costelytra giveni Coca-Abia & Romero-Samper), for which several ecotypes were analysed. In terms of bacterial assemblages, we found significant variation between the invasive and the non-invasive species. Three main groups of bacteria contributed to these differences, namely Oxalobacteracea, Rhizobiales, and Porphyromonadacea, with the last two potentially providing advantages in the exploitation of a new host plant. Among these bacteria, Porphyromonadacea were systematically present in high proportion in the gut of the invasive species C. giveni. The occurrence of these bacteria might have contributed to the initial capability of this insect to feed and benefit from newly introduced host plants. Furthermore, this study also revealed significant differences in the gut bacterial communities of four C. giveni ecotypes, supporting the hypothesis that part of the gut microbiota in this invasive phytophagous insect is likely to have been acquired horizontally from the newly exploited niche.
INTRODUCTION
Recently, Pélissié et al. (2018) stressed the importance of understanding the rapid evolutionary changes species undergo when they become invasive. These changes are such that they can sometimes result in speciation (e.g., Feder et al., 1998; Lee, 2002; Bourguet et al., 2014) despite the absence of geographic barriers. When considering phytophagous insects, this ‘sympatric’ speciation in invasive species can be the result of host race formation (Drès & Mallet, 2002; Lee, 2002; Forbes et al., 2017). From an ecological and evolutionary perspective, the process of sympatric speciation in invasive species could, in many instances, be summarised as follows (see Lefort et al., 2014): (1) host-shift initiation by host range expansion upon contact with a new host plant, given the ability (e.g., phenotypic plasticity) of the populations to maintain a suitable level of fitness on the novel host; followed by (2) host-shift completion by the newly formed ecotype (sensu Kim & McPheron, 1993), where some level of evolutionary changes has prevailed in the ecotype feeding on the new host, until the ability to effectively use the ancestral host is lost; resulting in (3) the formation of genetically distinct host races, or so-called biotypes (sensu Kim & McPheron, 1993); and finally in (4) speciation through genetic divergence over the speciation continuum (Powell et al., 2013) (Figure 1). This situation could be regarded as an example of ecological speciation, where an ecological change (i.e., host-shift) contributes to the divergence (Forbes et al., 2017). To date, the process and facilitation factors possibly supporting the required rapid adaptation (i.e., supporting the change in diet) and subsequent evolutionary changes in the event of a host-shift, have not been investigated in detail.
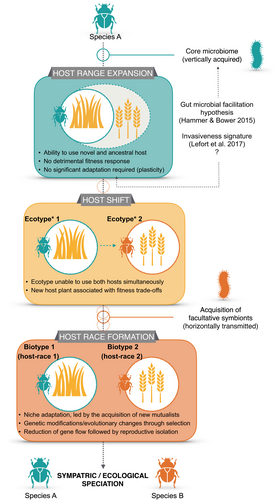
Several calls for a better understanding of the role of gut microbiome in invasive species have been made recently (Bahrndorff, 2016; Lefort et al., 2017). On the sympatric speciation continuum described above, the gut microbiota of phytophagous insects might mediate the process at several levels. First, it might play a key role in the initiation of the process, when phenotypic plasticity is required for the initial population to expand its diet to a new and chemically defended host (Figure 1). This ability is likely dependent on the presence of a specific set of host-symbionts, as proposed in the ‘gut microbial facilitation hypothesis’ (Hammer & Bowers, 2015), and serves as a source of phenotypic variation. For instance, chemical compounds such as monoterpenes, diterpenes, phenolics, and alkaloids, which are involved in plant defence, can be metabolized by bacteria (Malecky et al., 2012; Marmulla & Harder, 2014; Hammer & Bowers, 2015; Vilanova et al., 2016). At this stage, the gut microbiota is likely to be inherited – i.e., transmitted vertically – and serves as a basis for the ‘gut microbiota invasiveness signature’ (Lefort et al., 2017).
The gut microbiota is also likely to play a major role during the host-shift completion phase, where new sets of host-symbionts could be acquired horizontally and contribute to the evolutionary changes required for the ecotype or biotype to be regarded as a distinct host race (Figure 1). The ‘Red Queen’ hypothesis (Van Valen, 1973) provides a mechanistic explanation for the effects of microbiota, whether pathogen or mutualists, in shaping animal evolution (Shapira, 2016). For example, a species might evolve to increase its own fitness by increasing that of its mutualistic partners (Sachs et al., 2004; Dethlefsen et al., 2007). By contributing not only to the fitness, but also to the general health of the ecotype through better host development, fecundity, metabolism, immunity, and/or behaviour – see Engel & Moran (2013) for an extensive review of insect gut microbiota functions –, newly acquired host-symbionts could play a major role in the genetic differentiation of a population, as facilitators of niche adaptation (Shapira, 2016).
It has been demonstrated that some phytophagous insects possess specific gut microorganisms that facilitate the digestion of specific compounds in the plant cell wall, such as lignocellulose a complex network of lignin, cellulose, and hemicellulose (Jang & Kikuchi, 2020). Among these insect species, some have differentiated hindgut morphology that provides suitable microhabitats for the microorganisms responsible for the digestion of lignocellulosic matter (Ebert et al., 2021). This characteristic has been reported several times in scarab beetle larvae (Cazemier et al., 2003; Andert et al., 2010; Huang & Zhang, 2013).
Costelytra giveni Coca-Abia & Romero-Samper (Coleoptera: Scarabaeidae, Melolonthinae), historically called Costelytra zealandica (White) and commonly known as the New Zealand grass grub (Coca-Abia & Romero-Samper, 2016), has been a major pest of agro-ecosystems in New Zealand for decades. Both the larvae and the adult of this endemic species are economically problematic, and despite the existence and the discovery of new active biocontrol agents (Hurst et al., 2018; Glare & O'Callaghan, 2019), C. giveni remains a problem across the country. A better understanding of the processes that enable its pre-eminence across New Zealand ecosystems and its evolution would help consolidate existing pest control strategies and help develop future biocontrol solutions. It has been established previously that following host-shift events, several more damaging ecotypes of this species now prevail in agricultural areas on both islands of New Zealand (Lefort et al., 2014).
This study aims at exploring the variation in bacterial assemblages of the hindgut microbiota of Costelytra species, which represents a first step to understand the putative role of the gut bacteria in shaping sympatric speciation in invasive phytophagous scarab beetles.
MATERIALS AND METHODS
Insect sampling
Three types of ecosystems in which C. giveni is most often encountered, and therefore considered as representative of the diet breath of the insect, have been targeted for this study, namely native grasslands (its historical habitat), exotic pastures, and vineyards. Third instars of four ecotypes were collected from a set of five locations (Table 1) and snapped frozen at −80 °C. In addition, third instars of the closely related but rarely damaging Costelytra brunneum (Broun), also found in native grasslands and co-occurring with C. giveni, were collected to serve as an outgroup for this study (Table 1).
| Site location (NZ) | Coordinates | Site description (dominant plants) | Species sampled | Ecotype | n |
|---|---|---|---|---|---|
| Lincoln (South Island, SI) | 43°64'04″S, 172°47'82″E | Mixed exotic ryegrass (Lolium spp.) | C. giveni | Exotic pasture (SI) | 20 |
| Castle Hill (SI) | 43°12'20″S, 171°42'16″E | Native tussock grassland (Poa cita) close to the margin of beech forest (Nothofagus spp.) | C. brunneum | n.a. | 20 |
| Cass (SI) | 43°02'10″S, 171°45'40″E | Native tussock grassland (P. cita) | C. giveni | Native grassland | 20 |
| Awatere Valley (SI) | 41°44'00″S, 173°52'00″E | Vines (Vitis sp.) | C. giveni | Vineyard | 20 |
| Taupo (North Island, NI) | 38°40'02”S, 176°03'47″E | Mixed exotic ryegrass (Lolium spp.) | C. giveni | Exotic pasture (NI) | 20 |
Gut dissection and DNA extractions
Frozen larvae were thawed before sterilisation by immersion in 70% EtOH for 30 s followed by a rinse in sterile phosphate-buffered saline (PBS). For each individual larva, the hindgut was dissected using sterilized tools under a laminar flow hood. The DNA contained in each hindgut was then individually extracted using a commercial ZR Tissue & Insect DNA MicroPrep extraction kit (Zymo, Irvine, CA, USA), following published methodology (Lefort et al., 2012). DNA concentration in the extracts was checked using a Qubit fluorometer with a dsDNA HS Assay kit (Life Technologies, Carlsbad, CA, USA).
DNA sequencing on the MiSeq System
The gene of interest for this study was 16S rRNA, a prokaryotic gene, which was used to determine the bacterial composition of each dissected gut. This gene was amplified using fusion primers comprising 16S V3-V4 region primers – i.e., 16S V3-V4 forward CCTACGGGNGGCWGCAG (Claesson et al., 2010) and 16S V3-V4 reverse GACTACHVGGGTATCTAATCC (Muyzer et al., 1993) –, as well as overhang adapter as recommended by the manufacturer (Illumina, San Diego, CA, USA). PCR products were purified and impurity was removed using magnetic AMPure beads; DNA concentration was standardised before sequencing on Illumina MiSeq System. This last step was performed by New Zealand Genomics Limited (NZGL, Dunedin, New Zealand).
Bio-informatic analysis
The Illumina sequences were checked through the SolexaQA++ v.3.1.4 (Cox et al. 2010). Prior to analysis, the pooled amplicons were split with fastq_multx, and SolexaQA++ to ensure that the reads were still paired. The identification of 16S amplicons was performed as follows: the paired-end reads were merged using VSEARCH v2.0.3. (Rognes et al., 2016). To ensure that the reads were of a reasonable quality, reads with a quality score <3 were trimmed. Merged 16S sequences that were shorter than 200 bp as well as merged sequences which had more than one expected error per read (maxee = 1.0) (Edgar & Flyvbjerg, 2015) were also discarded. All sequences that occurred only once in the overall dataset were discarded, the data were then de-replicated (i.e., all non-unique sequences were removed, to make downstream computation faster). These unique sequences were then clustered with a 97% identity threshold using the cluster_fast command in VSEARCH. Finally, Chimeric sequences were removed using the Greengenes v.13_8_99 and Silva databases as a reference. The representative molecular operational taxonomic units (MOTUs) were then BLAST searched (Camacho et al., 2009) against the NCBI nucleotide database. The percentage identity threshold was conservatively set at 98% to assign species names to MOTUs (Boyer et al., 2012; Waterhouse et al., 2014). For the 16S rDNA gene, prior to performing data analysis in the software Primer-E, MOTUs were grouped at family level. Within each sample, any family composed by five or less MOTUs and that displayed strictly less than five reads, was regarded as non-robust and accounted for as absent.
Statistical analysis
All statistical analyses were performed with Primer-E. The non-metric multi-dimensional scaling (nMDS) plots for bacterial composition of the gut were produced, after applying a presence/absence pre-treatment and using the Bray-Curtis coefficient (Bray & Curtis, 1957) to build the dissimilarity matrix. Kruskal's stress value was used to determine the efficiency of sample placement in nMDS plots. Significant differences in bacterial gut community assemblages were determined using a one-way analysis of similarities (ANOSIM) after 10000 permutations. This procedure was applied to compare gut bacteria assemblages between the two species and between ecotypes of the invasive species. In the latter case, a post-hoc SIMPER test was performed to identify bacterial taxa allowing the discrimination between the four ecotypes.
RESULTS
Gut bacterial assemblages
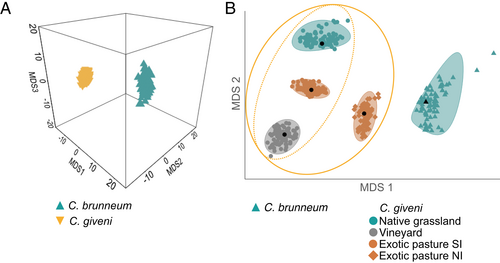
In total 929 MOTUs were produced. After grouping MOTUs at family level and removal of non-robust data, 55 taxonomic groups were retained (Figure 2). Differences in bacterial assemblages were identified between the non-invasive species (C. brunneum) and the invasive species (C. giveni), regardless of the ecotype (ANOSIM: R = 0.364, P < 0.001; Figure 3A). Differences were also observed between the bacterial assemblages of the various ecotypes included within the invasive species (ANOSIM: R = 0.553, P < 0.001; Figure 3B).

Interspecific variation
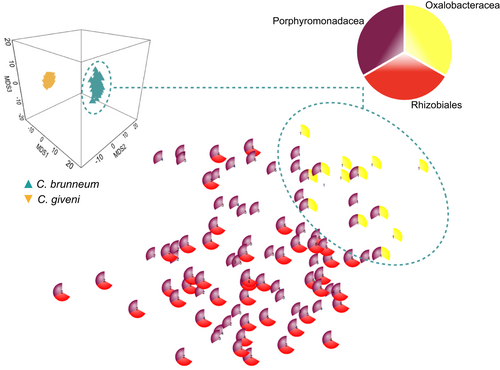
The average Bray Curtis dissimilarity between all pairs of C. brunneum and C. giveni (regardless of the ecotype) was 43.5%. Bacteria from the Oxalobacteracea family were only present in the non-invasive species C. brunneum (Figure 4). On the other hand, bacteria from the order Rhizobiales appeared to be more characteristic of the bacterial assemblages in the invasive species C. giveni (Figure 4). The third group of bacteria of interest explaining the dissimilarities between the composition of the gut microbiome of the two scarab species was the Porphyromonadacea (Figure 4). This bacterial family comprised over 21.2% of the total DNA reads in C. giveni and, unlike in the non-invasive species, was always present in the gut of individuals from the invasive species. BLAST searches with the corresponding MOTUs as queries revealed that all Porphyromonadacea belonged to the genus Dysgonomonas.
DISCUSSION
Invasiveness signature
By analysing the bacterial assemblages of the gut microbiota of two congeneric scarabs, we found significant variation between an invasive and a non-invasive species. More specifically, we identified three groups of bacteria that contributed to these differences. Oxalobacteracea bacteria were only present in the hindgut of the non-invasive scarab species. This family is in part composed by nitrogen-fixing bacteria associated with plant roots (Baldani et al., 2014). Plant-associated bacteria, especially endophytes, may survive stomach digestion (Ramírez-Puebla et al., 2013), and can therefore be retrieved from the stomach content of root-feeding insects. Further insight into the diet of the collected larvae would help to explain this disparity. The native grassland ecotype of C. giveni might feed on other varieties of plants than C. brunneum, despite occurring in similar habitat.
Bacteria from the order Rhizobiales appeared to be more characteristic of the gut of the invasive scarab species. This order comprises many gut-symbiont bacteria that are well known for their ability to fix nitrogen (Neuvonen et al., 2016). For example, Rhizobiales bacteria supply additional nitrogen to insects feeding on nitrogen-poor plant tissues in other insect species (e.g., turtle ants, Cephalotes spp.) (Kautz et al., 2013). The presence of this order and its associated metabolic function could counterbalance the absence of Oxalobacteracea bacteria in the invasive C. giveni.
The third major group contributing to the differences of bacterial assemblages between the invasive and the non-invasive scarab was the Porphyromonadacea. This family of bacteria was represented exclusively by Dysgonomonas bacteria which were systematically present in C. giveni unlike in C. brunneum. These bacteria were also very abundant as they represented 21.2% of all DNA reads in C. giveni. Such high abundance has been reported in the gut of other Coleoptera – e.g., the desert dung beetle Pachysoma striatum Castelnau, 26.3% of the reads (Franzini et al., 2016) –, including invasive species – e.g., larvae of the red palm weevil, Rhynchophorus ferrugineus Olivier, 25.3% of the reads in Zhang et al. (2014) and 21.8% of the reads in Tagliavia et al. (2014) –, but also in other groups of insects – e.g., the fungus-growing termite Macrotermes annandalei (Silvestri) (Zhang et al., 2014). It is likely that Dysgonomonas bacteria have been specifically selected as a response to adaptation to exotic weeds, as they are present, but less abundant, in the ecotype feeding on the native grasses. Their increase in abundance may have played a role in the invasive success of ecotypes feeding on exotic plants. Bacteria from the Porphyromonadacea family have been reported to ferment glucose and hemicellulose as a sole carbon source (Yang et al., 2014; Pramono et al., 2015), which suggests possible roles in providing readily metabolizable substrates for ingestion by the host, through the degradation of these compounds (Ceja-Navarro et al., 2015; Franzini et al., 2016; Berasategui et al., 2017). This could be regarded as an adaptive advantage and the sheer presence of such bacteria in the core microbiome makes it a candidate for an invasiveness signature in phytophagous insects (Figure 1).
Horizontally transmitted gut bacteria
Our analysis revealed significant bacterial assemblage differences among the four ecotypes of the invasive C. giveni. Pairwise post-hoc tests revealed that populations sampled in exotic pastures in the South Island and North Island were more similar than the two populations sampled in different habitats of the South Island. This result was somewhat surprising because the South Island ecotypes are genetically closer together than they are with the North Island one (Lefort et al., in prep.). However, it supports the hypothesis that part of the gut microbiota may be acquired horizontally through the diet of the insect (Engel & Moran, 2013). Such horizontal acquisition could have played a key role in the adaptation of C. giveni to its new niche, leading to the formation of host races (Lefort et al., 2014). It is also important to note that Costelytra larvae are recognized as non-obligate root feeders and have been reported, in certain situations, to grow on humus or organic material (Sutherland et al., 1975; Lefort, 2013). As insect gut microbiota might be partially acquired from the environment, and in dwelling life-stages especially from the soil, the similarities observed within the ecotypes feeding in exotic pastures might as well be the result of microbial soil composition similarities. Such similarities in soil composition are likely to result from the degradation of the dominant plant on the collections sites (i.e., clover) (Mawdsley & Bardgett, 1997). For instance, over a quarter of the gut microbiota OTUs of larvae of Popillia japonica Newman were derived directly from the soil microbiota (Chouaia et al., 2019). Hence, such acquisition of key microbiota from the environment, and in particular through feeding on new host plants, might play a role in the completion of a putative sympatric speciation in this species (Figure 1).
Concluding notes
The high proportion of Dysgonomonas spp. in the core gut microbiota of C. giveni might have contributed to its initial capability to feed and benefit from introduced host plants such as clover (Lefort et al., 2015a, 2015b). Other bacteria may have been acquired horizontally, probably from the newly exploited niche, and these have played a role in the adaptation of the insect. We hypothesise that the presence of Dysgonomonas bacteria in the gut microbiota of phytophagous insects could constitute an ‘invasiveness signature’. This preliminary insight into the putative roles of gut bacteria shaping the invasion success of C. giveni consolidates previous hypotheses and opens research towards next-generation biocontrol solutions (Lefort et al., 2017).
AUTHOR CONTRIBUTIONS
Marie-Caroline Lefort: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (supporting); methodology (lead); project administration (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Travis Glare: Conceptualization (supporting); writing – review and editing (supporting). Didier Bouchon: Project administration (supporting); writing – review and editing (supporting). Stéphane Boyer: Conceptualization (supporting); funding acquisition (lead); methodology (supporting); project administration (supporting); writing – review and editing (supporting).
ACKNOWLEDGMENTS
This work was supported by a Unitec Strategic Research Fund (RI16001). We thank Mauricio Gonzales-Chang (Aysén University, Chile) and Michael Quintern (MyNoke, New Zealand) for providing grass grub larvae. We also thank Kerry-Lee Pigg for technical help in the laboratory.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available upon publication of the manuscript at the following address: 10.6084/m9.figshare.21154561




