Flight muscle structure and flight capacity of females of the long-distance migratory armyworm, Spodoptera frugiperda: effect of aging and reproduction, and trade-offs between flight and fecundity
Abstract
Insects in flight achieve the highest metabolism rates and wing beat frequency known in the animal kingdom, but how they do this is not fully understood. Here, we demonstrate the structure and arrangement of flight muscles in the thorax of Spodoptera frugiperda Smith & Abbot (Lepidoptera: Noctuidae). Dorsoventral direct flight muscles are stronger and more complicated than dorsoventral indirect flight muscles, which may be important for the moth to swiftly control the trajectory. The abundance of giant mitochondria and the extensive tracheolar system in flight muscle are two prominent features that are well adapted to the special requirements of flight. Parameters of flight muscle and flight capacity were lower in young females, and then increased with time and peaked in middle-aged females. Mating accelerated the deterioration of the structure and function of flight muscles. Virgin females showed lower ovarian development rate but higher flight capacity than mated females. Further analysis found that female flight capacity was significantly negatively correlated to her fecundity, suggesting a trade-off between flight and reproduction. The resource allocation to flight and egg production may be the result of a balance between the intensity of natural and sexual selection on both traits.
INTRODUCTION
Insects are the most successful group of animals on earth and some insects are major agricultural pests, which may be partially due to the fact that most of them have the ability to fly (Vigoreaux, 2006; Glaeser et al., 2017). However, of all the organisms that can fly, the flight mechanisms of insects are possibly the least understood. The flight of insects involves complex mechanical dynamics, fuel utilization, and energy metabolism (Vigoreaux, 2006; Teulier et al., 2016; Glaeser et al., 2017).
Insect flight is driven by well adapted direct (DFMs) and indirect flight muscles (IFMs) (Vigoreaux, 2006). Direct flight muscles attach directly to the wings and mainly function in controlling the trajectory of the wings, whereas IFMs power the oscillating wing movements by distorting the insect's thorax (Vigoreaux, 2006). The flight muscles occupy most of the space in the thorax and are usually the best developed of the muscles in the insect body (Iwamoto, 2011; Klowden, 2013). The growth and maintenance of the flight muscles is a resource-dependent process (Vigoreaux, 2006; Iwamoto, 2011; Klowden, 2013). In order to support the weight of an insect in flight, total flight muscle mass must be greater than 12% of body mass (Klowden, 2013). In the fruit fly, flight muscle mass amounts to approximately 30% of total body mass (Vigoreaux, 2006). Furthermore, the frequency of wing beat during flight in insects can be very high, up to 1000 Hz, and thus insect flight is an enormously energy-demanding process (Beenakkers et al., 1984). The transitions from rest to flight in birds and from rest to running at maximum speed in mammals are accompanied by a 7–14-fold increase in metabolic rate, whereas in insects it is 50–100× from rest to flight (Beenakkers et al., 1984). Insects may use carbohydrates, fats, and proline to fuel flight, which can be different between species and between stages of flight (Thompson & Suarez, 2009). In some migratory insect species, such as various locusts and moths, flight is initially fueled by carbohydrates, and a long-distance flight will activate the fatty acid oxidation pathway to support the enduring energy demand (Arrese & Soulages, 2010).
On the other hand, reproduction in insects also is an extremely resource- and energy-demanding process, which may incur substantial costs on other processes and even survival (Labbadia & Morimoto, 2015; Rodrigues & Flatt, 2016; Shih et al., 2020). Female insects usually produce huge numbers of large eggs that are rich in protein, lipid, and carbohydrate stores (Lorenz & Gaede, 2009). For example, eggs of the cricket Gryllus bimaculatus De Geer contain 21% of lipid, 15% of protein, and 2% of carbohydrate (glycogen plus free carbohydrate) (Lorenz, 2003). Also studies in moths suggest that the oviposition process and behaviors are likely to incur energy costs, which may negatively affect other functions and lifespan (Xue et al., 2010; Yu et al., 2014).
Ecologists often consider female insects with respect to an energy allocation budget between flight and reproduction, particularly in long-distance migratory species (Lorenz & Gaede, 2009). The resources diverted to flight mussels may reduce the resources canalized to egg production (or vice versa), constraining their adaptive evolution (Gibbs & Van Dyck, 2010). For example, in the brown planthopper Niparvata lugens (Stål), when conditions favor reproduction, wing polyphenic species produce adults that either have no wings or short, non-functional wings, reflecting a physiological and evolutionary trade-off between migration and reproduction triggered by environmental conditions (Lin et al., 2016). Alatae of the pea aphid, Acyrthosiphon pisum Harris, took off for flight when the protein content of the flight muscles attained the maximum; after migratory flight, the protein content of the flight muscles tended to decrease and the body weight to increase, followed by the onset of larviposition (Kobayashi & Ishikawa, 1993). Reproduction can induce flight muscle degradation, thereby diminishing flight ability (Azizi et al., 2009). In Dysdercus cingulatus (Fabricius), proteins from degenerating flight muscles can be reused for egg production (Nair & Prabhu, 1985). Female flight performance in Spodoptera exigua (Hübner) is negatively correlated to the number of deposited eggs (Jiang et al., 2010).
The fall armyworm, Spodoptera frugiperda Smith & Abbot (Lepidoptera: Noctuidae), is a worldwide migratory pest (FAO, 2019; CABI, 2020; Congdon et al., 2021). This pest is native to subtropical and tropical regions of the Americas (Kumar et al., 2009) and was first found in India in 2018 and then spread throughout Asia soon after (FAO, 2019; CABI, 2020). Long-distance migration is the key determinant of the invasion status and evolutionary success of S. frugiperda (Johnson, 1987; Westbrook et al., 2016; Nagoshi et al., 2019). A laboratory flight performance test exhibited a strong flight capacity in S. frugiperda, flying up to 163 km and for >46 h (Ge et al., 2021a). Migratory flight may affect reproductive fitness in S. frugiperda; it shortens the pre-oviposition period and reduces egg production (Ge et al., 2021b), and flight distance and duration are negatively correlated with the number of eggs laid in S. frugiperda (Ge et al., 2021c). Ovarian dissections further revealed that over half of migrant females were mated and 46–54% had initiated oviposition, indicating potential trade-offs between reproduction and migratory flight in this moth pest (Ge et al., 2021c).
In this study, using optical and electron microscopes, the macroscopic and microscopic structure of flight muscles in the thorax were studied to provide a deeper basic understanding of the structure and function of the flight muscles in this long-distance migrator. The changes in the ultrastructure of the flight muscles in association with aging and reproduction were further tested. Possible links between structure and flight function and between flight capacity and reproduction were also tested and discussed. This study not only provides new information on the flight muscle anatomy in a migratory moth, but also contributes to the recognition of the migratory strategy of S. frugiperda.
MATERIALS AND METHODS
Insects
Spodoptera frugiperda (maize strain) larvae were reared on artificial diet (Li et al., 2006) at 28 ± 1 °C, 60–80% r.h., and L14:D10 photoperiod, in the IPM Laboratory of Southwest Forestry University (Kunming, China). To ensure virginity and age, male and female pupae were sexed based on morphological characteristics (Dong et al., 2019) and were then caged separately. Adult eclosion was recorded daily and the eclosion day was recorded as ‘0 days old’. The moths were provided 10% honey solution as food.
Flight muscle anatomy and effect of aging and reproduction
For optical microscope observation and photography, the adult moth was soaked in absolute ethanol overnight and then the flight muscle was dissected under a Leica M165FC stereo microscope (Leica Microsystems, Wetzlar, Germany) and its structure was photographed using a Leica DFC495 digital camera (Leica Camera, Wetzlar, Germany). To better show the structure of flight muscles, the flight muscles were drawn based on observations and photos.
A JEM-1011 transmission electron microscope (JEOL, Akishima, Japan) was used to view and compare the ultrastructure of flight muscles from different treatments. To test the effect of aging on flight muscles, 1-, 3-, 5-, 7-, 9-, and 11-day-old virgin female moths were sampled. To test the effect of mating on flight muscle, 3-day-old virgin females were allowed to mate once with 3-day-old virgin males (males were removed immediately after mating and mated females were caged individually) afterwards mated females were sampled at 5, 7, and 9 days old (we did not have 11-day-old mated females in the samples because mated females died before they were 11 days old). Three females were sampled for each sampling time point and each female was used as a replicate (i.e., n = 3). The thoracic dorsal-longitudinal muscle (Figure 1) was dissected under a stereo microscope as above. The obtained samples were then treated according to the method outlined by Chen et al. (2015). Samples were first fixed in 3.5% glutaraldehyde solution and then in 1% osmic acid, followed by gradient dehydration using ethyl alcohol and acetone. After the penetration and embedding by using 618 epoxy resins, samples were trimmed and sectioned using a Leica EM UC7 ultramicrotome (Leica Microsystems). Finally, sections were stained by citrate-uranium and then observed with the electron microscope.
Following above age and mating treatments, the thorax was also sampled (feet and wings were removed) and its fresh weight was weighed to the accuracy of 0.0001 g by using a Sartorius MSX electronic balance (Sartorius, Germany). Thirty females were sampled for each sampling time point and each female was used as a replicate (n = 30).
In the above experiments, the moths were provided 10% honey solution as food until sampling.
Flight capacity and effect of aging and reproduction
After eclosion, the number of ovarian mature eggs increased with time in S. frugiperda females. Similar to some other moths (e.g., Xu & Wang, 2011), mated S. frugiperda females start to lay many fertile eggs (hatchable), whereas aged virgin females may also lay some unfertile eggs (that cannot hatch). To test the lifetime egg production of virgin females, newly eclosed (0 days old) females were collected and individually caged in plastic boxes, as above. Each box was provided a paper strip (15 × 20 cm) folded in zig-zag fashion as an oviposition substratum and 10% honey solution as food. Boxes were changed daily for all replications and eggs laid by each female were counted daily. To test the lifetime egg production of mated females, individually caged virgin females were allowed to mate 1× at 3 days old with virgin males, as above. Mated females were caged individually and their eggs laid were counted daily. A moth was considered a replicate and 20 moths were used for virgin or mated females (n = 20). The moths were provided 10% honey solution as food.
Virgin and mated females were treated and caged individually, as above. Virgin females were sampled at 1, 3, 5, 7, 9, and 11 days old, whereas mated females were sampled 5, 7, and 9 days old for flight capacity tests. The moths were provided 10% honey solution as food and their eggs laid were counted daily before sampling. Flight capacity was measured by using a computer-monitored flight mill (Jiaduo Industry & Trade, Hebi, Henan, China) and followed the method used by Chen et al. (2015). For each replicate, a moth was anesthetized using CO2 and then adhered to the tip of the cantilever of the mill via the pronotum of the moth by using Supertite glue (Gymcol Adhesives, Pinghu, Zhejiang, China). Each test was conducted for 9 h during the scotophase and all tests were conducted under the conditions mentioned above. A moth was considered a replicate and 40 moths were used for each sample (n = 40). The total number of flight mill revolutions was recorded and the flight distance (km) for each moth was computed using custom-made software.
After the flight test, females were dissected to count ovarian mature eggs, following the methods outlined by Edwards (1954). The ovaries were separated from the female's abdomen in a drop of 1% saline solution on a glass slide under a dissecting microscope. The separated ovaries were immersed in 1% acetocarmine for 10 s to stain the eggs. Stained eggs were classified as immature and unstained eggs as mature. This is because the chorion of mature eggs prevents penetration of the stain, whereas immature eggs absorb the stain.
Statistical analysis
If data were normally distributed, sarcomere length, myofibril area, thorax weight, number of eggs laid, number of ovarian mature eggs, and total number of eggs (i.e., eggs laid + ovarian mature eggs) were analysed by ANOVA followed by Fisher's least significant difference (LSD) test for multiple comparisons. If data were not normally distributed, despite various transformation trials, they were analysed by the nonparametric Kruskal-Wallis (K-W) test followed by Dunn's procedure for multiple comparisons (Zar, 1999).
Because flight distance, flight duration, and flight speed are intercorrelated (Scheiner, 2001), data on flight capacity were analysed by using a multivariate ANOVA (MANOVA) followed by Fisher's LSD test for multiple comparisons between treatments.
The relationship between flight capacity and egg production was first detected separately in virgin or mated females of the same age (day) by using a regression analysis. However, no significant correlation between flight capacity and reproduction was found. We thus pooled all observations of mated and virgin females of the same age for further regression analysis.
All analyses were conducted using IBM-SPSS v.25.0 (IBM, Armonk, NY, USA). The significance threshold was set at α = 0.05.
RESULTS
Structure of flight muscles in the thorax
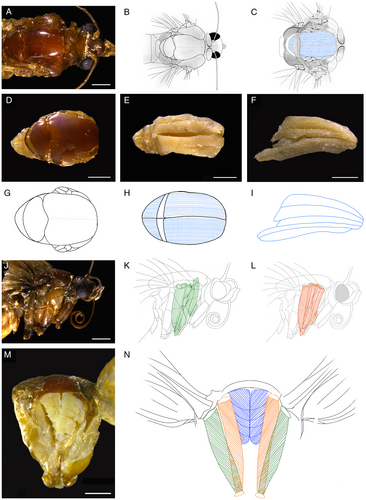
Dorsal, lateral, and cross-sectional observations revealed one dorsal longitudinal IFM, a pair of dorsoventral DFMs, and a pair of dorsoventral IFMs. The dorsal longitudinal IFM laid mostly under the cover of the tergum (Figure 1A–I), and from the dorsal view it can be divided into two parts (left and right; Figure 1E, H) and from the lateral view it can be recognized as five layers (Figure 1F, I). One dorsoventral DFM was connected to the upper (basilar of wing) and lower (sternum) surfaces of the insect thorax (Figure 1 K, M, N), and the other dorsoventral IFM was connected to the upper (tergum) and lower (sternum) surfaces (Figure 1 L, M, N) of the insect thorax. Both dorsoventral DFMs and the IFM of left or right side were composed of five columnar muscles, with the DFMs being thicker and more complicated than the IFM.
Ultrastructure of flight muscle and effect of aging and reproduction
In longitudinal view, the myofibril sarcomeres were cylindrical in shape (Figure 2A–C), and in cross-section, the myofibrils were elliptical in shape (Figure 2D–F). Measurements showed a similar age-related change pattern on sarcomere length and myofibril cross-section area (Figure 3A, B): sarcomere length and myofibril cross-section area were lower in 1-day-old females, which increased with time and peaked in 5-day-old females, and then declined with aging (K-W, sarcomere length: χ2 = 327.433; myofibril area: χ2 = 68.742, both d.f. = 5, P < 0.0001). Mating also showed significant effects on sarcomere length: mated females have shorter sarcomeres than virgin females at 5 days old (K-W: χ2 = 13.020, d.f. = 1, P < 0.0001), 7 days old (χ2 = 71.841, d.f. = 1, P < 0.0001), and 9 days old (χ2 = 9.863, d.f. = 1, P = 0.002). Mated females also had a significantly smaller myofibril area than virgin females at 5 days old (K-W: χ2 = 8.565, d.f. = 1, P = 0.003); this difference was not significant at 7 days old (ANOVA: F1,113 = 0.043, P = 0.84) or 9 days old (F1,138 = 1.629, P = 0.20). Flight muscle myofibrils are composed of thick and thin filaments, each thick filament was enclosed by six thin myosin filaments that were arranged equidistantly in a hexagon pattern (Figure 2G).
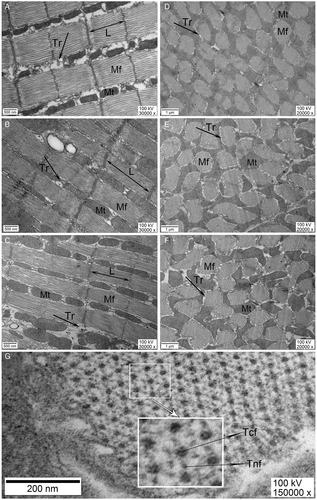

From the longitudinal view (Figure 2A–C), the flight muscle mitochondria were mostly rodlike and parallel to the myofibril, but some were oval or irregular in shape. In cross-section (Figure 2D–F), mitochondria are mostly irregular in shape and the boundaries between mitochondria are often unclear. Between mitochondria and myofibril, there are horizontally or vertically arranged tracheoles. The myofibrils appear to be surrounded by mitochondria and tracheoles.
Weight of thorax and effect of aging and reproduction
The fresh weight of the thorax decreased with aging, both in virgin (K-W: χ2 = 35.238, d.f. = 5, P < 0.0001) and mated (ANOVA: F2,87 = 16.352, P < 0.0001) females (Figure 3C). Mating furthermore accelerated thoracal weight loss – at 5 days old, the weight loss was not significant (F1,58 = 1.655, P = 0.20); however, at 7 and 9 days old, mated females have lower weights than virgin females (7 days old: F1,58 = 29.020; 9 days old: F1,58 = 23.795, both P < 0.0001).
Flight capacity and effect of aging and reproduction
Within virgin females, the flight capacity is low in 1-day-old females and peaked in 3-day-old females (Table 1a, Figure 3D–F). Within mated females, the flight capacity is high in 5-day-old females and then declined with aging (Table 1b; Figure 3D–F). Comparing virgin to mated females, mated females have lower flight capacity than virgin females at 9 days old (Table 1e, Figure 3D–F), whereas no significant difference was found at 5 or 7 days old (Table 1c,d; Figure 3D–F).
| Model | Description | Parameter | d.f. | F | P |
|---|---|---|---|---|---|
| a | Effect of age on flight capacity of virgin females | Whole model | 15,858 | 5.096 | <0.0001 |
| Flight distance | 5,286 | 14.421 | <0.0001 | ||
| Flight duration | 5,286 | 14.807 | <0.0001 | ||
| Flight speed | 5,286 | 2.199 | 0.055 | ||
| b | Effect of age on flight capacity of mated females | Whole model | 6,338 | 4.562 | <0.0001 |
| Flight distance | 2,112 | 9.348 | <0.0001 | ||
| Flight duration | 2,112 | 11.369 | <0.0001 | ||
| Flight speed | 2,112 | 2.882 | 0.060 | ||
| c | Difference of flight capacity between virgin and mated females at 5 days old | Whole model | 3,290 | 0.095 | 0.96 |
| d | Difference of flight capacity between virgin and mated females at 7 days old | Whole model | 3,279 | 1.017 | 0.39 |
| e | Difference of flight capacity between virgin and mated females at 9 days old | Whole model | 3,207 | 4.358 | 0.007 |
| Flight distance | 1,69 | 1.667 | 0.20 | ||
| Flight duration | 1,69 | 1.849 | 0.18 | ||
| Flight speed | 1,69 | 4.044 | 0.048 |
Egg development and oviposition in mated and unmated females
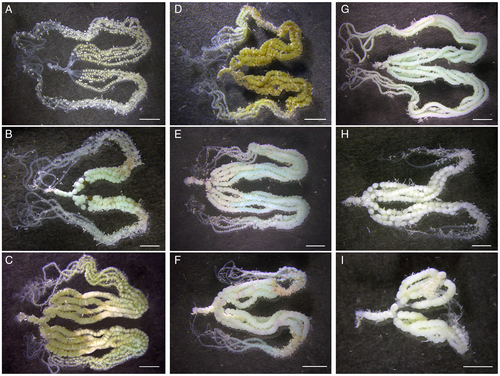
In virgin females, the number of ovarian mature eggs increased with time and peaked before death (K-W: χ2 = 69.555, d.f. = 5, P < 0.0001) (Figures 3G and 4). Mating promoted egg maturation, with the number of ovarian mature eggs peaking in 5-day-old females and then quickly declining (ANOVA: F1,62 = 28.227, P < 0.0001) (Figures 3G and 4). Virgin females can lay some eggs before death and the number of eggs laid increased with time (χ2 = 68.435, d.f. = 5, P < 0.0001) (Figure 3H). Mated females started to lay a large number of eggs and the number increased with time (F2,62 = 57.873, P < 0.0001) (Figure 3H). The total number of eggs (= eggs laid + ovarian mature eggs) increased with time both in virgin (χ2 = 75.642, d.f. = 5) and mated (F2,62 = 26.205, both P < 0.0001) females (Figures 3I and 4).
Relationship between flight capacity and fecundity
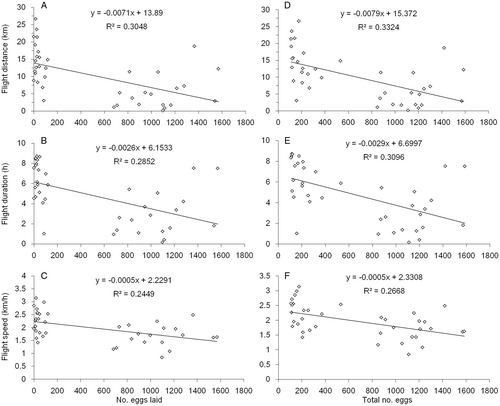
After pooling of the observations of mated and virgin females of the same age, analysis indicated that the flight capacity of 9-day-old females was negatively correlated to the number of eggs laid (flight distance: F1,36 = 15.78, P = 0.00033; flight duration: F1,36 = 14.36, P = 0.00033; flight speed: F1,36 = 11.67, P = 0.0016; Figure 5A–C) and the total number of eggs (flight distance: F1,36 = 17.92, P = 0.00015; flight duration: F1,36 = 16.15, P = 0.00029; flight speed: F1,36 = 13.10, P = 0.00090; Figure 5D–F), but not to the number of ovarian mature eggs. Also, no significant correlation was found between flight capacity and reproduction in 5- and 7-day-old females.
DISCUSSION
In the present study, based on photomicrography and drawing, we showed the structure and arrangement of (dorsoventral) DFMs and (dorsoventral and dorsal longitudinal) IFMs in the thorax of S. frugiperda. The wings are raised by the contraction of dorsoventral IFMs and lowered by a contraction of the dorsal longitudinal IFMs (Klowden, 2013). We also noticed that the dorsoventral DFMs are stronger and more complicated than the dorsoventral IFMs in S. frugiperda. For a moth trying to escape from an attack, the ability to rapidly change the trajectory may represent an important selection criterion for the DFMs (Vigoreaux, 2006; Yang, 2020).
Flight muscle ultrastructure studies further revealed that the abundance of giant mitochondria and the extensive tracheolar system are two of the most prominent features, well adapted to the special requirements of flight (Vigoreaux, 2006). Virtually every mitochondrion is in contact with or is encircled by terminal tracheoles, which increases the efficiency of respiration in the flight muscles (Wigglesworth & Lee, 1982). In cross-section, the myofibrils were elliptical whereas mitochondria were mostly irregular in shape, with myofibrils being encircled by mitochondria in S. frugiperda, which is similar to the results found in other lepidopterans such as Spodoptera litura (Fabricius) (Yang, 2020) and Danaus plexippus (L.) (Wensler, 1977). However, in the dipterans Bactrocera dorsalis (Hendel) (Chen et al., 2015), Phormia regina (Meigen) (Sacktor & Shimada, 1972), and Aedes sp. (Lehane & Laurence, 1977), and the hemipteran Nilaparvata lugens (Stål) (Wan et al., 2013; Liu et al., 2017), mitochondria were mostly oval to round, and less in contact with myofibrils. Moreover, in cross-section myofibrils are larger than mitochondria in the African migratory locust, Locusta migratoria L. (Papidze et al., 2015). However, whether these differences are related to energy metabolism rate or flight capacity needs further study.
Flight muscle fibers are composed mainly of large, easily dissociable myofibrils (Tiegs, 1955). Each myofibril consists of ca. 1000 thick and 3000 thin filaments, organized into structures called sarcomeres. The thick filaments consist primarily of the motor molecules myosin, which interact with the thin filaments' actin molecules, and function in the sliding motion between the two sets of filaments. Biochemical study suggested that the activities of most enzymes involved in fuel metabolism are much higher in insect flight muscles than in vertebrate skeletal muscles (Crabtree & Newsholme, 1975). Glycolytic enzymes in insect flight muscle have a high affinity for myofibril and may exist as part of a complex that assists in the local generation of ATP (Sullivan et al., 2003). These prominent physiological and biochemical features ensure insects can fly with high frequency and superb skills.
In this study, we found the fresh weight of thorax to decrease with aging in S. frugiperda females. A similar age-related change pattern was shown for parameters of flight muscle and flight capacity: their values were lower in young females, increased with time and peaked in middle-aged females. Similar results have also been found in S. litura (Yang, 2020) and B. dorsalis (Chen et al., 2015). These results not only suggest that flight muscles have some development after eclosion, both in species with a short adult period (such as S. frugiperda and S. litura, with an adult life span of about 10 days) and in species with a long adult period (such as B. dorsalis, with an adult life span of about 70 days), they also imply that myofibril thickness and sarcomere length may be important for flight performance.
Traits that influence organisms' fitness often are physiologically and genetically interrelated (Stearns, 1992, 2000; Schwenke et al., 2016). Therefore, it is likely that promoting the fitness value of one trait may cause a corresponding reduction in the fitness value of another. Reproduction and flight (especially long-distance migratory flight) may restrict each other, with enhanced reproductive activity and fecundity limiting flight capacity, and vice versa (Roff & Fairbairn, 2007; Nespolo et al., 2008). The trade-off between reproduction and flight is likely due to alternative allocation of limiting energetic resources because both reproduction and flight are extremely resource- and energy-demanding processes (Labbadia & Morimoto, 2015; Rodrigues & Flatt, 2016; Shih et al., 2020). Most lepidopteran species do not feed on a protein or lipid source as adults; even in those nectar-feeding species, only a small amount of protein or lipid may be obtained by adults (Gilbert, 1972; Baker & Baker, 1973). As a consequence, most materials for reproduction and survival must be obtained during the larval stage. Lepidoptera females, therefore, have a limited nutrition and energy supply and are likely to incur trade-offs between flight and reproduction.
In S. frugiperda, mating showed negative effects on parameters of flight muscle and flight capacity: mated females have shorter sarcomeres and smaller myofibril areas, as well as lower flight capacity than virgin females. Therefore, a trade-off between reproduction and flight may exist in S. frugiperda females. Trade-offs between reproduction and flight in insects may be due to an overlap in the resources used by flight muscles with those used during egg production (Gibbs & Van Dyck, 2010). Previous studies have shown that the energy materials for insect flight – including carbohydrates (principally trehalose), fats (mainly diacylglycerol), and proline (an amino acid) – mainly come from the fat body (Arrese & Soulages, 2010). On the other hand, egg development involves substantial mobilization of reserves from the fat body to the ovaries (Shiao et al., 2008; Arrese & Soulages, 2010). Moreover, mating- and oviposition-related physiological and behavioral processes are also energy costly (Xue et al., 2010; Yu et al., 2014). In S. frugiperda, virgin females showed slower ovarian development and egg production than mated females. Further, the flight capacity was negatively correlated to the number of eggs laid and total eggs in 9-day-old females. A previous study in S. frugiperda also showed that female flight distance and duration negatively correlated with the number of deposited eggs (Ge et al., 2021c). These results further support the hypothesis of trade-offs between reproduction and flight, in which mating is an important switch of resources reallocation between flight and egg production.
Overall, our study provides detailed insight into the structure and function of flight muscles and into the trade-off between reproduction and flight in S. frugiperda. Aside from providing new information on the life history and influencial factors in a migratory moth, our findings also contribute to the development of monitoring strategies and management plans for this newly invasive pest in Asia.
ACKNOWLEDGEMENTS
We acknowledge Science and Technology Planning Project in Key Areas of Yunnan Province (202001BB050002), Basic Research Projects of Yunnan Province (202201AS070025), Joint Special Project of Yunnan Province for Agricultural Basic Research (2018FG001-002), and National Natural Science Foundation Program of China (31760635) for funding this research.
AUTHOR CONTRIBUTIONS
Ping Guo: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); resources (equal); software (equal); writing – original draft (equal). Hong Yu: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review and editing (equal). Jin Xu: Conceptualization (equal); formal analysis (equal); funding acquisition (equal); project administration (equal); resources (equal); supervision (equal); writing – review and editing (equal). Yong-He Li: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal). Hui Ye: Conceptualization (equal); funding acquisition (equal); resources (equal); supervision (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this article. Other related data are available on request from the corresponding author.




