Revisiting the hymenopteran diploid male vortex: a review of avoidance mechanisms and incidence
Abstract
All hymenopterans (bees, ants, wasps, and sawflies) have haplodiploid sex determination. Generally, this involves haploid males developing from unfertilized eggs and diploid females developing from fertilized eggs, but diploid male production (DMP) frequently occurs. Some species have complementary sex determination (CSD), in which heterozygotes of a csd locus or loci are diploid females, hemizygotes are haploid males, and homozygotes are typically sterile diploid males. These diploid males underlie the diploid male vortex (DMV), the phenomenon of inbreeding reducing csd allelic diversity and causing increasingly higher production of sterile diploid males until the population dies out. The DMV has been cited as an extinction risk for many species across the order and a danger to both human-controlled and natural hymenopteran populations important in biological control, pollination, and endangered species conservation. However, it has been unclear how frequently it occurs. We review the known mechanisms for DMV avoidance. Many of these mechanisms are linked to lifestyle, and so we structure our investigation around eusocial lifestyle and non-eusocial (solitary or gregarious) species. We also review documented DMV incidence to make an inference about its prevalence. There are many means to avoid inbreeding or diploid male production, including eusocial-exclusive polygyny, polyandry, and diploid queen execution. For both eusocial and non-eusocial species these include biological mechanisms such as multi-locus CSD or non-CSD sex determination, diploid male inviability or brood removal, partial diploid male fecundity, and mating avoidance of kin or diploid males; or population genetic mechanisms such as gene flow and dispersal, balancing selection of csd alleles (with some being more prominent in eusocial vs. non-eusocial species). Documented cases of DMV are uncommon, and incidence is often tied to exacerbatory conditions such as habitat fragmentation and host declines. With this review we suggest that due to numerous avoidance mechanisms, DMV risk may not be as high as previously believed, and requires specific circumstances. In doing so we aim to better inform hymenopteran breeding and conservation efforts.
INTRODUCTION
Potentially more than a million species of bees, ants, wasps, and sawflies comprise the Hymenoptera (Forbes et al., 2018), collectively representing enormous ecological and economic importance (Latty & Dakos, 2019). For instance, pollinators are crucial for the health of natural environments and for human crop production (Quezada-Euán et al., 2018; Vrabcová & Hájek, 2020; van Huis, 2021) and many hymenopteran parasitoids are used as biocontrol agents, exceeding the number of species used and market share value of all other insect orders combined (van Lenteren et al., 2018; Leung et al., 2020). Across this incredible diversity is the universal trait of haplodiploid sex determination.
Most species have arrhenotokous parthenogenesis: unfertilized eggs develop into haploid males and fertilized eggs develop into diploid females (Heimpel & de Boer, 2008). Haplodiploidy also occurs in other groups (e.g., other insects, mites, rotifers, and nematodes), representing around 15% of all animal species (Liu & Smith, 2000). It is often suggested that haplodiploids have greater resilience against inbreeding depression because deleterious recessive alleles are purged from the population in the haploid state (for hymenopterans, the male) (Werren, 1993). Although this cannot protect against female-specific disadvantage (Whitehorn et al., 2009b), this has resulted in the idea that hymenopterans are generally less vulnerable to extinction than diploid systems. This in turn may have resulted in less investigation or effort invested into their conservation (Zayed & Packer, 2005; van Wilgenburg et al., 2006).
However, individuals with aberrant ploidy, typically diploid males, frequently appear in numerous and phylogenetically diverse hymenopteran species. Over 15 years ago, Zayed & Packer (2005) published a seminal paper on the diploid male vortex (DMV). They proposed that inbreeding leads to a significantly increased extinction risk in species with complementary sex determination (CSD). This is the first described (Whiting, 1943) and best-characterized (Beye et al., 2003) hymenopteran sex determination mechanism. In CSD, sex is regulated through one sex locus (sl-CSD) or multiple sex loci (ml-CSD), although it is unknown which is the ancestral state (Asplen et al., 2009). Heterozygosity results in the development of females, hemizygosity results in haploid males, and homozygosity results in diploid males (Whiting, 1943; Asplen et al., 2009). Generally, high allelic richness for these sex loci warrants high production of haploid males and diploid females (de Boer et al., 2015). However, when inbreeding or genetic bottleneck occurs, as is common for small endangered populations or breeding populations with founder effects, genetic diversity declines, leading to diploid male production (DMP) (Zayed & Packer, 2005). Diploid males are almost always either inviable or effectively sterile (Harpur et al., 2013). When they are inviable, this has the least impact on a population, because they cannot mate and will therefore only reduce the number of reproductives in their own generation. In contrast, when effectively sterile diploid males can mate with females, they typically only produce diploid sperm that usually creates inviable triploid zygotes, causing the female proportion to decline in the next generation (van Wilgenburg et al., 2006; Harpur et al., 2013). As the population becomes smaller and increasingly inbred, the proportion of diploid males increases with every generation until it goes extinct (Zayed & Packer, 2005).
The conditions of the DMV are: females produce offspring at a low rate and fixed sex ratio, females cannot exercise mate choice, and the population is fragmented. The risk for DMV would logically be especially pronounced for species with sl-CSD. Species with ml-CSD are less likely to be homozygous for all csd loci (de Boer et al., 2008), and any analogous effects for non-CSD species have gone largely unexamined (Leung et al., 2019). Figure 1 illustrates these various sex determination systems. In sharp contrast to haplodiploidy generally protecting against inbreeding depression, the DMV for hymenopterans with CSD represents an extreme case of it. It has been suggested special attention must be paid to the conservation of these species and that the rate of DMP can be used as a measure of a population’s genetic health (Zayed & Packer, 2001, 2005; Darrouzet et al., 2015; Betti & Lee, 2020). The DMV has been alluded to be a widespread problem for various hymenopteran groups including wild pollinators (e.g., Steffan-Dewenter et al., 2005; Faria et al., 2016; López-Uribe et al., 2017), in colonies reared for crop pollination and honey production (e.g., Whitehorn et al., 2009a; Zareba et al., 2017; Betti & Lee, 2020), and those used in biological control (e.g., de Boer et al., 2012; Fauvergue et al., 2012; Leung et al., 2020). However, it is unclear how common inbreeding avoidance, other DMP preventative mechanisms, and DMV incidence actually are.
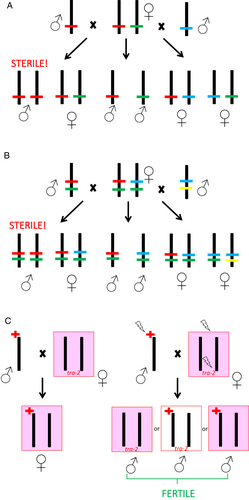
The DMV is broadly referenced across hymenopteran literature, potentially reflecting impact for many taxa. However, given its extreme consequence, strong evolutionary pressure might also exist to counter it. In this paper we review the literature on DMP and DMV avoidance mechanisms and documented DMV incidence, considering both theoretical models and empirical case studies. In doing so we aim to provide clearer resolution on how broadly the DMV affects hymenopteran populations, and guide further research on their breeding, ecology, and conservation. We distinguish discussion on eusocial vs. non-eusocial species due to these lifestyles having different conditions and degrees of conspecific interaction, which are reflected in evolution of different kinds of DMP and DMV avoidance mechanisms. It should be noted that a CSD mechanism, the primary driver of the DMV, has not been confirmed or ruled out for all the species discussed in this review, but we note what is known about sex determination in these cases.
THE SIGNIFICANCE OF LIFESTYLE TO DIPLOID MALE PRODUCTION AND THE DIPLOID MALE VORTEX
The Hymenoptera can be split between eusocial and non-eusocial species, with the latter consisting of solitary and gregarious species. This is not a phylogenetic distinction, as eusociality evolved independently multiple times (Peters et al., 2017). Complementary sex determination also commonly occurs for both groups and is also not a monophyletic trait although it is believed to be the ancestral hymenopteran sex determination system (de Boer et al., 2008; Asplen et al., 2009). However, these lifestyles differ in their degree of conspecific interaction, morphological and caste variation, and basic reproductive biology that corresponds to some distinction of DMP and DMV avoidance mechanisms. Accordingly, we provide an introduction to the basic biology of eusocial and non-eusocial species and then divide our discussion for these two general categories.
Eusocial species are defined by co-habiting adults, shared juvenile care, few reproductive individuals, and overlapping generations (Wilson, 1971). It includes 300–400 bee species (Apidae), ca. 900 wasp species (in Sphecidae and Vespidae), and all the ants (Formicidae; although eusociality has been secondarily lost for a few derived species) (Plowes, 2010). Eusociality falls along a gradient. Highly eusocial species have morphologically well-defined castes with one or several reproducing queens and many completely sterile female workers in large, long-lived colonies. The weakly eusocial species have less morphological distinction between reproductive and non-reproductive individuals and form small and short-lived colonies. For some eusocial species, the workers may also have some reproductive ability or the potential to become queens themselves, with unmated female workers producing haploid male offspring (Bourke, 1988; Wenseleers et al., 2004; Helantera & Sundstrom 2007; Cardinal & Danforth, 2011). Workers successfully mating with males and producing diploid female offspring is not known to occur. The number of males they contribute to the colony varies greatly by species and circumstance. For example, the ovaries of honeybee workers are inactivated by brood and queen pheromone, and the death of a queen can result in ovary reactivation in these unmated females and thus increased male production (Traynor et al., 2014). It is unclear how such worker-produced males might impact DMP or DMV outcomes, e.g., whether they mate with intra- or inter-colony queens. To our knowledge, this has not been investigated to date.
The non-eusocial solitary and gregarious species are far more numerous and include the megadiverse and monophyletic parasitoids (Peters et al., 2017). The categorization of solitary vs. gregarious species usually references development. Solitary species have individualized juvenile development, with eggs being oviposited singly (in the case of parasitoids, on or within hosts). Gregarious species are characterized by many offspring developing within a single host and therefore competing for resources (Pennacchio & Strand, 2006). Upon emergence, many gregarious species mate with proximate siblings before dispersing from the natal patch (Hamilton, 1967; Hardy, 1994). In adulthood both solitary and gregarious species may temporarily live together with con-specifics in large groups. Unlike eusocial species, this is not necessarily cooperative. Although there may be benefits such as increased reproductive rate (Itô et al., 1988), this behavior has been attributed to the ‘selfish herd’ theory (Hamilton, 1971), with aggregation reducing individual susceptibility to predation or parasitization (Wcislo, 1984; Itô et al., 1988; Landi et al., 2002) (although this itself may be an evolutionary precursor to eusociality; Rosenheim, 1990).
Although some characteristics are shared between these groups (i.e., primitively eusocial species with gregarious species, quasi-gregarious solitary species with gregarious species), in general terms a eusocial, colony-forming lifestyle vs. a non-eusocial lifestyle represents strongly contrasting reproductive strategies and degrees of con-specific decision-making. This in turn influences what outcomes are possible in concerning inbreeding and diploid male interactions. As such, we frame our review on DMP and DMV avoidance around these hymenopteran lifestyles. Note that although the distinction of solitary vs. gregarious also indicates different levels of intraspecific interaction and there are some aspects of DMP and DMV specific to each, we organize this section by mechanism rather than discussing each separately. That is because there is less specificity in mechanisms for these two, relative to the eusocial species, and more overlap in general biology (e.g., parasitoidism).
AVOIDANCE MECHANISMS OF THE EUSOCIAL HYMENOPTERA
The reproductive output of most eusocial Hymenoptera is high (Shik et al., 2012), seemingly violating the restricted reproduction requirement of the original DMV model. However, the effective population sizes of these species are typically small because most offspring produced are non-reproductive female workers (Romiguier et al., 2014). Life-history optimality models, validated by field data, predict that queens first build up colonies by exclusively or primarily producing workers before switching to producing reproductives right before the breeding season (Macevicz & Oster, 1976). The timing and degree of overlap in caste production varies among species, but this phenomenon is for example strongly defined in bumblebees. Queens have a distinct ‘switch point’ (Duchateau & Velthuis, 1988), when they stop producing diploid female workers and start producing reproductive haploid drones (males) and diploid queens (Vidal et al., 2021). We did not find any formal investigations on how timing of caste production influences the DMV. Still, it is perhaps important to note before reviewing the existing literature on DMV that this basic tenet of eusocial biology may inherently guard against the DMV. Diploid male production would be concentrated in the early phase from the diverted development of would-be diploid female workers, and may not be as likely to encounter young queens in the new breeding season as haploids.
All studied eusocial species are known or likely CSD species, and diploid male avoidance in these taxa was discussed well before the DMV was described. For example, polyandry (females mating with more than one mate) and polygyny (multiple queens) is a common but variably expressed trait across and even within eusocial species (Ratnieks, 1990; Pamilo et al., 1994). The load hypothesis suggests that they evolved to reduce the number of diploid males produced, because a monandrous, monogynous queen of a sl-CSD species mated with a brother or a diploid male would result in only male offspring and prevent colony establishment (Crozier & Page, 1985). One extrapolation of this hypothesis is that if there is high mortality for diploid males early on in their development, their impact to the population is minimal (Pamilo et al., 1994). Under these circumstances monogyny and monandry would be less deleterious and polygyny and polyandry would not be predicted. This has been reflected in the range of diploid male frequency in Formica ants, for which lower or no diploid male colonies indicates brood removal. High numbers of diploid males (up to 10% of all males) occurred in polygynous colonies of Formica aquilonia Yarrow, Formica polyctena Foerster, and Formica truncorum Fabricius; some were present in monogynous/weakly polygynous Formica rufa L., Formica lugubris Zetterstedt, and F. truncorum; and they were completely absent from strongly monoandrous and monogynous colonies of Formica exsecta Nylander and Formica pratensis Retzius (Pamilo et al., 1994). In contrast, the diploid male brood is not removed in Solenopsis invicta Buren, so the female worker fraction is reduced and the upkeep of mature diploid males strains colony resources. Thus, diploid-producing laboratory colonies of the monogynous fire ant S. invicta go extinct (Ross & Fletcher, 1986). However, counter to this pattern, Apis mellifera L. and Apis cerana Fabricius honeybees remove diploid male brood but are polyandrous (Woyke, 1963, 1980). Many subsequent studies on diploid male impacts on eusocial species, including those on the DMV, still focus on diploid male viability and queen reproductive behavior.
Several studies investigate whether diploid males are prevented from reaching adulthood. In field-collected samples of the leaf-cutting ant Atta sexdens (L.) haploid males were morphologically distinguishable from diploid males based on size (Armitage et al., 2010). When the workers were assayed for the presence of diploid males, few were found, even though corresponding worker brood was ca. 10% diploid males. This would indicate that workers recognize and eliminate diploid male brood, preventing them from reaching adulthood, which also occurs for Apis honeybees (Woyke, 1963; Ratnieks, 1990). For the tropical fire ant Solenopsis geminata (Fabricius), in addition to founding nests with other queens to mitigate DMP, the queen herself cannibalizes her diploid male brood, possibly to recover nutrients for further reproduction (Lenancker et al., 2019). The mechanisms by which workers of various species identify diploid males are unclear. It has been suggested that they do not actually recognize the ploidy level of males, but find male larvae where female worker brood should be (Thiel et al., 2014). An analysis of haploid and diploid A. mellifera larvae did not find any unique cuticular compounds on the diploid males, but the different proportions are apparently sufficient for workers to recognize and remove brood from cells (Santomauro et al., 2004). Alternatively, although hymenopteran diploid males are generally not larger than haploids or are only slightly bigger (Leung et al., 2019), morphological differences for haploid and diploid males occur for various species – e.g., wing morphologies of Bombus terrestris (L.) bumblebees (Gerard et al., 2015); epiproct anal plates of A. mellifera larvae (Santomauro & Wolf, 2002) – and could form the bases of how diploid males are recognized and removed. However, as most eusocial species conduct much of their life history in low-light nests, the use of morphological cues for diploid male recognition is not as likely as those based on chemical communication. Chemical cues are indeed used extensively to facilitate various interactions between nestmates for foraging, brood rearing, defense, and suppressing reproduction in workers (Richard & Hunt, 2013).
Other species have an even more aggressive strategy. Workers of the stingless bees Scaptotrigona depilis (Moure) (Vollet-Neto et al., 2017), Melipona quadrifasciata Lepeletier (Ratnieks, 1990), and Melipona scutallaris Latreille (Alves et al., 2011) execute queens that produce diploid males. In the case of S. depilis, the presence of juvenile adult males does not initiate this behavior. Authors measured cuticular hydrocarbons (CHCs), known to be important for Hymenoptera in individual recognition for haploid and diploid males. Significant differences were only found on the 10th day of adulthood onward, which corresponds with when diploid male-producing queens are executed (Vollet-Neto et al., 2017). It is possible that for other species chemosensory mechanisms for diploid male detection are only active when they reach maturity. For the fire ant S. invicta, which does not have brood removal, juvenile diploid males have ‘diploid female-like’ expression as pupae and switch to ‘haploid male-like’ expression as adults (Nipitwattanaphon et al., 2014). If this occurs for other hymenopterans, it could allow juvenile diploid males to resemble female brood and escape worker detection.
The ability of queens to identify and mate discriminate against diploids and brothers is inconsistent among species (Harpur et al., 2013). For instance, it is unclear whether diploid male A. sexdens mate with queens. However, Armitage et al. (2010) found that diploid males produce diploid sperm, which can only create triploid zygotes upon fertilization of a haploid egg that are almost always inviable for Hymenoptera (Zayed & Packer, 2005; Asplen et al., 2009). However, A. sexdens queens are polyandrous, so mating with a sterile diploid male would not prevent a reproductive female from producing female offspring if she also mated with a fertile haploid. Moreover, in species with polyandry, haploid sperm may outcompete diploid sperm or sperm with the same csd allele(s) as the egg, either actively by being better at fertilizing eggs or passively by resulting in more viable offspring (as suggested by the simulation study of Hein et al., 2009). Numbers and sperm health may also give haploid males an advantage, as A. sexdens diploid males produce 3.5× less and more elongated sperm than haploids (Armitage et al., 2010). Other polyploid males may also have impaired sperm; for example, diploid B. terrestris have larger, fewer sperm than haploid counterparts, in addition to smaller testes, and produce very few viable offspring (Duchateau & Mariën, 1995).
In species with monandrous queens, mating avoidance with diploid males or siblings is critical because no healthy sperm from an alternative mate is available. One such species is the sl-CSD bumblebee, B. terrestris. Under captive conditions, it takes longer for queens to mate with brothers than non-sibling males. This suggests that they are able to recognize brothers and adjust sexual behavior accordingly (Whitehorn et al., 2009b). Interestingly, another study found that there is no inbreeding avoidance in B. terrestris and inbred matings occur more quickly than outbred ones (Bogo et al., 2018). Additionally, a study on male cephalic labial gland secretions, which are key to pre-mating recognition by queens (Baer, 2003), found no significant differences in chemical composition between haploid and diploid males (Lecocq et al., 2017). Therefore, B. terrestris queens may not be able to recognize the ploidy level of males to discriminate against diploid mates, although their ability to recognize and avoid brother matings might contribute to reduction in inbreeding and DMP (Whitehorn et al., 2009b). Alternatively, inbreeding tolerance may be inherent (Bogo et al., 2018). There is a notable case of extreme genetic bottleneck for this species; an invasive population may have been founded by as few as only two individuals in Tasmania, Australia (originating from a population that was intentionally introduced in New Zealand). Paradoxically the population is not experiencing a DMV despite low genetic diversity (Schmid-Hempel et al., 2007).
Dispersal is an important means for avoiding diploid male production as it increases gene-flow and promotes higher allelic richness for csd loci (Faria et al., 2016). Although the assumption of the DMV is that no dispersal occurs (Zayed & Packer, 2005), the computational model of Hein et al. (2009) suggests that a dispersal rate of only 5% prevents the initiation of the DMV for a population, or even only 1% if diploid males are inviable. In this model even small populations can survive without significant loss of allelic richness over as much as 40 generations. In eusocial Hymenoptera, this is a particularly long time, because queens can live for as long as 30 years and produce offspring in widely spaced intervals (Vidal et al., 2021). In another model measuring the effects of male flight ability, csd richness, and dispersal barriers, dispersal is more important for the avoidance of DMP than csd allelic diversity (Winkert et al., 2019). Many eusocial Hymenoptera species have nuptial flights: queens and drones disperse away from their own colony and produce primarily non-reproductive females, which allows for very few sib-matings in the population (Vidal et al., 2021). Supporting these models, B. terrestris dispersal is thought to be the main contributor to the low rates of diploid male production (2%) (Lecocq et al., 2017). Vidal et al. (2021) observed a different form of mate choice and specialized dispersal in the ant Cardiocondyla elegans Emery. In this species, queens do not directly select a male to mate with themselves, but are subject to third-party sexual selection. Worker ants forage meters away from their colonies, and will carry their queen to the entrance of the other colonies so that she mates with an unrelated male. Incidentally, a close relative (Cardiocondyla obscurior Wheeler), inbreeds frequently, and whereas it has inbreeding depression effects of brood mortality, shorter queen lifespan, and more male-biased sex ratios, diploid males do not appear – a possible ml-CSD mechanism in these taxa could provide additional protection against the DMV (Schrempf et al., 2006).
Balancing selection has also been implicated in the prevention of diploid male production in eusocial species. Defined as strong negative frequency dependent selection, under balancing selection the diversity of sex-determining loci remains higher than that of declining neutral diversity. This is reflected in populations that have undergone bottlenecks and have become genetically depauperate overall still retaining high csd loci diversity and general population health. It has long been known that the csd locus is under balancing selection for the species it was first described, the honeybee A. mellifera (Charlesworth, 2004). More recent studies have also examined balancing selection as a factor for the invasion success of Asian A. cerana honeybees. Although diploid male frequency was not measured, populations in Australia (Gloag et al., 2017) and the wider Pacific region (Ding et al., 2021) were demonstrated to have fewer alleles for csd than native populations, and at skewed frequencies following invasion. However, in just a few years this skew was corrected. The authors use this documentation of balancing selection to explain why, despite a bottleneck founder effect, diploid male frequency would decrease rather than increase over time. Ding et al. (2017) note that extreme polyandry also occurs for A. cerana, so it may work in concert with balancing selection to prevent the DMV for this species.
A synthesis of these studies is that the eusocial Hymenoptera might be especially well positioned to avoid the DMV, either through DMP avoidance or tolerance to diploid male impacts. Indeed, diploid males are not unusual, usually representing <10% but accounting for up to 100% of males even in seemingly well-functioning colonies (Zayed & Packer, 2001; Cournault & Aron, 2009). For example, the primitively eusocial sl-CSD bee Halictus poeyi Lepeletier has both a high degree of inbreeding (18.2–100% of the male and female having the same csd allele, also known as matched matings) and diploid male production (9.1–50%), but is not negatively impacted for unknown reasons, with the species being abundant in its native Florida (USA) range (Zayed & Packer, 2001). One perspective might be that extreme reproductive limiting is already inherent for most species with the sterile worker class, so depending on context, the impacts of diploid males may not be that harmful. For example, diploid males of ants Hypoponera opacior (Forel) (Kureck et al., 2013) and Tapinoma erraticum (Latreille) (Cournault & Aron, 2009) both have diploid sperm and can produce a large portion of sterile triploid female offspring in their colonies. It is especially prevalent for H. opacior which has both winged and wingless reproductive forms, because wingless males including diploids often mate with siblings and practice mate-guarding (Kureck et al., 2013). However, these triploid daughters are viable and contribute to the colony as workers like diploid females. In the case of H. opacior, there is also evidence that a dispersal mechanism is in place to increase outbreeding: inbred colonies will shift investment into reproductives over workers, particularly in the outbreeding winged class (Kureck et al., 2012). The DMP and DMV avoidance mechanisms of the eusocial species of this section are summarized in Table 1.
| Group | Species | Population type | Observed DMP | DMV/inbreeding avoidance | Avoidance mechanism | Sex determination | References |
|---|---|---|---|---|---|---|---|
| Ant | Atta sexdens | Natural | x | x | DM brood removal; polyandry | Assumed sl-CSD | Armitage et al., 2010 |
| Cardiocondyla elegans | Natural | x | Third party sexual selection (queen transportation) | Possible ml-CSD | Vidal et al., 2021 | ||
| C. obscurior | Natural | x | Polygyny; possible ml-CSD | Possible ml-CSD | Schrempf et al., 2006 | ||
| Formica aquilonia | Natural | x | x | Polygyny | Assumed sl-CSD | Pamilo et al., 1994 | |
| F. exsecta | Natural | x | DM brood removal | Assumed sl-CSD | Pamilo et al., 1994 | ||
| F. lugubris | Natural | x | x | Polygyny | Assumed sl-CSD | Pamilo et al., 1994 | |
| F. polyctena | Natural | x | Polygyny | Assumed sl-CSD | Pamilo et al., 1994 | ||
| F. pratensis | Natural | x | DM brood removal | Assumed sl-CSD | Pamilo et al., 1994 | ||
| F. rufa | Natural | x | x | Polygyny | Assumed sl-CSD | Pamilo et al., 1994 | |
| F. truncorum | Natural | x | x | Polygyny | Assumed sl-CSD | Pamilo et al., 1994 | |
| Hypoponera opacior | Natural | x | x | Higher investment in winged reproductives in inbred colonies (dispersal) | Assumed sl-CSD | Kureck et al., 2012 | |
| Natural | x | x | Fertile DMs with worker-like triploid offspring | Assumed sl-CSD | Kureck et al., 2013 | ||
| Solenopsis geminata | Captive (invasive simulation) | x | x | Polygyny; DM brood removal (by queen) | Assumed sl-CSD | Lenancker et al., 2019 | |
| S. invicta | Natural | x | None, low DMP in native populations | sl-CSD | Krieger et al., 1999 | ||
| Natural (invasive) | x | None, DM producing colonies die out | sl-CSD | Ross & Fletcher, 1986* | |||
| Tapinoma erraticum | Natural | x | x | Fertile DMs with worker-like triploid offspring | sl-CSD | Cournault & Aron, 2009 | |
| Bee | Adrena scotica | Natural | x | x | ml-CSD; mate choice; sperm selection | ml-CSD | Paxton et al., 2000 |
| Apis cerana | Captive | x | x | Polyandry; DM brood removal | sl-CSD | Woyke, 1980 | |
| Natural (invasive) | x | Polyandry | sl-CSD | Ding et al., 2017 | |||
| Natural (invasive) | x | Balancing csd allele selection | sl-CSD | Gloag et al., 2017 | |||
| A. mellifera | Captive | x | x | None, extra resources rescue colonies, parasites and monoculture exacerbates DMP | sl-CSD | Betti & Lee, 2020 | |
| Captive | x | x | DM brood removal (by compound) | sl-CSD | Santomauro et al., 2004 | ||
| Captive | x | x | DM brood removal | sl-CSD | Woyke, 1963; Ratnieks, 1990 | ||
| Bombus florilegus* | Natural | x | None, decline and habitat fragmentation | Assumed sl-CSD | Takahashi et al., 2008* | ||
| B. muscorum* | Natural | x | None, decline and habitat fragmentation | Assumed sl-CSD | Darvill et al., 2006* | ||
| B. terrestris | Captive | x | None, no inbreeding avoidance; inbred matings occur faster than outbred matings | sl-CSD | Bogo et al., 2018 | ||
| Natural | x | High genetic variation (csd allelic variation) | sl-CSD | Duchateau et al., 1994 | |||
| Captive | x | None, morphological differences in haploid and diploid males | sl-CSD | Gerard et al., 2015 | |||
| Captive | x | Both inbreeding and outbreeding depression | sl-CSD | Gerloff & Schmid-Hempel, 2005 | |||
| Natural (Invasive) | None, population expansion despite extreme genetic bottleneck | sl-CSD | Schmid-Hempel et al., 2007 | ||||
| Captive and Natural | x | None, queens cannot tell difference between haploid and diploid male labial compounds | sl-CSD | Lecocq et al., 2017 | |||
| Captive | x | x | Kin discrimination | sl-CSD | Whitehorn et al., 2009b | ||
| B. terrestris* | Captive | x | None, DM producing nests too small to survive | sl-CSD | Bortolotti et al., 2020 | ||
| B. terricola* | Natural | None, decline correlation with inbreeding and positive selection for immunity genes | sl-CSD | Kent et al., 2018 | |||
| Halictus poeyi | Natural | x | None, high inbreeding and DMP does not affect population for unknown reasons | Assumed sl-CSD | Zayed & Packer, 2001 | ||
| Melipona quadrifasciata | Captive | x | DMP queen execution | Assumed sl-CSD | Kureck et al., 2013 | ||
| M. scutellaris | Natural and Captive | x | x | Extra resources rescue colonies; DMP queen elimination | Assumed sl-CSD | Noguiera-Neto, 2002 | |
| Melipona spp. | Captive | x | None, suggested 44 colony minimum to avoid DMV | Assumed sl-CSD | Ratnieks, 1990 | ||
| Captive | x | None, extra resources rescue colonies, high inbreeding tolerance | Assumed sl-CSD | Kerr, 1985 | |||
| Scaptotrigona depilis | Captive | x | x | DMP queen execution | sl-CSD | Vollet-Neto et al., 2017 | |
| Wasp | Polistes dominulus | Natural (invasive) | No csd allele bottleneck; inbreeding advantage | Assumed sl-CSD | Liebert et al., 2010 | ||
| P. chinensis antennalis | Natural (invasive) | x | None; invasion success despite genetic bottleneck | Assumed sl-CSD | Tsuchida et al., 2002, 2014 | ||
| Vespa velutina nigrithorax* | Natural (invasive) | x | None, high DMP | Assumed sl-CSD | Darrouzet et al., 2015 | ||
| Vespula germanica | Natural (Invasive) | x | x | Kin discrimination | Assumed sl-CSD | Masciocchi et al., 2020 |
- An asterisk denotes DMV incidence. ‘Natural’ refers to a population observed in the field, or materials taken directly from the field. ‘Captive’ refers to laboratory-reared populations. Avoidance mechanisms are specific to each reference. sl-CSD, ml-CSD: single-locus, multi-locus complementary sex determination.
AVOIDANCE MECHANISMS OF THE NON-EUSOCIAL HYMENOPTERA
The general biology of non-eusocial (solitary and gregarious) Hymenoptera is markedly different from that of the eusocial species. This group is dominated by the parasitoids, which are mostly monandrous (Harpur et al., 2013) and cannot alleviate inbreeding or DMP effects with polyandry. Also, eusocial species bred by humans are mainly used as honey producers or pollinators, but the applied relevance of many parasitoid wasps, including CSD species, lies in their usage as biocontrol agents (Nair et al., 2018; Leung et al., 2020; Nonaka & Kaitala, 2020). Although diploid males are rarely seen in natural native populations of non-eusocial species (Boulton et al., 2015), introduced and captive breeding populations (e.g., biocontrol agents and endangered species) are believed to be vulnerable to the DMV because of founder effects (Stouthamer et al., 1992; de Boer et al., 2012; Leung et al., 2020). And yet, whereas CSD species are more easily detected (determined through the presence or absence of diploid males with inbreeding assays as in, e.g., Cowan & Stahlhut, 2004; Schrempf et al., 2006; Ma et al., 2013) most solitary and gregarious hymenopterans are non-CSD. This is due to the extremely speciose parasitoid superfamily Chalcidoidea (22 500–500 000 species; Heraty et al., 2013) along with the moderately sized Cynipoidea superfamily lacking CSD entirely (Beukeboom et al., 2007; Heimpel & de Boer, 2008; Asplen et al., 2009). Other parasitoid superfamilies have a mix of CSD and non-CSD species, or have not been tested for its presence (Verhulst, 2010). However, diploid males are known for some non-CSD species (e.g., Whiting, 1960; Ma et al., 2013, 2015) and may occur more widely. Therefore, although we focus on CSD species here due to their basis for the original DMV concept and research bias for these taxa, we also discuss known diploid male impacts of non-CSD species.
Venturia canescens (Gravenhorst) is a sl-CSD solitary endoparasitoid of Lepidoptera eggs and is used in the biological control of several moth pests of stored cereal products (Eliopoulos, 2006). In a captive population of V. canescens, inbreeding did not only result in diploid male production, but also in delayed egg maturation in females (Vayssade et al., 2014). This may impair the capability of females to disperse, which would only increase the rate of inbreeding and speed up the increase of diploid male production. A field study by Collet et al. (2016) measured the genetic diversity of several V. canescens populations. They found that the genetic diversity in smaller isolated island populations and captive populations was lower than those on the mainland, which would have more gene flow. As expected, DMP was significantly higher for these populations and decreased population growth rate. However, no evidence was found for (near) extinction of such populations. Similarly, for the solitary and highly specialized solitary orchid bees that are probably sl-CSD (Giangarelli et al., 2015), there is no significantly increased extinction risk for island populations and DMP is minimal even with low genetic diversity, making diploid males a poor indicator for population health for these taxa, such as Euglossa cordata (L.) (Boff et al., 2014) and Euglossa dilemma Bembé & Eltz and Euglossa viridissima Friese (Soro et al., 2017).
The role of mate discrimination in solitary and gregarious Hymenoptera in DMP avoidance has also been explored. Assortative mating against closely related individuals would prevent DMP and the downstream DMV. Collet et al. (2020) performed a choice experiment for V. canescens that offered females a sib and non-sib male. Females did not seem to recognize kin or discriminate against them for mating. However, for field observations, the frequency of mating between siblings was much lower than between non-related individuals. As in their previous study (Collet et al., 2016) they emphasized the high dispersal ability of males, which would result in fewer sib encounters and less inbreeding (Collet et al., 2020). However, Metzger et al. (2010) found evidence for female-based kin discrimination in this species. Like Collet et al. (2020), they found no significant difference in a sib vs. non-sib choice experiment, but in a no-choice experiment, females mated twice as frequently with non-sib males. Conversely, Chuine et al. (2015) tested for males discriminating against mating with related females. Males of V. canescens actively search for females, being attracted to a combination of volatile chemicals and kairomones released by the females and their host residues (Chuine et al., 2015). Males spent less time in patches previously occupied by related females (Chuine et al., 2015), so both sexes may contribute inbreeding avoidance ability in V. canescens. The females of the gregarious CSD sawfly Neodiprion lecontei (Fitch) also suffer a fitness cost from inbreeding as diploid male larvae are inviable; they may circumvent this to a degree through kin discrimination, as they are less likely to mate with sibs than non-sibs in no-choice experiments (Harper et al., 2016).
But if reproductively impaired diploid males are already in the population, can females avoid mating with them to prevent the DMV? A review of hymenopteran haploid vs. diploid male mating competitions indicates a pattern of females not distinguishing between them (Harpur et al., 2013). However, there are some exceptions. Fauvergue et al. (2015) tested the mating success of diploid vs. haploid V. canescens males under simulated natural conditions. Their courtship behaviors were similar, but diploid males were less successful at mating. The diploid males that did successfully mate did not produce viable offspring, as males of this species have non-functional diploid sperm and thus are effectively sterile (Chuine et al., 2015; Collet et al., 2016). Thiel et al. (2014) tested whether female mating discrimination accounts for an unexpectedly low number of diploid males in captive populations (Whiting, 1943; Petters & Mettus, 1980; Ode et al., 1997) of the gregarious ectoparasitoid Bracon brevicornis Wesmael, a biocontrol agent of larval lepidopteran pests that likely has CSD (Ferguson et al., 2020). No discrimination against diploid males was found. Rather, in Bracon spp., high diploid male brood mortality rate seems to account for their low adult incidence. Diploid male brood mortality is 35% in B. brevicornis, significantly higher than that of diploid females (Thiel et al., 2014), and as high as 90% in a close relative, Bracon hebetor Say (Whiting, 1943; Petters & Mettus, 1980; Ode et al., 1997). For B. hebetor, it is also known that the diploid sperm of diploid males are incapable of penetrating eggs (MacBride, 1946).
Studies have examined whether Cotesia glomerata (L.), a gregarious ectoparasitoid of Lepidoptera and a common biocontrol agent (Le Masurier & Waage, 1993), uses kin discrimination to prevent inbreeding depression. Because kin recognition can be mediated through female perception of cuticular hydrocarbons (CHCs), Ruf et al. (2010) looked for CHC differences in related and unrelated males. Significant differences were not found, which corresponds to females not showing a preference in a mate choice experiment. It has been suggested that chemosensory profile differences between haploid and diploid males may exist in volatiles instead (Herzner et al., 2006; Metzger et al., 2010; Chuine et al., 2015). As these are environmentally released signals that can mix, females may become confused distinguishing between relative and non-relative males in close proximity. In comparison, in the sl-CSD solitary bee-killer wasp Philanthus triangulum Fabricius, males scent mark territories to attract females, which may allow females to differentiate between kin and non-kin (Herzner et al., 2006).
Females of C. glomerata also disperse away from their natal patches, which could be to avoid breeding with brothers but may be for other reasons. Ruf et al. (2011) isolated kin competition, food availability, habitat variability, and inbreeding avoidance as explanatory variables, and found that inbreeding avoidance accounts for 30% of female dispersal behavior. This may partially underlie the unexpectedly low frequency of diploid males in wild populations, particularly as juvenile mortality does not occur for this species (Ruf et al., 2013). Similar to eusocial species, dispersal may be a general DMV avoidance mechanism for many parasitoids, which often display single sex dispersal. They also commonly have low sexual receptivity upon hatching and will only mate after a significant period of time when most individuals have already left their natal patch. Additionally, one sex can have a delayed hatching, reducing the chances of mating with a sibling from the same host (Boulton et al., 2015). However, some species are inbreeding-prone, such as the sl-CSD solitary wasp Euodynerus foraminatus (de Saussure), which has 55–77% sibling matings and yet paradoxically has few diploid males in the population for unknown reasons (Cowan & Stahlhut, 2004). A general synthesis from these various studies might be that although mate choice is a theoretical diploid male avoidance mechanism, the current evidence does not seem to suggest that it plays a major role in this. Rather, dispersal seems more effective and prevalent.
Unusually, diploid Cotesia vestalis (Haliday) (de Boer et al., 2007), C. glomerata (Elias et al., 2009), and Cotesia flavipes Cameron (Trevisan et al., 2016) males are fertile and produce many female offspring (that are triploid), although at lower numbers than haploids. This has only been observed in a few other species such as Mastrus ridens Horstmann, a sl-CSD gregarious ectoparasitoid and biocontrol agent of codling moth (Zaviezo et al., 2018), and Nasonia vitripennis (Walker), a non-CSD gregarious parasitoid of blowfly pupae sometimes used as a biocontrol agent for stable flies (Kaufman et al., 2001). It is unknown how these viable triploid females might impact populations. However, the genus Cotesia interestingly has diverse sex determination mechanisms; C. glomerata has sl-CSD (Zhou et al., 2006), C. vestalis has ml-CSD (de Boer et al., 2007), Cotesia rubecula (Marshall) is a ml-CSD (two loci) species that can functionally resemble a sl-CSD species if there is a genetic bottleneck (de Boer et al., 2012), and C. flavipes and Cotesia sesamiae (Cameron) do not seem to be sl-CSD unless this sex determination mechanism is obscured by absolute diploid male mortality (Niyibigira et al., 2004). Although polyploid assays have not been performed for all these species, the range of sex determination systems in this species complex may be helpful for more direct comparisons of how each kind impacts diploid male and triploid female contributions to the DMV.
Several DMV avoidance mechanisms have been documented in solitary bees. In the case of the solitary bee Lasioglossum leucozonium (Schrank), a presumed sl-CSD species, a severe bottleneck occurred when this European species invaded North America. Nonetheless, a comparison of neutral microsatellite markers and sex-determining alleles determined that the latter is under balancing selection. This may explain L. leucozonium’s invasive success despite the North American population likely originating from a single mated female and 30% of diploid offspring being diverted into male development (Zayed et al., 2007). In another bee, Adrena scotica Perkins, the inbreeding rate is as high as 44%, which would theoretically mean a corresponding high rate of diploid male production of 11%. However, the frequency of diploid males is only 0.3% (Paxton et al., 2000). It has not been examined further as to why this is the case. Paxton et al. (2000) speculated that A. scotica has ml-CSD or that unmatched sperm selection may occur in this species. But notably, unlike other solitary bees, females of this species can occasionally nest together for aggregate care of brood (Armitage et al., 2010; Thiel et al., 2014). As this behavior could be considered precursory to primitive eusociality, we speculate that A. scotica may have DMP avoidance mechanisms like those used by eusocial species, possibly to do with the larger number of caregiver females interacting with diploid males from various families.
Host effect has rarely been studied as a contributory factor to DMP and the DMV, but a recent study investigated the gregarious hyperparasitoid Mesochorus cf. stigmaticus (presumed to be sl-CSD) (Nair et al., 2018). This species almost exclusively parasitizes Hyposter horticola (Gravenhorst), which is itself an egg-larval parasitoid of the butterfly Melitaea cinxia (L.). Fluctuations of M. cinxia populations have significant effect on the population sizes and the amount of diploid male production of M. cf. stigmaticus. A model based on field-collected data series shows that hyperparasitoid populations can go extinct from DMP (Nair et al., 2018), but extinction events are for very local metapopulations. The authors suggest that dispersal and balancing selection in favor of rare csd alleles prevents complete regional extinctions (Nair et al., 2018), in line with previously suggested models (Weis et al., 2017). Another more recent model demonstrates a role for host availability in parasitoid DMV likelihood, in addition to the already established preventative factors of balancing selection, partial diploid male fertility, and dispersal (Nonaka & Kaitala, 2020).
Only a few of the probably numerous non-CSD mechanisms have been identified in Hymenoptera. Their diversity is consistent with the large variety of animal sex determination systems overall (Bachtrog, 2014; Beukeboom & Perrin, 2014). Although there are fewer studies on non-CSD species, known DMP in these species is also reliant on sex determination, but the effects are unlikely to be as harmful as those for CSD species. The gregarious blowfly pupal parasitoids of Nasonia lend insight on this matter. Nasonia have the only other well characterized hymenopteran sex determination system, maternal effect genomic imprinting sex determination (MEGISD). In short, a maternally transmitted factor in the oosome (transformer) and a paternally inherited active gene overriding a silenced maternal copy (wasp overruler of masculinization) is needed for female development (Verhulst, 2010; Zou et al., 2020). All of the four described species of Nasonia have high inbreeding tolerance. Indeed, males even emerge from hosts a day before females and wait by the exit hole to mate with sisters (Moynihan & Shuker, 2011). Stable female isolines can be established and there is evidence for outbreeding depression in laboratory populations (Luna & Hawkins, 2004). Diploid males do not arise through inbreeding, but rather have appeared spontaneously in laboratory stocks (Whiting, 1960) and are generated with knockdowns of genes in the female developmental pathway (Verhulst, 2010; Geuverink et al., 2017; Zou et al., 2020). They are unusually fecund and produce viable and triploid daughters with reduced reproductive ability (Whiting, 1960; Beukeboom & Kamping, 2006; Leung et al., 2019; Leung, 2020). Although diploid males can be impaired in some ways such as reduced total fecundity and mating ability, they have not been documented in nature and can be produced only in limited numbers in the laboratory after the first generation (Leung, 2020). It is therefore unlikely that they imperil either natural or captive populations.
The non-CSD solitary Asobara are parasitoids of larval drosophilids and have thelytoky, a form of parthenogenesis that produces diploid female offspring (Kremer et al., 2009; Ma et al., 2013). In Asobara spp., DMP corresponds with low levels of Wolbachia endosymbiotic bacteria. The lower the titer, the higher the number of diploid males develop from what would otherwise become normal diploid females (Ma et al., 2013, 2015). Although these diploid males are sterile, they likely do not increase population extinction risk as females can reproduce without any males; indeed, Asobara japonica Belokobylskij has both sexually reproducing populations and asexually reproducing populations that are all-female (Kremer et al., 2009). Although studies on diploid males of non-CSD species are limited, they seem to suggest that the DMV is a less likely risk compared to CSD species (Leung et al., 2019). Even though the diploid males themselves are individually disadvantaged, they have not been problematic for the many non-CSD taxa that are active or candidate biocontrol agents either in control efficacy or in production. This includes for example the facultatively gregarious Trichogramma spp., a global biocontrol agent powerhouse used against a large range of lepidopteran pests (van Lenteren et al., 2018).
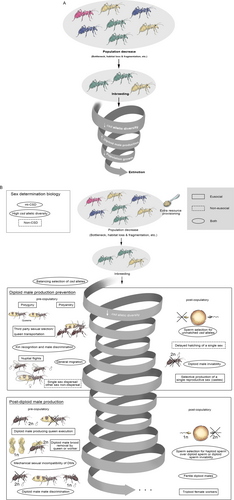
Across the variety and prevalence of hymenopteran DMP and DMV avoidance mechanisms (visually represented in Figure 2, which also distinguishes pre- and post-copulatory mechanisms to contextualize where they occur in an organism’s life history) more of these strategies are unique to the eusocial insects than the solitary species. This may be attributed to their lifestyle: for example, unlike solitary species, eusocial species care for their broods, which makes it possible for them to recognize diploid males before they reach adulthood. Specific queen and non-reproducing individuals (workers) behaviors, and their interactions among each other, making mechanisms such as third-party sexual selection and diploid male-producing queen execution possible. Figure 2 shows incompatibility of reproductive organs as a ‘mechanism’ found only in solitary species, but merely because it has only been observed in a solitary species so far. As there are also morphological differences between haploid and diploid males of eusocial species (Santomauro & Wolf, 2002; Armitage et al., 2010; Gerard et al., 2015), it is likely that physical reproductive impairment of diploid males also occurs. The DMP and DMV avoidance mechanisms of the non-eusocial (solitary and gregarious) species of this section are summarized in Table 2.
| Group | Species | Lifestyle | Population type | Observed DMP | DMV/inbreeding avoidance | Avoidance mechanism | Sex determination | References |
|---|---|---|---|---|---|---|---|---|
| Bee | Euglossa annectans | Solitary | Natural | x | None, high DMP from habitat fragmentation and reduced genetic diversity | Assumed sl-CSD | Frantine-Silva et al., 2021b | |
| E. cordata | Solitary | Natural | x | x | Lower DM than expected, proposed balancing csd allele selection and gene flow | Assumed sl-CSD | Boff et al., 2014 | |
| E. dilemma | Solitary | Natural | x | Lower DM than expected | Assumed sl-CSD | Soro et al., 2017 | ||
| E. viridissima | Solitary | Natural | x | Lower DM than expected | Assumed sl-CSD | Soro et al., 2017 | ||
| Natural (invasive) | Adaptation to new host plant | Assumed sl-CSD | Liu & Pemberton, 2009 | |||||
| Hylaeaus anthracinus | Solitary | Natural (reintroduction) | None, successful reestablishment in high-quality habitat vs. failed reestablishment with invasive predator | Unknown | Magnacca, 2020 | |||
| Lasioglossum leucozonium | Solitary | Natural (invasive) | x | x | Balancing selection of sex-determining (presumed csd) alleles | Assumed sl-CSD | Zayed et al., 2007 | |
| Parasitoid | Asobara spp. | Solitary | Captive and natural | x | None, non-CSD, DM males in absence of Wolbachia | Non-CSD | Kremer et al., 2009; Ma et al., 2015 | |
| Bracon brevicornis | Gregarious | Captive | x | x | DM mortality | sl-CSD | Thiel et al., 2014 | |
| B. hebetor | Gregarious | Captive | x | x | DM mortality | sl CSD | Whiting, 1943, Petters & Mettus, 1980; Ode et al., 1997 | |
| Cotesia flavipes | Gregarious | Captive | None, no DM observed | Not sl-CSD (?) | Niyibigira et al., 2004 | |||
| Captive | x | x | Fertile DMs | Not sl-CSD (?) | Trevisan et al., 2016 | |||
| C. glomerata | Gregarious | Captive | x | x | Fertile DMs | sl-CSD | Elias et al., 2009 | |
| Captive | None, no kin discrimination | sl-CSD | Ruf et al., 2010 | |||||
| Captive | x | Female dispersal | sl-CSD | Ruf et al., 2011 | ||||
| Captive and natural | x | Lower DM than expected; proposed sperm selection for unmatched csd alleles | sl-CSD | Ruf et al., 2013 | ||||
| Captive | x | x | Possible DM inviability (dependent on cross) | sl-CSD | Zhou et al., 2006 | |||
| C. rubecula* | Solitary | Natural (biocontrol) | x | None, ml-CSD becomes sl-CSD like from bottleneck | ml-CSD | de Boer et al., 2012 | ||
| C. sesamiae | Gregarious | Captive | None, no DM observed | Not sl-CSD (?) | Niyibigira et al., 2004 | |||
| C. vestalis | Solitary | Captive | x | Fertile DMs; ml-CSD | ml-CSD | de Boer et al., 2007, 2008 | ||
| Natural | x | x | ml-CSD | ml-CSD | de Boer et al., 2015 | |||
| Mastrus ridens | Gregarious | Captive | x | x | Fertile DMs | Assumed sl-CSD | Zaviezo et al., 2018 | |
| Mesochorus cf. stigmaticus* | Solitary | Natural | x | None, local metapopulation extinctions from host fluctuation | Assumed sl-CSD | Nair et al., 2018 | ||
| Nasonia vitripennis | Gregarious | Captive | x | x | Fertile DMs, non-CSD | MEGISD | Beukeboom et al., 2007; Leung et al., 2019 | |
| Venturia canescens | Solitary | Captive | x | x | Kin discrimination by males | sl-CSD | Chuine et al., 2015 | |
| Captive and natural | x | None, higher DMP in lower diversity and fragmented populations | sl-CSD | Collet et al., 2016 | ||||
| Captive and natural | x | x | Male dispersal; kin discrimination | sl-CSD | Collet et al., 2020 | |||
| Captive | x | x | DM mating discrimination | sl CSD | Fauvergue et al., 2015 | |||
| Sawfly | Neodiprion lecontei | Gregarious | Captive | x | x | Kin discrimination; DM mortality | sl-CSD | Harper et al., 2016 |
| Wasp | Euodynerus foraminatus | Solitary | Captive and natural | x | x | Fertile DMs | sl-CSD | Cowan & Stahlhut, 2004 |
| Philanthus triangulum | Solitary | Natural | x | Kin discrimination | sl-CSD | Herzner et al., 2006 |
- An asterisk denotes possible DMV incidence. ‘Natural’ refers to a population observed in the field, or materials taken directly from the field. ‘Captive’ refers to laboratory-reared populations. Avoidance mechanisms are specific to each reference. sl-CSD, ml-CSD: single-locus, multi-locus complementary sex determination; MEGISD, maternal effect genomic imprinting sex determination.
DIPLOID MALE VORTEX INCIDENCE IS RARE AND IS ASSOCIATED WITH OTHER FACTORS
The DMV is purportedly more likely to occur for populations under atypical stress, such as populations of invasive species, captive breeding populations of honey-producers, pollinators, and biocontrol agents, and populations that are experiencing habitat fragmentation, climate change stress, host loss, or pesticide effects (Zayed & Packer, 2005). These populations are all logically more vulnerable to inbreeding, genetic bottlenecks, and founder effects. Without reiterating the studies already discussed above, here we discuss what evidence exists for the DMV occurring either in nature or in captivity in these situations.
Hymenopteran invasions are pervasive due to human activity. Ant invasions in particular have been highly successful and are responsible for the decline of numerous native species (Holway et al., 2002). Having undergone csd allele loss, invasive populations may even exhibit a majority of diploid males, as with the eusocial sl-CSD Chinese hornet Vespa velutina nigrithorax De Buysson introduced to France (Darrouzet et al., 2015), and the South American ant S. invicta that has spread globally (Ross et al., 1993). In the case of the latter, there is a clear contrast of DMP rate in native populations at 13.1% (Krieger et al., 1999) to introduced ones at 83.3% (Ross et al., 1993). Although it has been suggested that DMP in invasive populations predict extinction, no specific cases are known (although laboratory colonies of S. invicta with DMP go extinct; Ross & Fletcher, 1986). A review on invasive eusocial hymenopterans notes that many successful invaders have DMP management strategies, and that allelic richness can be restored with de-novo mutations and secondary invasions. However, they also point out that invasions that quickly go extinct may never be documented, and in such cases it cannot be known whether a DMV was a driving factor (Hagan & Gloag, 2021). Inbreeding avoidance and kin recognition has been observed, for example, for the invasive eusocial wasp Vespula germanica (Fabricius), which avoids aggregating with nest-mates over non-nestmates (Masciocchi et al., 2020). Regardless, there may even be benefits to inbreeding as a founder effect, as with the invasive eusocial paper wasp Polistes dominulus (Christ). This species interestingly has not undergone a bottleneck in csd diversity post-invasion (Johnson & Starks, 2004; Liebert et al., 2005), but expresses less agonistic behavior mating with a nestmate vs. a non-nestmate. This may assist in stabilizing the population in the early founding stage (Liebert et al., 2010). It is notable that in contrast the related sl-CSD Japanese species Polistes chinensis antennalis Pérez has undergone a severe bottleneck following its introduction to New Zealand, but is also a successful invader although it has increased diploid male and triploid female frequency (Tsuchida et al., 2002, 2014). Interestingly these colonies also produce some triploid males (Tsuchida et al., 2014). This is a rare phenomenon also known to occur in the invasive ant S. invicta (Murakami et al., 2021) and widespread bumblebee B. terrestris (Ayabe et al., 2004). Of P. chinensis antennalis, the authors speculate that these triploid males have triploid sperm and produce tetraploid females, although how triploid males affect the DMV dynamic has not been well explored for any species. Nevertheless, we reinforce the caveat that invasive population DMVs may be underreported if extinctions occur before observation.
Species bred in captive conditions are considered particularly vulnerable to inbreeding and the DMV because they start from a limited number of individuals. For A. mellifera honeybees, the upkeep of diploid males is theoretically costly for the colony, and yet it seems that their effect is negligible in both models and in practice as long as the colony is well provisioned (Betti & Lee, 2020). An argument might be that A. mellifera is particularly well protected due to polyandry (Ratnieks, 1990) and diploid male brood removal (Woyke, 1963). However, the Melipona stingless bees (used for honey production in the Amazon) are monogynous and do not remove diploid male brood (Ratnieks, 1990). Its presumed high vulnerability to diploid male effects led to the proposal that keepers maintain a minimum of 44 colonies to prevent the population from dying out (Kerr, 1985). And yet, with extra resources (and worker executions of diploid-producing queens), it is possible to build and maintain medium-to-large expanding populations with a few or even a single starter colony (Nogueira-Neto, 2002). These and other highly inbred colonies survived throughout a 10-year observation period (Nogueira-Neto, 2002; Alves et al., 2011). Diploid males are frequently observed in B. terrestris colonies, bred for pollination of commercial crops (Gerard et al., 2015). Inbreeding itself generally does not endanger the survival of B. terrestris colonies, with inbred and outbred colonies performing equally in the field (Whitehorn et al., 2009a). In one study, a hibernating queen experiences a slight increase in mortality from having mated with a brother, but this can be offset by earlier mating and hibernation; furthermore, outbreeding depression is as common as inbreeding depression (Gerloff & Schmid-Hempel, 2005). However, B. terrestris may be a rare example of true DMV events, with DMP-producing colonies having reduced fitness, offspring production, colony growth, and field survival time (Whitehorn et al., 2009a). Another bumblebee, presumed sl-CSD Bombus terricola Kirby, a once common North American native pollinator now in decline, has also undergone population-wide inbreeding. Recent selection for immunity genes hints that this may be due to pathogenic stress, and with up to 40% matched csd allele matings, authors speculate that it will be subject to a DMV in the near future (Kent et al., 2018).
Biocontrol agents are similarly subject to inbreeding concerns. In many cases the genetic diversity of these populations cannot be supplemented with more individuals from the origin due to current trade laws complicating importation of biological materials (Cock et al., 2010; Lommen et al., 2017; Leung et al., 2020). And yet, so far there have not been any reports of captive industrial populations suffering inbreeding effects. It could be that these effects exist but are not considered problematic because target production or control goals were met, or private interests simply do not publicize DMVs occurring for their biocontrol agents. Alternatively, it could be that at least some companies follow the advice of Roush & Hopper (1995) and Bai et al. (2005), and preserve allelic diversity by maintaining multiple genetically distinct populations or isolines (the latter would only apply to non-CSD species; Stouthamer et al., 1992) that are only mixed periodically upon application. There are thus few examples of biocontrol DMVs, but an exception may be a classical biocontrol program of C. rubecula released in North America. Due to a genetic bottleneck, this ml-CSD species shows a high rate of diploid male production, effectively becoming a sl-CSD-like species due to near population-wide homozygosity of one of its two csd loci (de Boer et al., 2015). If this trend continues, with the other locus progressively losing allelic diversity, C. rubecula may undergo a true DMV. However, another contributory factor is that there is a high degree of hyperparasitoidism for this biocontrol agent (McDonald & Kok, 1991; Weis et al., 2016), so population declines may not be due to DMP effects alone or primarily.
Conservation programs for Hymenoptera are not common, but this may change in the face of a global crisis of biodiversity and biomass loss for pollinators (Goulson et al., 2008; Potts et al., 2010; Nicholson & Egan, 2020) and insects in general (Fonseca, 2009; Schachat & Labandeira, 2021). However, a thriving population of the endangered solitary yellow-face bee Hylaeus anthracinus (Smith) was founded with only 100 individuals reintroduced (translocated) to a site in their historical range. This population benefited from high native floral diversity and a lack of predators whereas two other attempted reintroductions died out from invasive bigheaded ants, Pheidole megacephala (Fabricius) (Magnacca, 2020). Habitat quality and not founder effect therefore was the determinant for population extinction in this case. This is consistent with the greater trend for global pollinator losses, for which the DMP and the DMV have not been directly implicated, although it is possible they contribute. Researchers more directly cite synergistic stress from habitat fragmentation, climate change, pesticide usage, alien species, and flower and host loss (Potts et al., 2010; Latty & Dakos, 2019). It is not a give-in that even extreme effect from one or more of these factors prompts the DMV either. For instance, host availability should have an immediate effect on its distribution of highly host-specific species. This can be seen in solitary bees that are highly specialized such as the euglossine orchid bees, a group defined by male behavior of collecting volatiles and in the process pollinating co-associated orchid species, in order to attract female mates (Faria et al., 2016). There is one case of higher DMP for an orchid bee (Euglossa annectans Dressler) due to habitat fragmentation and reduced genetic diversity (Frantine-Silva et al., 2021b), but E. cordata (Boff et al., 2014), E. dilemma, and E. viridissima (Soro et al., 2017) do not experience population declines from the same factors. In fact, there is also at least one case of an orchid bee (E. viridissima) becoming invasive and the primary pollinator of an invasive plant (Solanum torvum Swartz), demonstrating that even the most specialized of hymenopterans can decouple from their host plant and adapt (Liu & Pemberton, 2009).
In summary, although it is possible there is a publication bias towards identification of avoidance mechanisms, there are few definite cases of population declines from the DMV. Possible examples cite additional exacerbatory factors with it being unclear whether DMP is the primary cause. For example, monoculture and pesticides worsen what would otherwise be insignificant DMP effects on honeybees (Betti & Lee, 2020). The question becomes, under what circumstances would a DMV actually occur? The answer might be that taxa must lack DMP and DMV preventative mechanisms, the total variety and frequency of which is unknown as only a fraction of the immense hymenopteran diversity has been assessed. But more generally, several models suggest that Zayed & Packer (2005) underestimated population survival time because gene flow occurs for most populations (Nonaka & Kaitala, 2020). This assumption of low gene flow in simplified models may have caused the DMV to be predicted more often than it actually occurs in reality. Moreover, even though the time to reach adulthood for most Hymenoptera is relatively short (i.e., a few weeks), the generation time can be very long, especially in eusocial species. To illustrate this, if a small population of ants produces reproducing offspring once a year and it takes 40 generations before significant loss of genetic diversity occurs (Hein et al., 2009), it would take over 40 years before inbreeding effects would become evident.
FUTURE OUTLOOKS
- Delineate full CSD molecular mechanisms to better link them to functional outcomes. A csd locus has only been identified in sl-CSD A. mellifera, encoding an SR-type (RNA splicing) protein (Beye et al., 2003). Whereas the presence of CSD is detectable with inbreeding assays and the number of csd loci estimable by the number of generations needed for diploid males to appear (Cowan & Stahlhut, 2004; Schrempf et al., 2006; Ma et al., 2013), for all other species these loci are only putative in nature. Surprisingly, the increasing availability of sequenced genomes for CSD species – e.g., B. terrestris and Bombus impatiens Cresson (Sadd et al., 2015), Cotesia congregata (Say) (Gauthier et al., 2021), C. glomerata (Pinto et al., 2021), B. brevicornis (Ferguson et al., 2020) – has not yet resulted in the delineation of additional CSD mechanisms, although candidate regions have been suggested (B. terrestris, Gadau et al., 2001; B. brevicornis, Ferguson et al., 2020). Knowing the location, identity, and function of csd genes would give better insight for downstream consequences of homozygosity. For example, if there is partial redundancy with other genes this could perhaps explain why the DMV is not as severe as predicted in some cases. Furthermore, knowledge about genomic processes would elucidate means to prevent csd loci from becoming homozygous and DMP, e.g., through gene conversion or crossing-over mechanisms.
- Identify any taxonomic patterns to DMP and DMV effects. Theoretically there may be evolutionarily conserved clustering, making it easier to predict extinction risk by phylogenetic placement. And yet this cannot be assumed, because insect sex determination mechanisms and downstream DMP consequences are highly variable even for related species (Beukeboom & Perrin, 2014). Besides the previously described variation in csd loci number for Cotesia spp., Muscidifurax spp. do not have MEGISD despite being in the same family as Nasonia spp. (Wang, 2021), and the non-CSD genus Asobara (Ma et al., 2015) is in the same family as sl-CSD Bracon spp. (Whiting, 1943), for example. Because of the sheer diversity of Hymenoptera there is a corresponding lack of comprehensive representation of the whole order for DMP and DMV studies. However, as knowledge increases, it will become clear whether or not species relatedness correlates to similarity in DMV incidence and avoidance mechanisms.
- For biological control, know whether the sex determination mechanism is CSD a priori and, if possible, use a ml-CSD or non-CSD species. Although this review recovered little solid evidence for DMV in biocontrol breeding or field performance, it is still important to account for biocontrol agent’s sex determination. Logically the latter two better protected against increased diploid male production (and the corresponding fewer females to control the pest) in introduced populations in case of bottlenecks. Based on species diversity of various applicable groups there are abundant non sl-CSD biocontrol agent candidates (Beukeboom et al., 2007; Forbes et al., 2018). Alternatively, for sl-CSD biocontrol agents, careful monitoring of the genetic variation or simultaneous maintenance of separate lines in captivity prior to admixture for a field population can help to prevent DMVs.
- Our review suggests that the high presence of DMs in a population does not necessarily point towards extinction risk or even population declines. However, for conservational efforts of natural populations of specialized species, it may still be helpful to monitor the frequency of DMP if combined effects with loss of associated hosts and habitats represent a real threat. For example, extreme habitat loss has driven two native bumblebee species – Bombus muscorum (L.) (Darvill et al., 2006) and B. florilegus Panfilov (Takahashi et al., 2008) – to produce as much as 50% diploid males, possibly reflecting DMVs in progress, distinct in principle from invasive and human-maintained populations that can tolerate high DMP. In general, it is very important to promote connectivity in fragmented areas, to promote dispersal and gene flow (Hein et al., 2009; Frantine-Silva et al., 2021a).
- Increase study on host effect on the DMV for parasitoids. There have been some studies on how host plant presence impacts DMP for, e.g., pollinators (Whitehorn et al., 2009a; Kent et al., 2018) and the orchid bees (Liu & Pemberton, 2009). And yet, despite the tightly linked ecology of parasitoids and their hosts, there is surprisingly little research on how host dynamics impact parasitoid diploid male production and population health. The hyperparasitoid M. cf. stigmaticus experiencing local metapopulation extinctions due to fluctuations of the lepidopteran host of its own parasitoid host (Nair et al., 2018) indicates that there is justification for researching this topic further. As is, there is not enough information to judge whether host effect is generally important to DMV likelihood. Given the extreme biodiversity of parasitoid-host relationships, more knowledge is needed to establish host population relevance to parasitoid DMP frequency and DMV risk.
It is our aim that these recommendations provide a framework for further refinement of Zayed & Packer’s (2005) original conceptualization of the hymenopteran diploid male vortex.
AUTHOR CONTRIBUTIONS
Kelley Leung: Conceptualization (lead); supervision (lead); visualization (lead); writing – original draft (supporting); writing – review and editing (lead). Henk van der Meulen: Conceptualization (supporting); methodology (lead); writing – original draft (lead); writing – review and editing (supporting).
Acknowledgements
We thank Leo W. Beukeboom, Pauline R. Romeyer, and Fuyu Ye for textual improvements. We also thank Xuan Li for designing Figure 2. This work was supported by the Netherlands Organisation for Scientific Research project OCENW.KLEIN.333.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analysed for this manuscript, which is a review




